www.elsevier.com / locate / bres
Interactive report
Neuroprotective signaling and the aging brain: take away my food and
1let me run
*
Mark P. Mattson
Laboratory of Neurosciences, National Institute on Aging Gerontology Research Center, 5600 Nathan Shock Drive, Baltimore, MD 21224-6825, USA
Accepted 8 August 2000
Abstract
It is remarkable that neurons are able to survive and function for a century or more in many persons that age successfully. A better understanding of the molecular signaling mechanisms that permit such cell survival and synaptic plasticity may therefore lead to the development of new preventative and therapeutic strategies for age-related neurodegenerative disorders. We all know that overeating and lack of exercise are risk factors for many different age-related diseases including cardiovascular disease, diabetes and cancers. Our recent studies have shown that dietary restriction (reduced calorie intake) can increase the resistance of neurons in the brain to dysfunction and death in experimental models of Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and stroke. The mechanism underlying the beneficial effects of dietary restriction involves stimulation of the expression of ‘stress proteins’ and neurotrophic factors. The neurotrophic factors induced by dietary restriction may protect neurons by inducing the production of proteins that suppress oxyradical production, stabilize cellular calcium homeostasis and inhibit apoptotic biochemical cascades. Interestingly, dietary restriction also increases numbers of newly-generated neural cells in the adult brain suggesting that this dietary manipulation can increase the brain’s capacity for plasticity and self-repair. Work in other laboratories suggests that physical and intellectual activity can similarly increase neurotrophic factor production and neurogenesis. Collectively, the available data suggest the that dietary restriction, and physical and mental activity, may reduce both the incidence and severity of neurodegenerative disorders in humans. A better understanding of the cellular and molecular mechanisms underlying these effects of diet and behavior on the brain is also leading to novel therapeutic agents that mimick the beneficial effects of dietary restriction and exercise. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Genesis of neurons and glia
Keywords: Alzheimer’s disease; Calories; Heat shock protein; Oxidative stress; Mitochondria; Neurotrophic factor; Parkinson’s disease; Stroke
1. Low calorie intake and exercise: what’s good for the apply to neurodegenerative disorders including Alzheim-body is good for the brain er’s disease (AD), Parkinson’s disease (PD) and stroke. Before describing the findings that support the latter Clinical and epidemiological data unequivocally show statement, it is important to briefly review the extensive that overeating and lack of exercise increase the risk for data that document the beneficial effects of caloric restric-the most prevalent of age-related diseases including car- tion in the retardation of aging and disease.
diovascular disease, diabetes and cancers [1,23,26]. Con- The lifespans of laboratory mice and rats can be versely, a decrease in calorie intake and an increase in increased by up to 50% simply by reducing their calorie exercise can reduce risk for the same diseases. Emerging intake with maintenance of micronutrient intake [42]. Such data from our studies of animal models and from epi- dietary restriction (DR) also reduces the development of demiological data suggest that a similar scenario may age-related cancers and deficits in immune function. The cellular and molecular underpinnings of the beneficial
1 effects of DR are not yet fully understood, but may involve
Published on the World Wide Web on 24 August 2000.
reduced mitochondrial oxyradical production and / or in-*Tel.:11-410-558-843; fax:11-40-558-8465.
E-mail address: [email protected] (M.P. Mattson). creased expression of cytoprotective stress proteins. Might
DR also benefit the brain? Although benefits of DR on the that mimicks several features of PD [28]. Stroke models cardiovascular, immune and endocrine systems have been include transient or permanent occlusion of the middle demonstrated, its effects on the nervous system are only cerebral artery in rats and mice [4]. We have investigated now being studied. Studies of rats and mice maintained on the impact of DR on the neurodegenerative process in a DR feeding regimen suggest that DR may slow age- several of these animal models.
related molecular changes in the brain including increases In many of our studies we employed an alternate day in levels of glial fibrillary acidic protein and oxidative feeding DR regimen; this DR protocol results in an damage to proteins and DNA [8,11]. Moreover, DR approximately 30% reduction in calorie intake over time attenuates age-related deficits in learning and memory and extends the lifespans of rats and mice by 30–40%. ability and motor function in rodents [19,44]. Interestingly, Maintenance of rats on the alternate day DR feeding physical activity (which can reduce body weight) may also regimen for 2–4 months results in resistance of hippocam-counteract the adverse effects of aging and disease on the pal neurons to kainate-induced degeneration [3]. The brain. Thus, mice allowed access to a running wheel reduced damage to hippocampal neurons is correlated with exhibit increased neurogenesis and improved learning and a striking preservation of learning and memory in a water memory compared to ‘couch potato’ mice [45]. In addi- maze spatial learning task. In order to determine whether tion, when rodents are raised in an enriched environment in DR might counteract the pathogenic actions of mutations which they have many objects to play with and so on, in presenilin-1 and the amyloid precursor protein (APP), neurogenesis is enhanced and learning and memory ability we maintained presenilin-1 mutant knockin mice and APP
improved [22,37,47]. mutant transgenic mice on the alternate day feeding
regimen. We had previously shown that presenilin-1 muta-tions increase the vulnerability of hippocampal and cortical 2. Experimental and epidemiological support for neurons to excitotoxicity and apoptosis by a mechanism neuroprotective effects of dietary restriction involving enhanced calcium release from the endoplasmic reticulum [15,34]. Presenilin-1 mutant knockin mice that Because life expectancy is increasing, more and more had been maintained on DR for 3 months exhibited persons will suffer from age-related neurodegenerative increased resistance of hippocampal CA1 and CA3 neu-conditions with AD, PD and stroke being the most rons to excitotoxic injury compared to mice fed ad libitum prevalent. AD results from degeneration and death of [51]. Levels of oxidative stress in the hippocampus follow-neurons in brain regions involved in learning and memory ing kainate administration were lower the DR mice com-processes, such as the hippocampus and cerebral cortex pared to mice fed ad libitum, indicating that suppression of [29,30,40]. Degeneration of dopaminergic neurons in the oxidative stress may be one mechanism underlying the substantia nigra, with consequent motor dysfunction is the neuroprotective effect of DR. Thus, the neurodegeneration-defining feature of PD [21,41]. Stroke results from occlu- promoting effect of a mutation that causes AD can be sion or rupture of a cerebral blood vessel which damages counteracted by a reduced calorie diet.
the brain cells supplied by that vessel [4,10]. The impact of APP mutant mice exhibit progressive age-dependent these disorders on our society is emphasized by the fact deposition of Ab in their brains which is most prominent that more dollars are required to care for patients with AD, in the cerebral cortex and hippocampus [12,18]. In an PD and stroke than are spent on care for patients with initial test of the hypothesis that DR would suppress Ab
determin-ing if, under the latter conditions, as well as in APP mutant genesis of AD, PD, HD and stroke. The striking beneficial mice maintained on a pair-feeding DR regimen, deposition effects of DR in these experimental models suggest that of Ab in the brain is decreased. DR may prove beneficial in reducing the incidence and / or DR also has a beneficial effect in experimental models severity of many different human neurodegenerative dis-of the movement disorders. We have shown that the orders. The following epidemiological data support the vulnerability of midbrain dopaminergic neurons to MPTP latter possibility.
toxicity is decreased in mice maintained on DR [5]. More Recent epidemiological data suggest that individuals dopaminergic neurons survive exposure to MPTP, and with a low calorie intake may have reduced risk for AD deficits in motor function are markedly decreased in DR and PD. Analyses of different populations throughout the rats. Selective degeneration of neurons in the striatum of world reveals a strong relation between per capita food patients with Huntington’s disease (HD) is responsible for consumption and risk for AD [14]. For example, persons in their inability to control body movements properly. An China and Japan have relatively low calorie intakes (1600– animal model of Huntington’s disease involves administra- 2000 calories / day) as compared to persons in the United tion of the succinate dehydrogenase inhibitor (mitochon- States and Western Europe (2500–3000 calories / day). The drial toxin) 3-nitropropionic acid (3NP) to rats and mice. incidence of AD in China and Japan is approximately half Maintenance of rats on a DR regimen for several months that in the United States and Western Europe, although it prior to administration of 3NP results in increased resist- should be recognized that per capita food consumption is a ance of striatal neurons to 3NP and improved motor very poor measure of energy intake. It may also be the
function [3]. case that AD is underdiagnosed in countries such as China,
Amyotrophic lateral sclerosis (ALS) is a fatal disease and therefore the kinds of relationships established in such characterized by progressive degeneration of spinal cord cross-cultural comparisons are not conclusive. More com-motor neurons resulting in progressive paralysis. A small pelling evidence that caloric restriction can protect against percentage of cases of ALS result from mutations in the neurodegenerative disorders comes from prospective gene encoding the antioxidant enzyme Cu / Zn-superoxide studies of a large cohort of people living in New York dismutase. Transgenic mice expressing mutant Cu / Zn- City. It was found that those with the lowest daily calorie SOD exhibit progressive motor neuron degeneration and a intakes had the lowest risk for AD [35] and PD [27]. In clinical phenotype remarkably similar to ALS patients both of these studies nutrient intake was assessed using a [38]. We maintained Cu / Zn-SOD mutant mice on a DR semi-quantitative food-frequency questionnaire which in-regimen to determine the impact of a reduced calorie diet cluded 61 foods, use of vitamin and mineral supplements, on age of disease onset and disease progression. In contrast types of breakfast cereals consumed, type of fats used for to the beneficial effects of DR in the PD and HD models, frying and baking, use of sugar and salt, and alcohol. Each Cu / Zn-SOD mutant mice do not benefit from DR [38]. DR food had a fixed portion size, and nutrient content of each did not delay disease onset and, once mice became portion was estimated from USDA food composition data. symptomatic, disease progression was actually accelerated. Subjects were asked how often the consumed each food, These findings are important because they show that DR and were asked to report their usual dietary pattern over cannot overcome the pathogenic action of the Cu / Zn-SOD the last year. To control for possible disease-related mutation suggesting that the neurodegenerative cascade in changes in diet, the subjects were asked to report changes this mouse model is fundamentally different than that in in their dietary habits in the last 10 years. Finally, the the AD, PD and HD models. However, an alternative epidemiological data suggesting that overeating is a major interpretation is that not all populations of neurons benefit risk factor for stroke is quite compelling [2]. Our ex-equally from DR. From the clinical perspective these perimental data described above further suggest that a high findings are also of interest because it is well known that calorie intake can worsen outcome following stroke [49]. It increased energy intake has a beneficial effect on disease remains to be determined whether reduced calorie intake progression in ALS patients. will result in improved outcome following stroke in
We have also determined whether DR can modify humans. outcome in a rat stroke model in which the middle cerebral
artery is transiently occluded resulting in damage to the
cerebral cortex and striatum supplied by that artery, and 3. Training neurons to increase their endurance and associated motor dysfunction. Rats that had been main- power: the cellular and molecular mechanisms tained on DR for several months exhibited reduced brain underlying the beneficial effects of dietary restriction damage and improved behavioral outcome following tran- and exercise
sient occlusion of the middle cerebral artery [49]. DR is
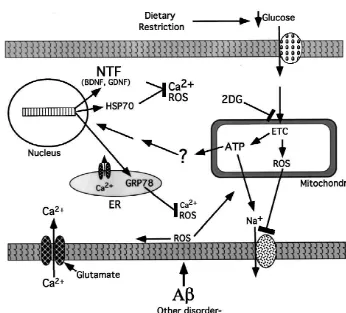
in aging and neurodegenerative disorders. Although the suggested that DR may induce a mild stress response in genetic and environmental factors that initiate the neurode- neurons, presumably because of a reduced energy (glu-generative process may differ among diseases, consider- cose) availability to the neurons. Such a ‘preconditioning’ able evidence suggests that a common set of alterations mechanism of action of DR is supported by our studies ensues that ultimately kills the neuron. Prominent among showing that metabolic stress induced by administration of the alterations are: increased oxidative stress involving 2-deoxyglucose (2-DG, a non-metabolizable analog of oxyradical-mediated damage to proteins, lipids and nucleic glucose) to animals fed ad libitum also increases resistance acids; impaired ability of the neuron to regulate ion of neurons to injury. For example, rats receiving 2-DG homeostasis resulting in aberrant increases in intracellular exhibit reduced damage to hippocampal neurons and calcium levels; impaired energy metabolism that may improved learning and memory ability following kainate result from and contribute to mitochondrial dysfunction; administration [24], and reduced damage to cortical and and activation of a cascade of molecular interactions called striatal neurons and improved behavioral outcome follow-apoptosis that involves proteins such as Par-4, Bcl-2 ing transient occlusion of the middle cerebral artery [49]. family members and caspases [33]. In AD the neurode- In addition, mice pretreated with 2-DG, exhibit decreased generative cascade can be initiated by the aging process, or damage to dopaminergic neurons in the substantia nigra by specific genetic mutations in APP, presenilin-1 or and marked attenuation of motor deficits [5]. 2-DG treat-presenilin-2. In each case there is increased production and ment resulted in increased expression of HSP-70 and extracellular deposition of a neurotoxic proteolytic peptide GRP-78. In cultured neurons 2-DG pretreatment suppres-product of APP called amyloid b-peptide (Ab). Ab ses oxidative stress, preserves mitochondrial function, promotes neuronal apoptosis and excitotoxicity by a stabilizes calcium homeostasis and attenuates neuronal mechanism involving membrane lipid peroxidation and death following exposure to excitotoxic, metabolic and impairment of ion-motive ATPases and glucose and gluta- oxidative insults [24].
mate transporters [30], PD may be caused primarily by Considerable evidence suggests that the neurodegenera-environmental factors, although a very small percentage of tive process results from alterations in synaptic terminals inherited cases have been linked to mutations in a synaptic that result in synaptic dysfunction and activation of protein called a-synuclein [41]. The neurodegenerative apoptotic and excitotoxic cascades [33]. We have found process may be triggered in dopaminergic neurons by that cortical synaptosomes prepared from rats maintained factors that induce oxidative stress such as iron and on DR exhibit increased resistance to oxidative and dopamine metabolites. Oxidative stress and perturbed metabolic insults, as indicated by relative preservation of calcium regulation are also intimately involved in the glucose and glutamate transport and mitochondrial func-neurodegenerative cascades that occur in HD and stroke. tion [16]. Synaptosomes from rats given 2-DG are also Because DR increases neuronal resistance to dysfunction more resistant to various insults [17]. The content of and death in each of the just-mentioned disorders, it seems HSP-70 and GRP-78 in the synaptosomes is increased likely that the mechanism underlying this neuroprotective suggesting that levels of neuroprotective proteins increase effect of DR somehow impinges upon the shared features locally in synaptic compartments in response to DR of the neurodegenerative cascades. [16,17]. Thus, DR bolsters the ability of synapses to cope
Data from the animal studies described above clearly with oxidative and metabolic insults.
of a BDNF blocking antibody into the lateral ventricles of numbers of newly generated cells in the dentate gyrus were rats maintained on DR significantly attenuates the protec- quantified by unbiased stereological methods. There was tive effect of DR [7]. no difference in numbers of newly generated cells at the 1 Work performed during the past decade has established day time point. However, significantly more BrdU-positive that the adult brain contains populations of cells that are cells remained at the 3 week time point in the DR rats capable of dividing and then differentiating into neurons or compared to the ad libitum-fed rats suggesting that DR glial cells, a process called neurogenesis. In rodents and promotes survival of newly generated neural cells (Figs. 1 primates such neural stem cells are most abundant in the and 2). Although not yet established, it is conceivable that subventricular zone and the dentate gyrus of the hippocam- BDNF plays a role in the enhanced survival of newly-pus [36]. The presence of stem cells in the adult brain generated neural stem cells in the dentate gyrus of rats suggests that they may provide a reserve of neural cells maintained on DR because BDNF is known to have a that can be used to replace cells that die as the result of similar effect on other populations of neural stem cells various injuries and diseases. Indeed, the proliferation of [50].
neural stem cells can be stimulated by traumatic, ischemic and excitotoxic brain injuries. Interestingly, an ‘enriched’
environment and physical exercise can also enhance 4. Conclusions neurogenesis [22,47] suggesting that the stem cells can
respond not only to injury, but also to increased functional In many different experimental animal models DR demands upon neural circuits. We have found that DR can increases resistance of neurons to the kinds of adverse modulate numbers of newly generated neural cells in the conditions believed to promote the neurodegenerative brains of rats [25]. Rats that had been maintained on ad process. Findings from animal studies are supported by libitum and DR (alternate day feeding) regimens for 3 epidemiological data, and together strongly suggest that months were given five daily injections of the DNA reduced calorie intake increases resistance of the nervous precursor bromodeoxyuridine (BrdU). Rats were killed system to disease. DR may exert its beneficial effects by either 1 day or 3 weeks after the last BrdU injection and inducing a mild ‘stress response’ which results in the
behavioral functions of the mouse, Arch. Biochem. Biophys. 333 (1996) 189–197.
[9] K. Duff, C. Eckman, C. Zehr, X. Yu, C.-M. Prada, J. Perez-Tur, M. Hutton, L. Buee, Y. Harigaya, D. Yager, D. Morgan, M.N. Gordon, L. Holcomb, L. Refolo, B. Zenk, J. Hardy, S. Younkin, Increased amyloid-b42(43) in brains of mice expressing mutant presenilin 1, Nature 383 (1996) 710–713.
[10] M. Endres, K. Fink, J. Zhu, N.E. Stagliano, V. Bondada, J.W. Geddes, T. Azuma, M.P. Mattson, D.J. Kwiatkowski, M.A. Mos-kowitz, Neuroprotective effects of gelsolin during murine stroke, J. Clin. Invest. 103 (1999) 347–354.
[11] C.E. Finch, Morgan, Food restriction and brain aging, in: M.P. Mattson, J.W. Geddes, (Eds.), The Aging Brain, JAI Press, Adv. Cell Aging Gerontol. 2 (1997) 279–297.
[12] D. Games, D. Adams, R. Alessandrini, R. Barbour, P. Berthelette, C. Blackwell, T. Carr, J. Clemens, T. Donaldson, F. Gillespie, Al-zheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein, Nature 373 (1995) 523–527. [13] C. Geula, C.K. Wu, D. Saroff, A. Lorenzo, M. Yuan, B.A. Yankner, Aging renders the brain vulnerable to amyloid beta-protein neuro-toxicity, Nature Med. 4 (1998) 827–831.
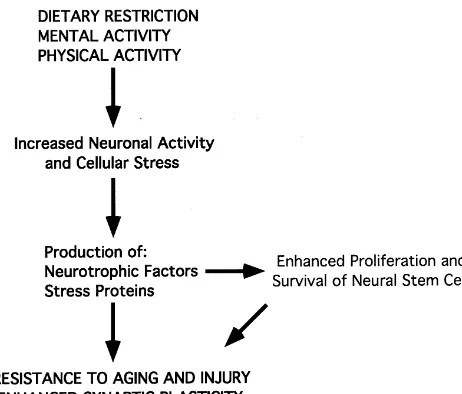
Fig. 2. The beneficial effects of dietary restriction, and mental and
[14] W. Grant, Dietary links to Alzheimer’s disease, Alz. Dis. Rev. 2 physical activity, may involve shared signaling pathways involving a mild
(1997) 42–55. stress response and production of neurotrophic factors. This pathway can
[15] Q. Guo, W. Fu, B.L. Sopher, M.W. Miller, C.B. Ware, G.M. Martin, promote survival and plasticity of neurons, and may enhance
neuro-M.P. Mattson, Increased vulnerability of hippocampal neurons to genesis.
excitotoxic necrosis in presenilin-1 mutant knockin mice, Nature Med. 5 (1999) 101–107.
[16] Z. Guo, A. Ersoz, D.A. Butterfield, M.P. Mattson, Beneficial effects expression of genes that encode proteins such as neuro- of dietary restriction on cerebral cortical synaptic terminals: pre-trophic factors and heat-shock proteins that serve to servation of glucose and glutamate transport and mitochondrial suppress oxyradical production and stabilize cellular cal- function after exposure to amyloid beta-peptide, iron, and
3-nitro-propionic acid, J. Neurochem. 75 (2000) 314–320. cium homeostasis. When extrapolated to humans, the data
[17] Z. Guo, Mattson, In vivo 2-deoxyglucose administration preserves obtained from animal studies suggest that moderate levels
glucose and glutamate transport and mitochondrial function in of DR in adult life (in the range of 1800–2200 calories / cortical synaptic terminals after exposure to amyloidb-peptide and day) may dramatically reduce the incidence and severity of iron: evidence for a stress response, Exp. Neurol. (2000) in press. AD, PD and stroke. [18] K. Hsiao, P. Chapman, S. Nilsen, C. Eckman, Y. Harigaya, S.
Younkin, F. Yang, G. Cole, Correlative memory deficits, Ab
elevation, and amyloid plaques in transgenic mice, Science 274 (1996) 99–102.
[19] D.K. Ingram, R. Weindruch, E.L. Spangler, J.R. Freeman, R.L. References
Walford, Dietary restriction benefits learning and motor performance of aged mice, J. Gerontol. 42 (1987) 78–81.
[1] M. Brochu, E.T. Poehlman, P.A. Ades, Obesity, body fat dis- [20] J.L. Jankowsky, P.H. Patterson, Cytokine and growth factor in-tribution, and coronary artery disease, J. Cardiopulm. Rehabil. 20 volvement in long-term potentiation, Mol. Cell. Neurosci. 14 (1999)
(2000) 96–108. 273–286.
[2] L.L. Bronner, D.S. Kanter, J.E. Manson, Primary prevention of [21] P. Jenner, C.W. Olanow, Understanding cell death in Parkinson’s stroke, N. Engl. J. Med. 333 (1995) 1392–1400. disease, Ann. Neurol. 44 (1998) S72–S84.
[3] A.J. Bruce-Keller, G. Umberger, R. McFall, M.P. Mattson, Food [22] G. Kempermann, H.G. Kuhn, F.H. Gage, More hippocampal neu-restriction reduces brain damage and improves behavioral outcome rons in adult mice living in an enriched environment, Nature 386 following excitotoxic and metabolic insults, Ann. Neurol. 45 (1999) (1997) 493–495.
8–15. [23] H.E. Lebovitz, Type 2 diabetes: an overview, Clin. Chem. 45 (1999) [4] U. Dirnagl, C. Iadecola, M.A. Moskowitz, Pathobiology of is- 1339–1345.
chaemic stroke: an integrated view, Trends Neurosci. 22 (1999) [24] J. Lee, A.J. Bruce-Keller, Y. Kruman, S.L. Chan, M.P. Mattson, 391–397. 2-deoxy-D-glucose protects hippocampal neurons against excitotoxic [5] W. Duan, M.P. Mattson, Dietary restriction and 2-deoxyglucose and oxidative injury: evidence for the involvement of stress proteins,
administration improve behavioral outcome and reduce degeneration J. Neurosci. Res. 57 (1999) 48–61.
of dopaminergic neurons in models of Parkinson’s disease, J. [25] J. Lee, W. Duan, J.M. Long, D.K. Ingram, M.P. Mattson, Dietary Neurosci. Res. 57 (1999) 195–206. restriction increases survival of newly-generated neural cells and [6] W. Duan, Z. Zhang, D.M. Gash, M.P. Mattson, Participation of Par-4 induces BDNF expression in the dentate gyrus of rats. J. Mol.
in degeneration of dopaminergic neurons in models of Parkinson’s Neurosci. (2000) in press.
disease, Ann. Neurol. 46 (1999) 587–597. [26] F. Levi, Cancer prevention: epidemiology and perspectives, Eur. J. [7] W. Duan, J. Lee, Z. Guo, M.P. Mattson, Dietary restriction stimu- Cancer 35 (1999) 1912–1924.
lates BDNF production in the brain and thereby protects neurons [27] G. Logroscino, K. Marder, L. Cote, M.X. Tang, S. Shea, R. Mayeux, against excitotoxic injury, Nat. Neurosci. (2000) in press. Dietary lipids and antioxidants in Parkinson’s disease: a population-[8] A. Dubey, M.J. Forster, H. Lal, R.S. Sohal, Effect of age and caloric based, case-control study, Ann. Neurol. 39 (1996) 89–94.
Hashimoto, A. Takeda, Y. Sagara, A. Sisk, L. Mucke, Dopaminergic sporadic and familial forms of Alzheimer’s disease, Mol. Med. loss and inclusion body formation in alpha-synuclein mice: implica- Today 4 (1998) 151–157.
tions for neurodegenerative disorders, Science 287 (2000) 1265– [41] J.B. Schulz, J. Dichgans, Molecular pathogenesis of movement
1269. disorders: are protein aggregates a common link in neuronal
[29] M.P. Mattson, Experimental models of Alzheimer’s disease, Science degeneration?, Curr. Opin. Neurol. 12 (1999) 433–439.
& Medicine March /April (1998) 16–25. [42] R.S. Sohal, R. Weindruch, Oxidative stress, caloric restriction, and [30] M.P. Mattson, Cellular actions of beta-amyloid precursor protein and aging, Science 273 (1996) 59–63.
its soluble and fibrillogenic derivatives, Physiol. Rev. 77 (1997) [43] B. Stein-Behrens, M.P. Mattson, I. Chang, M. Yeh, R. Sapolsky, 1081–1132. Stress exacerbates neuron loss and cytoskeletal pathology in the [31] M.P. Mattson, K. Furukawa, Programmed cell life: anti-apoptotic hippocampus, J. Neurosci. 14 (1994) 5373–5380.
signaling and therapeutic strategies for neurodegenerative disorders, [44] J. Stewart, J. Mitchell, N. Kalant, The effects of life-long food Restorative Neurol. Neurosci. 9 (1996) 191–205. restriction on spatial memory in young and aged Fischer 344 rats [32] M.P. Mattson, O. Lindvall, Neurotrophic factor and cytokine sig- measured in the eight-arm radial and the Morris water mazes,
naling in the aging brain, in: M.P. Mattson, J.W. Geddes (Eds.), The Neurobiol. Aging 10 (1989) 669–675.
Aging Brain (JAI Press, Greenwich CT), Adv. Cell Aging Gerontol. [45] H. van Praag, G. Kempermann, F.H. Gage, Running increases cell 2 (1997) 299–345. proliferation and neurogenesis in the adult mouse dentate gyrus, Nat. [33] M.P. Mattson, Apoptosis in neurodegenerative disorders, Nature Neurosci. 2 (1999) 266–270.
Rev. Mol. Cell Biol. 1 (2000) in press. [46] J.M. Warrick, H.Y. Chan, G.L. Gray-Board, Y. Chai, H.L. Paulson, [34] M.P. Mattson, F.M. LaFerla, S.L. Chan, M.A. Leissring, P.N. N.M. Bonini, Related Articles Suppression of polyglutamine-me-Shepel, J.D. Geiger, Calcium signaling in the ER: its role in diated neurodegeneration in Drosophila by the molecular chaperone neuronal plasticity and neurodegenerative disorders, Trends Neuro- HSP70, Nat. Genet. 23 (1999) 425–428.
sci. 23 (2000) 222–229. [47] D. Young, P.A. Lawlor, P. Leone, M. Dragunow, M.J. During, [35] R. Mayeux, R. Costa, K. Bell, C. Merchant, M.X. Tung, D. Jacobs, Environmental enrichment inhibits spontaneous apoptosis, prevents
Reduced risk of Alzheimer’s disease among individuals with low seizures and is neuroprotective, Nat Med. 5 (1999) 448–453. calorie intake, Neurology 59 (1999) S296–S297. [48] Z. Yu, H. Luo, W. Fu, M.P. Mattson, The endoplasmic reticulum [36] S. Momma, C.B. Johansson, J. Frisen, Get to know your stem cells, stress-responsive protein GRP78 protects neurons against excitotox-Curr. Opin. Neurobiol. 10 (2000) 45–49. icity and apoptosis: suppression of oxidative stress and stabilization [37] M. Nilsson, E. Perfilieva, U. Johansson, O. Orwar, P. Eriksson, of calcium homeostasis, Exp. Neurol. 155 (1999) 302–314.
Enriched environment increases neurogenesis in the adult rat dentate [49] Z.F. Yu, M.P. Mattson, Dietary restriction and 2-deoxyglucose gyrus and improves spatial memory, J. Neurobiol. 39 (1999) 569– administration reduce focal ischemic brain damage and improve
578. behavioral outcome: evidence for a preconditioning mechanism, J.
[38] W.A. Pedersen, M.P. Mattson, No benefit of dietary restriction on Neurosci. Res. 57 (1999) 830–839.
disease onset or progression in amyotrophic lateral sclerosis Cu / Zn- [50] T. Zigova, V. Pencea, S.J. Wiegand, M.B. Luskin, Intraventricular superoxide dismutase mutant mice, Brain Res. 833 (1999) 117–120. administration of BDNF increases the number of newly generated [39] W.A. Pedersen, C. Culmsee, D. Ziegler, J.P. Herman, M.P. Mattson, neurons in the adult olfactory bulb, Mol. Cell. Neurosci. 11 (1998)
Aberrant stress response associated with severe hypoglycemia in a 234–245.