www.elsevier.com / locate / bres
Research report
Inhibition of evoked glutamate release by the neuroprotective 5-HT
1Areceptor agonist BAY x 3702 in vitro and in vivo
*
´
Frank Mauler , Thomas Fahrig, Ervin Horvath, Reinhard Jork
Bayer AG, PH-R CNS, Aprather Weg 18a, 42096 Wuppertal, FRG Accepted 26 September 2000
Abstract
Brain ischemia provoked by stroke or traumatic brain injury induces a massive increase in neurotransmitter release, in particular of the excitotoxin glutamate. Glutamate triggers a cascade of events finally leading to widespread neuronal cell damage and death. The aminomethylchroman derivative BAY x 3702 is a novel neuroprotectant which shows pronounced beneficial effects in various animal models of ischemic brain injury. As shown previously BAY x 3702 binds to 5-HT1A receptors of different species in subnanomolar range and is characterized as a full receptor agonist. In this study we investigated the influence of BAY x 3702 on potassium-evoked glutamate release in vitro and ischemia-induced glutamate release in vivo. In rat hippocampal slices BAY x 3702 inhibited evoked glutamate release in a dose-dependent manner (IC5051mM). This effect was blocked by the selective 5-HT1Areceptor antagonist WAY 100635, indicating that BAY x 3702 specifically acts via 5-HT1A receptors. In vivo, release of endogenous aspartate and glutamate was measured in the cortex of rats by microdialysis before and after onset of permanent middle cerebral artery occlusion. Single dose administration of BAY x 3702 (1mg / kg or 10mg / kg i.v.) immediately after occlusion reduced the increase and total release of extracellular glutamate by about 50% compared to non-treated animals, whereas the extracellular aspartate levels were not significantly affected. Inhibition of glutamate release may therefore contribute to the pronounced neuroprotective efficacy of BAY x 3702 in various animal models of ischemic brain damage. 2001 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Ischemia
Keywords: BAY x 3702; Glutamate release; Ischemia; Middle cerebral artery occlusion; Microdialysis
1. Introduction levels represents a non-physiological stimulus, triggering various intracellular processes including activation of Ischemic brain damage caused by stroke or traumatic lipases and nitric oxide synthase, formation of oxygen free brain injury is characterized by an immediate depletion of radicals and release of neurotransmitters as glutamate, cellular energy levels. Massive ion fluxes across the dopamine, serotonin etc. [11,19,22]. As a result of this plasma membrane and breakdown of the energy-driven cascade neurons finally die and represent new sources of membrane potential induces liberation of neurotransmitters neurotoxic glutamate [40].
into the extracellular space, in particular the excitotoxin Based on the prominent role of glutamate, strategies for glutamate [2,20,36,39,42]. Excess of glutamate leads to a development of pharmacological principles interfering with continuous activation of NMDA, AMPA and metabotropic the cascade of ischemia-mediated cell death have initially glutamate receptors, resulting in massive calcium influx focused on receptors, which trigger the neurotoxic effects and mobilization of intracellular calcium stores [32]. An caused by glutamate, i.e., on NMDA receptors including extensive and long-lasting rise in intracellular calcium their modulatory sites, and AMPA receptors [5]. Glutamate antagonists directly acting at the postsynaptic glutamate receptors show considerable side effects, including
hypo-*Corresponding author. Tel.: 149-202-36-4926; fax: 1
49-202-36-tension and psychotomimetic effects in humans, and can
5122.
E-mail address: [email protected] (F. Mauler). cause neurotoxic injury in animals [26,32,45]. So far, most
glutamate receptor antagonists investigated in clinical trials Krebs–Ringer solution and were kept at 378C for 30 min. revealed no therapeutic efficacy due to unfavorable risk– Slices were then transferred sequentially, 8 min each, to benefit ratio or lack of efficacy [13,28]. However, new Eppendorf tubes containing Krebs–Ringer solution (basal compounds acting via modulatory sites at the NMDA release), Krebs–Ringer solution containing 75 mM KCl receptor like the glycine receptor antagonist GV 150526 do (stimulated release), Krebs–Ringer solution (wash-out), not produce neuronal vacuolization or cognitive distur- Krebs–Ringer solution containing BAY x 3702 (effect of bances. In addition, clinical studies revealed that this BAY x 3702 on basal release), and Krebs–Ringer solution, compound is well tolerated, even at plasma levels, which containing both BAY x 3702 and 75 mM KCl (effect of displayed neuroprotective efficacy in preclinical models of BAY x 3702 on stimulated release). Controls without stroke. drug(s) were handled similar. The hippocampal slices were Alternative strategies to attenuate glutamate-mediated then dissolved in 2 N NaOH and protein content was toxicity have been set up. These approaches include determined according to a modification of the Lowry inhibition of second messenger cascades involved in method [21]. Glutamate content was determined glutamatergic signaling and blockade of ion channels fluorimetrically using the NAD/ glutamate dehydrogenase which may counteract excessive ischemia-induced neuro- reduction assay. In brief, 100 ml of each sample were nal depolarization. 5-HT1A receptor agonists have been added to 1 ml of an aqueous solution of 0.5 M glycine, 0.4
1
tested in various models of ischemic damage and have M hydrazine. Finally, 20ml of NAD (6.64 mg / ml) were revealed some neuroprotective efficacy in vivo and in vitro added and fluorescence measured at ex. 340 nm / em. 440 [14,34,35]. However, all compounds investigated so far nm. The formation of NADH was induced by addition of showed only restricted 5-HT1A receptor selectivity, affinity 10 ml glutamate dehydrogenase (10 mg / ml) and was and / or intrinsic activity. Recently, the amino- determined 5 min after reaction start. Fluorescence was methylchroman derivative BAY x 3702 was described as a converted into mmol glutamate by means of a respective novel highly potent 5-HT1A receptor full agonist with standard curve.
pronounced neuroprotective properties in various animal
models of ischemic brain injury [1,14–16,23,24,41]. 2.2.2. Permanent middle cerebral artery occlusion In the present study we investigated the effects of BAY ( pMCA-O)
x 3702 on glutamate release both in vitro and in vivo in
order to evaluate whether modulation of glutamatergic 2.2.2.1. Animals. Male Long–Evans rats (300–350 g, neurotransmission may be involved in the neuroprotective Mollegaards Breeding Center Ltd, Li Skensved, Denmark) properties of this compound. were allowed to adapt to housing conditions for at least 1 week before they were subjected to the study. They were housed in groups of 3–5 individuals in macrolon cages
2. Material and methods (type III, Ehret, Emmendingen, FRG) bedded with saw-dust. The animal housing room and the laboratory for 2.1. Chemicals and reagents surgery were climate controlled and continuously illumi-nated from 7.00 a.m. until 7.00 p.m. Room temperature All chemicals were of highest commercially available was 21.561.58C, relative humidity was 40–60%. Food
purity and were purchased from E. Merck, Darmstadt, (Altromin 1324, Altomin Spezialfutterwerk GmbH, Lage, FRG. BAY x 3702 (R-(2)-2-h4-[(chroman-2-ylmethyl)- FRG) and water were available ad libitum.
amino]-butylj-1,1-dioxo-benzo[d]isothiazolone
hydrochlo-ride) and WAY 100635 (N-[2-[4-(2-methoxyphenyl)-1- 2.2.2.2. Occlusion. Under general anesthesia (Isoflurane piperazinyl]ethyl]-N(2-pyridinyl)cyclohexane carboxamide 4–1.5% mixed with air as inhalational anesthetics) the trihydrochloride) were obtained from the Chemistry De- middle cerebral artery (MCA) was occluded unilaterally partment of Bayer AG, Wuppertal, FRG. Isoflurane was based on the surgical procedure of Bederson and co-obtained from Abbot GmbH, Wiesbaden, FRG. workers [4]. Briefly, the left temporal–parietal region of the head was shaved, the skin was disinfected and opened 2.2. Methods between the orbit and the external ear canal. Following a midline incision, the temporal muscle was divided and 2.2.1. Determination of glutamate release from pulled aside with surgical hooks to make the lateral aspect
between the olfactory tract and the inferior cerebral vein software (Perkin Elmer, Friedrichshafen, FRG; Version 3), by microbipolar electrocoagulation (Bipolator 50, Fischer and stored electronically and as hardcopy.
MET GmbH, Freiburg, FRG). To avoid recanalization the
occluded vessels were removed. The operation area was 2.2.5. Statistical analysis
covered with a small piece of sterile absorbable gelatin Statistical analysis of hippocampal glutamate release
sponge (Marbagelan , Behringwerke AG, Marburg, FRG). data was performed by ANOVA followed by Fisher’s LSD Muscle and skin wounds were closed with tissue glue post hoc analysis. P-values #0.05 were considered to be
(Histoacryl , B. Braun Melsungen, Melsungen, FRG). statistically significant. Statistical analysis for mi-During surgery and microdialysis the body temperature crodialysis probes was performed by repeated-measures was monitored and maintained between 36.5–37.58C with ANOVA with pairwise multiple comparison test using the a heating plate. LSD test (SAS version 6.12, SAS Institute Inc., USA).
Significance was accepted at the P,0.05 level. 2.2.3. Microdialysis
Three hours before occlusion of the MCA the mi- 2.2.6. Drug application
crodialysis probes (CMA10, 4 mm, Carnegie Medicine, The lyophilized formulation of BAY x 3702 was recon-Stockholm, Sweden) were implanted under general anes- stituted by adding of physiological saline. The drug-solu-thesia into the cortex (interaural line coordinates frontal tion or vehicle was administered intravenously (i.v.) as a
17.7, lateral 15.8, horizontal 16.8 [33]). Microdialysis bolus injection directly after pMCA-O. Application vol-probes were connected to a perfusion pump (CMA100, ume was 2 ml / kg body-weight. Control groups received Carnegie Medicine, Stockholm, Sweden) and to a fraction physiological saline only.
collector (CMA140, Carnegie Medicine, Stockholm, Sweden). Flow was set to 2 ml / min. The dialysis fibers
were perfused immediately after implantation with Krebs– 3. Results
Ringer solution consisting of 118 mM NaCl, 4.7 mM KCl,
2.5 mM CaCl , 1.2 mM MgSO , 1.2 mM NaH PO , 102 4 2 4 3.1. Glutamate release in vitro mM glucose, 25 mM NaHCO , pH 7.4. The dialysate of3
the first 90 min was discharged, samples were collected in During incubation of hippocampus slices in physiologi-15 min intervals 90 min before and 180 min after pMCA- cal buffer glutamate is spontaneously released into the O and stored at2708C until HPLC analysis. Localization surrounding medium. Mean basal release was |0.5 mmol
of the microdialysis probe was controlled microscopically glutamate / mg protein / min. Transfer of the tissue pieces
1
after each experiment. into high potassium buffer (75 mM K ) resulted in an 20–40% increase of glutamate accumulation in the 2.2.4. HPLC analysis of endogenous glutamate and medium. After wash out of excess of potassium, basal
aspartate glutamate release recovered to about 95% of that observed Aspartate and glutamate were analyzed using automated prior to stimulation. A second exposure of hippocampal precolumn o-phthaldialdehyde (OPA) derivatization and slices to elevated extracellular potassium concentration fluorescence detection. Twenty microliters of the dialysate caused an increase in extracellular glutamate concentra-was mixed with 25 ml H O and 252 ml internal standard tions. The increase in released glutamate was of similar (homocysteinic acid) and derivatized with 70 ml OPA amplitude as that observed after the first potassium-in-solution (120 mg OPA, 2 ml methanol, 20 ml 0.5 M duced stimulation. Basal glutamate release was not effect-borate, pH 9.5, 100 ml mercaptopropionic acid) in a ed by BAY x 3702 at concentrations up to 5mM. However, temperated rack (88C) using a L-7250 programmable BAY x 3702 inhibited potassium-stimulated release in a autosampler (Merck Hitachi, Darmstadt, FRG). After 3 dose-dependent manner (Fig. 1). Maximal inhibition was min the pH of the probe was shifted to pH 8.0 by adding achieved at 5–10mM, concentrations at which stimulated 150 ml of column buffer (50 mM sodium phosphate pH release was nearly completely blocked. The estimated IC50
3.2. Microdialysis
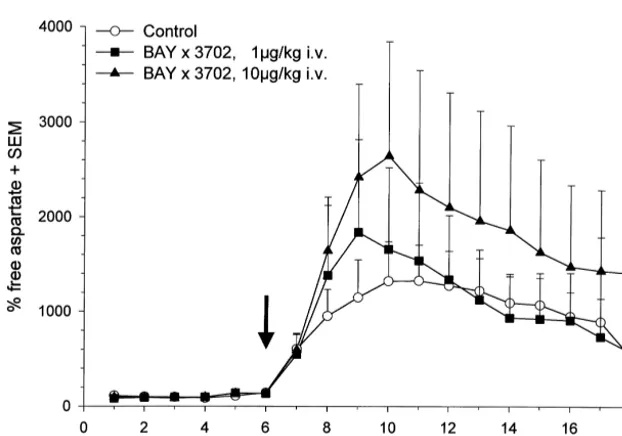
The effects of BAY x 3702 on glutamate and aspartate release are summarized in Figs. 3 and 4. In the control and treatment groups extracellular free glutamate (5.160.12 nM /ml dialysate) and aspartate (1.3260.07 nM /ml dialysate) base levels increased rapidly in a first phase within 30 min after onset of pMCA-O and peaked at 45 (treatment group) and at 105 min (control group). In the control group the maximal increase of free extracellular glutamate and aspartate over basal levels was 1300% (69.967.7 nM /ml dialysate) and 1000% (18.8262.6 nM /
ml dialysate), respectively.
Free extracellular glutamate levels were reduced but not totally blocked by i.v. bolus administration of 1 or 10
mg / kg BAY x 3702 immediately after pMCA-O. Applica-tion of 1 mg / kg reduced glutamate levels, which become statistically significant 90 min after occlusion. Application of 10 mg / kg showed a clear tendency to reduce the free glutamate levels under ischemic conditions but becomes significant at the last time point only. Comparison of total release of glutamate using area under curve (AUC)
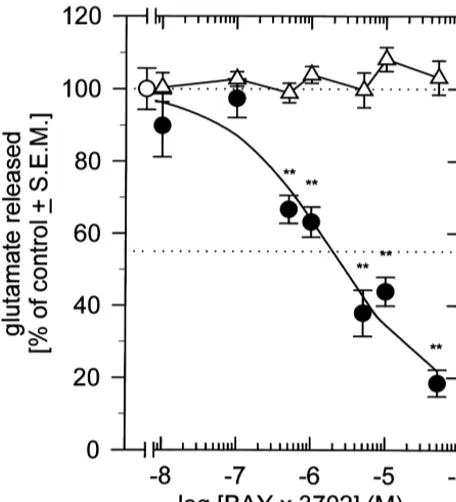
analy-Fig. 1. Inhibition of potassium-evoked glutamate release from rat hip- sis revealed similar results. Compared to control values the pocampal slices by BAY x 3702. Rat hippocampal slices were incubated
total glutamate release was reduced to 41.6610.5% at 1
without (D) or with increasing concentrations of BAY x 3702 (d) and
mg / kg (P50.068) and to 54.2614.1% at 10 mg / kg (P5 potassium-stimulated glutamate release was determined fluorimetrically.
0.188), respectively.
The mean amount of glutamate released / mg protein during the second
stimulation period (s) was set 100% and the other values calculated in The cortical aspartate levels were not diminished by
relation. Data are means6S.E.M. from 3 to 21 experiments carried out in BAY x 3702 but showed the tendency to increase. Statisti-duplicates. **P,0.01.
cal analysis did not revealed significant difference between control and treatment groups, as well as AUC analysis for the 1 mg / kg (108.9659.4%, P50.926) and 10 mg / kg (164696%, P50.513) group.
4. Discussion
The aminomethylchroman derivative BAY x 3702 is a novel neuroprotectant and has previously been character-ized as a highly potent and selective 5-HT1A receptor full agonist which bound to human and rat 5-HT1A receptors with nearly identical affinities [16]. Strong neuroprotective efficacy of BAY x 3702 has been demonstrated in a number of different animal models of acute ischemic brain injury [1,14,23,24].
Preclinical as well as clinical studies indicate that excitatory amino acids are massively increased in brain tissue after occlusive stroke [6]. Among others, therapeutic activity of BAY x 3702 in animal models of stroke and traumatic brain injury may therefore involve attenuation of
Fig. 2. Reversal of BAY x 3702-induced inhibition of glutamate release
extracellular free glutamate concentrations. The present
from rat hippocampal slices by WAY 100635. Rat hippocampal slices
were incubated with increasing concentrations of WAY 100635 in the experiments were conducted in order to study the effects of
presence or absence of 1 mM BAY x 3702. Potassium-stimulated BAY x 3702 on glutamate release from rat hippocampal glutamate release was determined fluorimetrically. The mean amount of slices in vitro and on ischemia-induced excitatory amino glutamate released during the second stimulation period was set 100%
acid release in a rat pMCA-O model in vivo.
and the other values calculated in relation. Data are means6S.E.M. from
BAY x 3702 suppressed the potassium-evoked glutamate
12 to 21 experiments carried out in duplicates. *P,0.05, n.s., not
concentration-depen-Fig. 3. Time-course of free extracellular glutamate before and after pMCA-O. Experiments were performed as described in Materials and methods. BAY x 3702 was applied i.v. as single bolus at 1mg / kg (j) and 10mg / kg (m) immediately after occlusion of the middle cerebral artery. Control animals (s) received vehicle alone. Surgery was performed between 75 and 90 min after start of sample collection, arrow indicates the start of the pMCA-O. Values are the mean6S.E.M. of at least six independent experiments. *statistically significant difference from control, P,0.05.
dent manner. These effects were mediated by activation of although this reduction only reached statistical significance 5-HT1A receptors because they were blocked by the for the lower dose group. This was a little bit surprising, specific 5-HT1A receptor antagonist WAY 100635. This in because studies addressing the neuroprotective efficacy of vitro observation was corroborated by the finding that BAY x 3702 in different animal models of brain ischemia administration of BAY x 3702 at doses displaying pro- revealed no difference between either dose. However, nounced neuroprotective efficacy in the pMCA-O model although the localization of the microdialysis probe was reduced the ischemia-induced increase in extracellular confirmed by histological analysis after each experiment, glutamate concentrations in cerebral cortex by about 50%, the relatively large individual variations of the infarcted
Fig. 4. Time-course of free extracellular aspartate before and after pMCA-O. Experiments were performed as described in Materials and methods. BAY x 3702 was applied i.v. as single bolus at 1mg / kg (j) and 10mg / kg (m) immediately after occlusion of the middle cerebral artery. Control animals (s)
cortical areas may have led to localization errors, i.e., parts In addition to a neuronal localization, 5-HT1A receptor of the probes may have been placed in parenchymal areas expression has also been shown in astroglial cells, both in which were not susceptible to pharmacological interven- vitro and in situ [44]. Although not yet demonstrated, tion by BAY x 3702. activation of astroglial 5-HT1A receptors may modulate Previous reports demonstrated that ischemia-induced glial glutamate transport by changing the conduction extracellular glutamate levels increased rapidly after onset properties of the glial cell membrane. An effect of BAY x of pMCA-O and declined thereafter, but did not reach base 3702 on glial glutamate transport can therefore not be levels as found in transient MCA-O models excluded.
[8,9,17,18,37,39]. Wahl and coworkers demonstrated that Ischemia-induced changes in extracellular aspartate glutamate release follows a multiphasic time-course in a levels were not affected by BAY x 3702 treatment. model of permanent focal cerebral ischemia, suggesting Comparable results with respect to extracellular free the involvement of a cascade of different mechanisms. The aspartate levels in rat cortex under ischemic conditions
21
first phase may represent the classical Ca -dependent have also been reported by Meldrum and coworkers [30]. glutamate release of neuronal origin due to ATP loss and They found that the sodium channel blocker BW1003C87
21
anoxic depolarization. The second phase was Ca -in- reduced glutamate release while showing no effect on free dependent and is thought to reflect glutamate release or aspartate concentrations. The reasons for this remain liberation from non-neuronal and / or disrupted cells [43]. unclear.
Recent studies revealed, that glutamate overflow in the Physiological effects resulting from 5-HT1A receptor second phase may be mainly mediated by ischemia-in- activation are not limited to the serotonergic system but duced reversal of the astrocyte glutamate transporter GLT- imply both hormonal and other neurotransmitter systems as 1 [37,38]. Using a transient MCA-O model, Seki and reflected by e.g., elevation of plasma ACTH, cortico-coworkers have shown, that block of GLT-1 with steroids or prolactin levels, and enhanced release of dihydrokainate significantly reduced ischemia-induced glu- noradrenaline and acetylcholine [3,27]. However, the tamate increase by more than 50% as measured by relationship between the above-mentioned 5-HT1A -medi-microdialysis in the striatum. Furthermore, analysis of the ated effects and neuroprotective efficacy remains un-data revealed a significant delay in the onset of the initial known. One consequence of these complex and intensive rise in extracellular glutamate concentrations. interactions and a well known pharmacological effect of Two principle basic mechanisms may account for the 5-HT1A agonists is the induction of hypothermia. As BAY x 3702-mediated decrease in extracellular glutamate: recently reported, hypothermia attenuates glutamate release (i) inhibition of glutamate release from synaptic vesicles or [7,29,31] resulting in significant neuroprotection [25]. A from glial cells provoked by reversed glutamate transport significant contribution of hypothermia to BAY x 3702 and / or (ii) stimulation of glutamate uptake. So far, we induced reduction of free glutamate seems to be unlikely have no experimental evidence for a direct interaction of for the following reasons: (i) body temperature of the BAY x 3702 with any of the currently known glutamate animals was maintained at 36.5–37.58C during the whole transporters, thus making it unlikely that BAY x 3702 experiment (see Methods), (ii) in the highest dose group activity was due to direct modulation of glutamate trans- temperature decrease is weak and not sufficient to at-port (increased uptake or block of reversed transat-port). tenuate glutamate release (218C at 10mg / kg, i.v. after 30 More likely, effects of BAY x 3702 on glutamate release min which raised to normal within further 30 min) [16], and / or uptake may be due to the hyperpolarizing activity (iii) temperature drop is negotiable in the low dose group of 5-HT1A receptor agonists, which in turn would lead to in which reduction of glutamate release was even more diminished vesicular neurotransmitter release. 5-HT1A pronounced.
receptors are located in areas which are highly sensitive to In conclusion, BAY x 3702 inhibits both potassium-damage induced by ischemic stroke or brain trauma, such evoked glutamate release in vitro and ischemia-induced as the hippocampus and the cerebral cortex, as well as on glutamate release in vivo. One putative molecular mecha-serotonergic cell bodies and dendrites in the raphe nuclei. nism involved may be 5-HT1A receptor-mediated hyperpo-Activation of 5-HT1A receptors results in neuronal hy- larization of the neuronal membrane resulting in reduced perpolarization via several mechanisms, e.g., by opening of depolarization-induced vesicular release of glutamate.
Inhi-21 1
G-protein-coupled and Ca -independent K channels bition of glutamate release may be a major activity [10,12], which leads to reduction or total block of neuronal involved in the pronounced neuroprotective effects of BAY firing rate. This has been shown for BAY x 3702 in dorsal x 3702 in animal models of acute, ischemic brain injury. raphe nucleus (DRN) using electrophysiological recordings
in vivo or from slice preparations in vitro. If administered
intravenously or into the bath solution BAY x 3702 Acknowledgements
induced an almost complete and long-lasting, but
Ginsberg, Effect of ischaemia on the in vivo release of striatal
References
dopamine, glutamate and g-aminobutyric acid studied by in-tracerebral microdialysis, J. Neurochem. 51 (1988) 1455–1464. [1] B. Alessandri, E. Tsuchida, R.M. Bullock, The neuroprotective
¨
[20] H. Hagberg, A. Lehmann, M. Sandberg, B. Nystrom, I. Jacobson, A. effect of a new serotonin receptor agonist, BAY x 3702, upon focal
Hamberger, Ischemia-induced shift of inhibitory and excitatory ischemic brain damage caused by acute subdural hematoma in the
amino acids from intra- to extracellular compartments, J. Cereb. rat, Brain Res. 845 (1999) 232–235.
Blood Flow Metab. 5 (1985) 413–419. [2] A.J. Baker, M.H. Zornow, M.S. Scheller, S.R. Skilling, D.H.
[21] C.R. Harrington, Lowry protein assay containing sodium dodecyl Smullin, A.A. Larson, R. Kuczenski, Changes in extracellular
sulfate in microtiter plates for protein determinations on fractions concentrations of glutamate, aspartate, glycine, dopamine, serotonin,
from brain tissue, Analyt. Biochem. 186 (1990) 285–287. and dopamine metabolites after transient global ischemia in rabbit
¨ ¨
[22] L. Hillered, A. Hallstrom, S. Segersvard, L. Persson, U. Ungerstedt, brain, J. Neurochem. 57 (1991) 1370–1379.
Dynamics of extracellular metabolites in the striatum after middle [3] N.M. Barnes, T. Sharp, A review of central 5-HT receptors and their
cerebral artery occlusion in the rat monitored by intracerebral function, Neuropharmacolgy 38 (1999) 1083–1152.
microdialysis, J. Cereb. Blood Flow Metab. 9 (1989) 607–616. [4] J.B. Bederson, L.H. Pitts, M. Tsuji, M.C. Nishimura, R.L. Davis, H.
´
[23] E. Horvath, K.-H. Augstein, Neuroprotection by the novel 5-HT
Bartkowski, Rat middle artery occlusion: evaluation of the model 1A
receptor agonist BAY x 3702 in the rat model of acute subdural and development of a neurologic examination, Stroke 17 (1986)
hematoma, J. Neurotrauma 14 (1998) 800. 472–476.
´
[24] E. Horvath, K.-H. Augstein, R. Wittka, Neuroprotective effect of the [5] P.A. Boxer, C.F. Bigge, Mechanisms of neuronal cell injury / death
novel 5-HT receptor agonist BAY x 3702 in a rat model of and targets for drug intervention, Drug Discovery Today 2 (1997) 1A
permanent focal cerebral ischemia and traumatic brain injury, Soc. 219–228.
Neurosci. Abstr. 23 (1997) 1923. [6] R. Bullock, A. Zauner, J. Woodward, H.F. Young, Massive persistent
[25] P.W. Huh, L. Belayev, W.Z. Zhao, S. Koch, R. Busto, M.D. release of excitatory amino acids following human occlusive stroke,
Ginsberg, Comparative neuroprotective efficacy of prolonged mod-Stroke 26 (1995) 2187–2189.
erate intraischemic and postischemic hypothermia in focal cerebral [7] R. Busto, W.D. Dietrich, M.Y. Globus, M.D. Ginsberg, Postischemic
ischemia, J. Neurosurg. 92 (2000) 91–99. moderate hypothermia inhibits CA1 hippocampal ischemic neuronal
[26] J. Kornhuber, M. Weller, Psychotogenicity and N-methyl-D-aspartate
injury, Neurosci. Lett. 101 (1989) 299–304.
receptor antagonism: implications for neuroprotective pharmaco-[8] S.P. Butcher, R. Bullock, D.I. Graham, J. McCulloch, Correlation
therapy, Biol. Psychiat. 41 (1997) 135–144. between amino acid release and neuropathologic outcome in rat
brain following middle cerebral artery occlusion, Stroke 21 (1990) [27] T. Koyama, Y. Nakajima, T. Fujii, K. Kawashima, Enhancement of 1727–1733. cortical and hippocampal cholinergic neurotransmission through [9] J. Chen, S.H. Graham, R.P. Simon, A comparison of the effects of a 5-HT1A receptor-mediated pathways by BAY x 3702 in freely
sodium channel blocker and an NMDA antagonist upon extracellular moving rats, Neurosci. Lett. 265 (1999) 33–36.
glutamate in rat focal cerebral ischemia, Brain Res. 699 (1995) [28] K.R. Lees, Cerestat and other NMDA antagonists in ischemic stroke,
121–124. Neurology 49 (1997) S66–S69.
[10] A. Colino, J.V. Halliwell, Differential modulation of three separate [29] C.M. Maier, K.v. Ahern, M.L. Cheng, J.E. Lee, M.A. Yenari, G.K. K-conductances in hippocampal CA1 neurons by serotonin, Nature Steinberg, Optimal depth and duration of mild hypothermia in a 328 (1987) 73–77. focal model of transient cerebral ischemia: effects on neurologic [11] G. Damsma, D.P. Boisvert, L.A. Mudrick, D. Wenkstern, H.C. outcome, infarct size, apoptosis, and inflammation, Stroke 29 (1998)
Fibiger, Effects of transient forebrain ischemia and pargyline on 2171–2180.
extracellular concentrations of dopamine, serotonin, and their metab- [30] B.S. Meldrum, J.H. Swan, M.J. Leach, M.H. Millan, R. Gwinn, K. olites in the rat striatum as determined by in vivo microdialysis, J. Kadota, S.H. Graham, J. Chen, R.P. Simon, Reduction of glutamate Neurochem. 54 (1990) 801–808. release and protection against ischemic brain damage by BW [12] M.F. Davies, R.A. Deisz, D.A. Prince, S.J. Peroutka, Two distinct 1003C87, Brain Res. 593 (1992) 1–6.
effects of 5-hydroxytryptamine on single cortical neurons, Brain [31] A. Mitani, K. Kataoka, Critical levels of extracellular glutamate Res. 423 (1987) 347–352. mediating gerbil hippocampal delayed neuronal death during hypo-[13] J. De Keyser, G. Sulter, P.G. Luiten, Clinical trials with neuroprotec- thermia: brain microdialysis study, Neuroscience 42 (1991) 661–
tive drugs in acute ischaemic stroke: are we doing the right thing?, 670.
Trends Neurosci. 22 (1999) 535–540. [32] J.W. Olney, J. Labruyere, M.T. Pirce, Pathological changes induced ´
[14] J. De Vry, H. Dietrich, T. Glaser, H.-G. Heine, E. Horvath, R. Jork, in cerebrocortical neurons by phencyclidine and related drugs, T. Maertins, F. Mauler, W. Opitz, D. Scherling, R. Schohe-Loop, T. Science 244 (1989) 1360–1362.
Schwarz, BAY x 3702, Drugs of the Future 22 (1997) 341–349. [33] G. Paxinos, C. Watson, The Rat Brain in Stereotactic Coordinates, [15] J. De Vry, K.R. Jentzsch, Discriminative stimulus properties of the 2nd Edition, Academic Press, New York, 1982.
5-HT1Areceptor agonist BAY x 3702 in the rat, Eur. J. Pharm. 357 [34] B. Peruche, C. Backhauß, J.H.M. Prehn, J. Krieglstein, Protective
(1998) 1–8. effects of 5-HT1A receptor agonists against neuronal damage
[16] J. De Vry, R. Schoe-Loop, H.-G. Heine, J.M. Greul, F. Mauler, B. demonstrated in vivo and in vitro, J. Neural Transm. 8 (1994) Schmidt, H. Sommermeyer, T. Glaser, Characterization of the 73–83.
aminomethylchroman derivate BAY x 3702 as a highly potent [35] J.H.M. Prehn, M. Welsch, C. Backhauß, J. Nuglisch, F. Ausmeier, C. 5-hydroxytryptamine1A receptor agonist, J. Pharm. Exp. Ther. 284 Karkoutly, J. Krieglstein, Effects of serotonergic drugs in ex-(1998) 1082–1094. perimental brain ischemia: evidence for a protective role of [17] J.L. Graham, S.E. Smith, A.G. Chapman, B.S. Meldrum, Effect of serotonin in cerebral ischemia, Brain Res. 630 (1993) 10–20.
Lamotrigine on microdialysate amino-acid concentration in the [36] S.M. Rothman, J.W. Olney, Glutamate and the pathophysiology of striatum after focal cerebral-ischemia in the rat, Brit. J. Med. 112 hypoxic–ischemic brain damage, Ann. Neurol. 19 (1986) 105–111. (1994) U 141. [37] Y. Seki, P.J. Feustel, R.W. Keller, B.I. Tranmer, H.K. Kimelberg, [18] S.H. Graham, J. Chen, F.R. Sharp, R.P. Simon, Limiting ischemic Inhibition of ischemia-induced glutamate release in rat striatum by injury by inhibition of excitatory amino acid release, J. Cereb. Blood dihydrokainate and an anion channel blocker, Stroke 30 (1999)
Flow Metab. 13 (1993) 88–97. 433–440.
Matsuoka, T. Nishizaki, N. Saito, The neuroprotective agent MS- Martinez, S. Kraydieh, R. Busto, Changes in amino acid neuro-153 stimulates glutamate uptake, Eur. J. Pharm. 386 (1999) 263– transmitters and cerebral blood flow in the ischemic penumbral
270. region following middle cerebral artery occlusion in the rat:
[39] H. Shimizu, S.H. Graham, L.H. Chang, J. Mintorovitch, T.L. James, correlation with histopathology, J. Cereb. Blood Flow Metab. 13 A.I. Faden, P.R. Weinstein, Relationship between extracellular (1993) 575–585.
neurotransmitter amino acids and energy metabolism during cerebral [43] F. Wahl, T.P. Obrenovitch, A.M. Hardy, M. Plotkine, R. Boulu, L. ischemia in rats monitored by microdialysis and in vivo magnetic Symon, Extracellular glutamate during focal cerebral ischaemia in resonance spectroscopy, Brain Res. 605 (1993) 33–42. rats: time course and calcium dependency, J. Neurochem. 63 (1994)
¨
[40] B.K. Siesjo, Pathophysiology and treatment of focal cerebral 1003–1011.
ischemia. Part II: mechanisms of damage and treatment, J. Neuro- [44] P.M. Whitaker-Azmitia, The discovery of serotonin and its role in surg. 77 (1992) 337–354. neuroscience, Neuropsychopharmacology 21 (1999) S2–S8. [41] B. Suchanek, H. Struppeck, T. Fahrig, The 5-HT1Areceptor agonist [45] M.A. Yenari, T.E. Bell, A.N. Kotake, M. Powell, G.K. Steinberg,