q
Chemistry of Sponges Part 20. For Part 19 see Wellington, K.D., Cambie, R.C., Rutledge, P.S., Bergquist, P.R., 2000. J. Nat. Prod. 63, 79.
*Corresponding author.
Biochemical Systematics and Ecology 29 (2001) 199}201
Constituents of the sponge
Thorecta reticulata
qRichard C. Cambie
*
, Allick R. Lal, Peter S. Rutledge,
Keri D. Wellington
Department of Chemistry, University of Auckland, Private Bag 92019, Auckland, New Zealand
Received 23 February 2000; accepted 5 March 2000
Keywords:Thorecta reticulata; Thorectidae; Furospinosulin 1; Cometin C; Lu!arin X; Pentaprenyl-quinone; PentaprenylhydroPentaprenyl-quinone; Petrosaspongiolide B.
1. Subject and source
Thorecta reticulata Bergquist is a sponge of the family Thorectidae (order Dic-tyoceratida) collected from the Kaikoura Peninsula, New Zealand, by SCUBA at a depth of 20 m. Taxonomic identi"cation was provided by Professor Dame Patricia Bergquist, School of Biological Sciences, University of Auckland, and a voucher specimen (P.O.R. 436) has been deposited in the Museum of New Zealand, Wellington.
2. Previous work
Previously, only seven compounds have been isolated fromThorectasponges, viz., two linear sesterterpenes and the carotenoid thorexanthin fromT. horridus, collected from the Bahamas (Fattorusso et al., 1991, 1992), two linear sesterterpenes from aThorecta species collected from the Bay of Islands, New Zealand (Kernan et al., 1991), and a linear sesterterpene and a sesquiterpene quinone plus its hydroquinone fromT. choanoidescollected from southern Australia (Bonny and Capon, 1994).
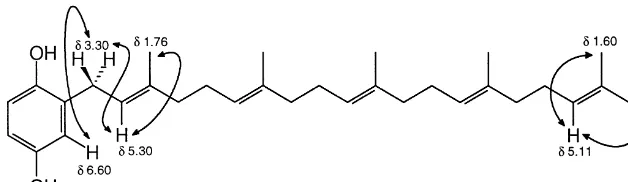
Fig. 1. COSY correlations for pentaprenylhydroquinone.
3. Present study
The freeze-dried sponge was extracted with CH
2Cl2and with MeOH. The CH2Cl2
extract was separated by#ash column chromatography on silica gel to yield furos-pinosulin 1 (0.07%) (Cimino et al., 1972), cometin C (0.004%) (Urban and Capon, 1992), lu!arin X (0.003%) (Butler and Capon, 1992), pentaprenylhydroquinone (0.01%) (Lumsdon et al., 1992), and pentaprenylquinone (0.005%) (Cimino et al., 1975). While the13C NMR spectrum of pentaprenylhydroquinone was identical with that published (Lumsdon et al., 1992), a COSY spectrum showed that the signals of a terminal methyl group and the terminal ole"nic proton in the1H NMR spectrum were incorrectly assigned and are correctly represented as in Fig. 1.
Flash-column chromatography of the MeOH extract on silica gel followed by particle size exclusion "ltration through Sephadex LH-20 gave the sesterterpene lactone petrosaspongiolide B (0.09%) (Lal et al., 1994).
4. Chemotaxonomic signi5cance
The isolation of furospinosulin 1, cometin C, lu!arin X, pentaprenylhydroquinone, and pentaprenylquinone is unexceptional and is in accordance with other compounds isolated fromThorectaspecies. The isolation of the hexacyclic sesterterpene petrosas-pongiolide B was unexpected from a Thorecta species. As such, the sponge was re-examined morphologically and the taxonomy was re-con"rmed asT. reticulata. To date petrosaspongiolides have only been isolated from the sponge Petrosaspongia nigracollected from New Caledonia (Lal et al., 1994; Paloma et al., 1997; Randazzo et al., 1998) which was introduced into the family Thorectidae by Bergquist in 1995.
References
Bergquist, P.R., 1995. Mem. Queensl. Mus. 38, 1. Bonny, M.L., Capon, R.J., 1994. Aust. J. Chem. 47, 539. Butler, M.S., Capon, R.J., 1992. Aust. J. Chem. 45, 1705.
Cimino, G., De Luca, P., De Stefano, S., Minale, L., 1975. Tetrahedron 31, 271.
Cimino, G., De Stefano, S., Minale, L., 1972. Tetrahedron 28, 1315.
Fattorusso, E., Lanzotti, V., Magno, S., Mayol, L., 1992. Z. Naturforsch. B Chem. Sci. 47, 1477. Fattorusso, E., Lanzotti, V., Magno, S., Mayol, L., De Rosa, M., Ialenti, A., 1991. Bioorg. Med. Chem. Lett.
1, 639.
Kernan, M.R., Cambie, R.C., Bergquist, P.R., 1991. J. Nat. Prod. 54, 265.
Lal, A.R., Cambie, R.C., Rickard, C.E.F., Bergquist, P.R., 1994. Tetrahedron Lett. 35, 2603. Lumsdon, D., Capon, R.J., Thomas, S.G., Beveridge, A.A., 1992. Aust. J. Chem. 45, 1321. Urban, S., Capon, R.J., 1992. Aust. J. Chem. 45, 1255.