Effects of benzidine and benzidine analogues on the growth
and nitrogenase activity of
Azotobacter
C. Pozo, M.V. Mart´ınez-Toledo, V. Salmerón, B. Rodelas, J. González-López
∗Departamento de Microbiolog´ıa, Environmental Microbiology Group, Facultad de Farmacia e Instituto del Agua, Universidad de Granada, 18071 Granada, Spain
Received 11 November 1999; received in revised form 2 March 2000; accepted 8 March 2000
Abstract
The effects of 5, 10, 25, 50mg benzidine, 4-aminobiphenyl or 3-3′-diaminobenzidine per ml of culture medium or gram of
soil was studied in dialysed-soil media, nonsterile soil incubated under aerobic conditions, and sterilized soil inoculated with
Azotobacter chroococcum. Microorganisms cultured in the presence of 4-aminobiphenyl or 3-3′-diaminobenzidine showed
lower nitrogenase activity, growth and levels of ATP, when compared with control cells. However, the presence of benzidine increased growth and biological activity ofAzotobacter, showing that these microorganisms can tolerate high concentrations of this compound. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Azotobacter; Benzidine; Azo dyes; Nitrogen fixation
1. Introduction
Commercial dyes are not generally considered as hazardous chemicals, with the exception of azo dyes. Azo dyes are synthetic organic colorants used for research purposes and in the textile, hair dying, pa-per making and food industries (Meyer, 1981). Large amounts of these compounds are discharged into the environment and are not readily degraded un-der aerobic conditions (Michaels and Lewis, 1985). The toxicity of azo dyes for microbial populations in aquatic environments is well documented and affects the processes of biotransformation and mineralization of organic compounds (Chung and Stevens, 1993). The impact of azo dyes on soil environments has been investigated to a lesser extent, but it has been reported
∗Corresponding author. Tel.: +34-958-24-3870/+
34-958-24-9966; fax:+34-958-24-6235.
E-mail address:[email protected] (J. Gonz´alez-L´opez)
that benzamines released from their degradation are toxic for several soil bacteria (Chung et al., 1997).
Benzidines are used in the manufacturing of azo dyes, and are also released from them through azo re-duction by intestinal and environmental microorgan-isms (Chung et al., 1992; Chung and Stevens, 1993). Benzidines have mutagenic and carcinogenic activ-ity and may accumulate in the food chain (Kriek, 1979). The presence of these chemicals in the environ-ment poses serious public health and environenviron-mental problems. However, the effects of benzidines on the biological activity of selected microorganisms in the environment have not been thoroughly investigated (Chung et al., 1998).
Microorganisms can be used as bioindicators for the assessment of chemical risk to ecosystems (Martinez-Toledo et al., 1998). The use of a bacterial assay system to test the toxicity of an environmental chemical is based on its effect on the growth or via-bility of the test microorganism in the system (Gaur
and Bhattacherjee, 1991). Azotobacter chroococcum
are the major group of soil aerobic N2-fixing bacteria
that keep N cycling in the biosphere (Tchan and New, 1984) and have been often used for testing the effects of various environmental toxicants on growth and ni-trogen fixation (Martinez-Toledo et al., 1990). Chung et al. (1998) examined the nitrogen-fixing capacity of
Azotobacter vinelandii grown in chemically-defined media in the presence of benzidine and benzidine analogues. We report the effect of benzidine and chemical analogues (benzidine, 4-aminobiphenyl and
3-3′-diaminobenzidine) onA. chroococcumgrown in
dialysed-soil and agricultural soils (nonsterile or ster-ile), to elucidate the effects of these substances on the
biological activity of Azotobacter, both in pure and
mixed populations such as those in soil.
2. Materials and methods
2.1. Soil samples
Soil samples used for dialysed-soil, sterile and non-sterile soil experiments, were collected from the top 10 cm of a maize field near Granada, Spain. Soil sam-ples were sieved through a 2 mm mesh screen and
stored at 4◦C before use. The soil was a typical
Xe-rorthent with loam texture, containing 50% sand, 30% silt, 20% clay, 0.12% total N and 3.77% organic
mat-ter, with pH=7.1.
2.2. Microorganisms and culture media
The bacterium used in dialysed-soil and sterile soil wasA. chroococcumstrain H23. This microorganism
was isolated from the roots ofZea mays(Hybrid AE
703) by Martinez-Toledo et al. (1985). The compo-sition of Burk’s N-free medium amended with 0.5%
glucose, and the procedure for enumeration of
Azoto-bacterspp. in the soil samples, were earlier described by Martinez-Toledo et al. (1991).
2.3. Experiments using dialysed-soil media
Agricultural soil was air dried and sieved through a 2 mm screen, and 15 g amounts were placed in-side dialysis tubing. Tubes were tied at both ends and
placed in 50 ml of distilled water in 250 ml Erlenmeyer
flasks, and autoclaved at 121◦C for 30 min. The
dial-ysis bags filled with soil were left in the flask cul-tures as a source of nutrients. The dialysate remained clear; soil particles did not spill out of the dialysis bags and, when bacteria grew, they did not penetrate the bag. The sterile equilibrated system had a pH of 7.0. Experiments were initiated by adding 0.5% glu-cose and chemicals at concentrations of 0, 5, 10, 25
and 50mg ml−1. An inoculum (about 108 cells ml−1,
optical density 0.6 at 550 nm) from a culture on N-free medium, was washed twice in sterile phosphate buffer (150 mM, pH 7.0) and then added to 250 ml
Erlen-meyer flasks. The cultures were then incubated at 28◦C
for 24, 48 and 72 h on a rotary shaker at 100 rpm. Growth curves were determined by plate counts on N-free agar media prepared by the addition of 1.5% (w/v) agar. Five replicates were prepared for each cul-ture medium. The nitrogenase activity was determined using the acetylene reduction assay (ARA) method (Hardy et al., 1968). Aliquots (3 ml) of the cultures were added to 30 ml tubes and sealed with rubber stop-pers. After 10% of the atmosphere had been replaced
by C2H2, the tubes were held at 28◦C and 0.5 ml gas
samples were assayed for C2H4after 0.5, 1 and 2 h by
injection into a Perkin-Elmer model 8420 gas chro-matograph fitted with a Poropak T column and a H-flame ionization detector. Acetylene was generated im-mediately before use from Ca carbide and water. The
ethylene contamination of the C2H2was known (about
2 nmol ml−1) and accounted for in final calculations.
Dimethyl sulfoxide (DMSO) was used to extract
ATP. Samples consisting of 50ml of the bacterial
cul-tures after growth for 24, 48 and 72 h were injected
into 8 mm×50 mm polypropylene reaction tubes
con-taining 50 ml of DMSO. After incubation for 1 min,
the samples were stored at −20◦C until used for
measurement of ATP. The procedure suggested by Chapman et al. (1971) was used for measuring ATP. Reference curves for ATP were linear within the range
from 10−5 to 10−12mg ml−1 of ATP in the reaction
mixture. Light output due to ATP was measured with a Turner 20 photometer.
2.4. Experiments using nonsterile soil
tube and 0.1 ml of distilled water was added (50–60% of field capacity). Each tube was amended with 0.1% (w/w) glucose and 0.1 ml amounts of appropriate con-centrations of benzidine and benzidine analogues were injected.
The growth ofAzotobacterspp. after aerobic
incu-bation for 24, 48 and 72 h was determined by plate counts using N-free agar media (Martinez-Toledo et al., 1985). Five replicates were prepared from each
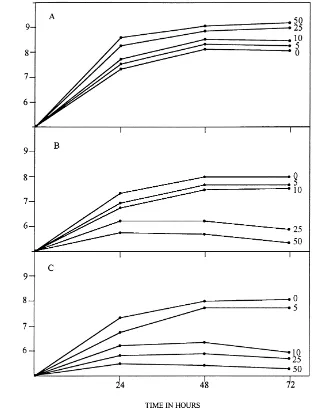
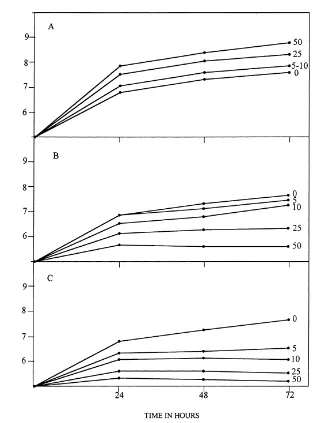
Fig. 1. Growth of Azotobacter chroococcum in the presence of concentrations ranging from 0 to 50mg ml−1 of (A) benzidine; (B)
4-aminobiphenyl and (C) 3-3′-diaminobenzidine in dialysed-soil medium with 0.5% glucose. The experiment was carried out five times.
tube. Nitrogenase activity was determined by ARA as described earlier.
2.5. Sterile soil experiments
Azotobacter chroococcum was grown in N-free medium amended with 0.5% glucose on a shaker
with gentle agitation at 28◦C for 24 h. After three
cells were harvested by centrifugation at 10,000×g
for 10 min and resuspended in sterile phosphate buffer (150 mM, pH 7.0, optical density of 0.6 at 550 nm). To investigate the effects of benzidine and benzidine
analogues on growth and ARA of A. chroococcum,
tubes containing 2 g of soil (five replicate 2 g samples were included in these experiments) were autoclaved
at 121◦C for 1 h on each of two successive days.
Each tube was then inoculated with 0.5 ml of cell suspensions of the bacterium, followed by the addi-tion of 1% (w/w) glucose. Concentrated soluaddi-tions of benzidine and benzidine analogues were prepared as described earlier and added to the tubes to give the
desired amounts. Growth and ARA of A.
chroococ-cumin the presence of chemicals were determined as
indicated earlier.
2.6. Statistical analysis
Statistical analysis of data obtained through this study was performed using STATGRAPHICS V. 5.0 (STSC, Rockville, MD, USA) software package. Analysis of variance (ANOVA) was conducted. Least significant differences between means (Student’s
t-test) were calculated at 95% confidence intervals.
Table 1
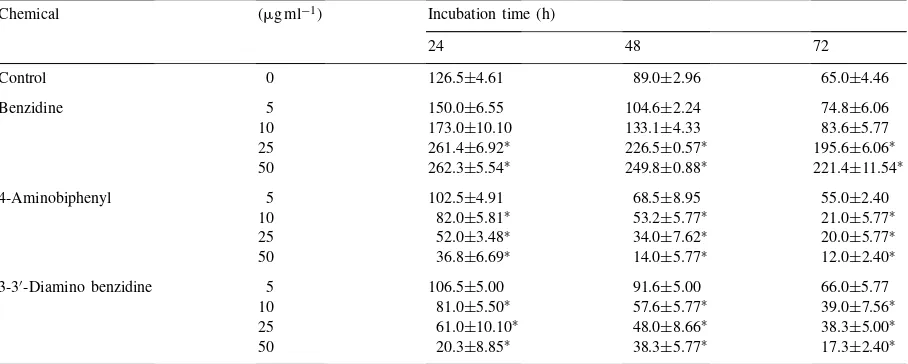
Ethylene production (nmol h−1 per 108 cells) by Azotobacter chroococcum in the presence of benzidine, 4-aminobiphenyl and 3-3′-diaminobenzidine in a dialysed-soil medium amended with 0.5% glucose
Chemical (mg ml−1) Incubation time (h)
24 48 72
Control 0 126.5±4.61 89.0±2.96 65.0±4.46
Benzidine 5 150.0±6.55 104.6±2.24 74.8±6.06
10 173.0±10.10 133.1±4.33 83.6±5.77
25 261.4±6.92∗ 226.5±0.57∗ 195.6±6.06∗
50 262.3±5.54∗ 249.8±0.88∗ 221.4±11.54∗
4-Aminobiphenyl 5 102.5±4.91 68.5±8.95 55.0±2.40
10 82.0±5.81∗ 53.2±5.77∗ 21.0±5.77∗
25 52.0±3.48∗ 34.0±7.62∗ 20.0±5.77∗
50 36.8±6.69∗ 14.0±5.77∗ 12.0±2.40∗
3-3′-Diamino benzidine 5 106.5±5.00 91.6±5.00 66.0±5.77
10 81.0±5.50∗ 57.6±5.77∗ 39.0±7.56∗
25 61.0±10.10∗ 48.0±8.66∗ 38.3±5.00∗
50 20.3±8.85∗ 38.3±5.77∗ 17.3±2.40∗
∗Significantly different from control (p
<0.001); values are means±standard error of five replicates.
3. Results
Growth of A. chroococcum in a dialysed-soil
medium amended with benzidine was increased com-pared with the growth obtained in unamended media (Fig. 1). The presence of benzidine in dialysed-soil media with 0.5% glucose stimulated ARA. The degree of stimulation was related to the concentration of the
chemical: the presence of 5, 10, 25 and 50mg ml−1
of benzidine in dialysed-soil medium caused 18, 36, 106 and 107% stimulation, respectively, after 24 h (Table 1). Under these culture conditions, the ATP
content of A. chroococcum indicated that levels of
ATP were also increased when compared with control cells grown on unamended media (Table 3).
Growth ofA. chroococcumH23 in dialysed-soil
me-dia amended with 4-aminobiphenyl or 3-3′
-diamino-benzidine was drastically affected (Fig. 1). The
presence of 4-aminobiphenyl or 3-3′-diaminobenzidine
clearly reduced ARA (Table 1). Concentrations of 5,
10, 25 and 50mg ml−1 of 4-aminobiphenyl, caused
19, 45, 59 and 72% inhibition, respectively, after 24 h of incubation. Concentrations of 5, 10, 25 and
50mg ml−1of 3-3′-diaminobenzidine, caused 16, 36,
52 and 84% inhibition, respectively, after 24 h. ATP
Table 2
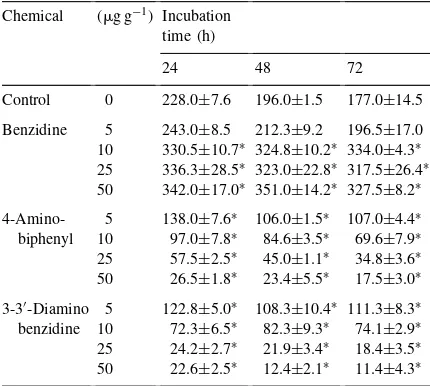
Ethylene production (nmol h−1 per 108 cells) by Azotobacter spp. in the presence of benzidine, 4-aminobiphenyl and 3-3′
-diaminobenzidine in nonsterile agricultural soil amended with 0.5% glucose
Chemical (mg g−1) Incubation
time (h)
24 48 72
Control 0 228.0±7.6 196.0±1.5 177.0±14.5
Benzidine 5 243.0±8.5 212.3±9.2 196.5±17.0 10 330.5±10.7∗ 324.8±10.2∗ 334.0±4.3∗
25 336.3±28.5∗ 323.0±22.8∗ 317.5±26.4∗
50 342.0±17.0∗ 351.0±14.2∗ 327.5±8.2∗
4-Amino- 5 138.0±7.6∗ 106.0±1.5∗ 107.0±4.4∗
biphenyl 10 97.0±7.8∗ 84.6±3.5∗ 69.6±7.9∗
25 57.5±2.5∗ 45.0±1.1∗ 34.8±3.6∗
50 26.5±1.8∗ 23.4±5.5∗ 17.5±3.0∗
3-3′-Diamino 5 122.8±5.0∗ 108.3±10.4∗ 111.3±8.3∗
benzidine 10 72.3±6.5∗ 82.3±9.3∗ 74.1±2.9∗
25 24.2±2.7∗ 21.9±3.4∗ 18.4±3.5∗
50 22.6±2.5∗ 12.4±2.1∗ 11.4±4.3∗ ∗Significantly different from control (p
<0.001); values are
means±standard error of five replicates.
media amended with 5–50mg ml−1 of
4-amino-biphenyl or 3-3′-diaminobenzidine was significantly
decreased with 25–50mg ml−1 of 4-aminobiphenyl
and 10–50mg ml−1 of 3-3′-diaminobenzidine, when
compared with control cells grown on unamended media (Table 3).
Growth of Azotobacter spp. in nonsterile soil
amended with benzidine was affected compared with growth obtained in unamended agricultural soil (Fig.
2). The presence of 10–50mg ml−1in nonsterile soil
stimulated ARA (Table 2). Our data indicate that different concentrations of benzidine could positively affect growth and nitrogenase activity in agricultural soils.
When Azotobacter spp. were cultured in nonster-ile soils under aerobic conditions in the presence of
4-aminobiphenyl or 3-3′-diaminobenzidine, strong
in-hibition of growth and ARA was observed (Fig. 2 and
Table 2). Concentrations of 5, 10, 25 and 50mg ml−1
caused a significant decrease of growth and nitro-genase activity after 24, 48 and 72 h. The degree of inhibition was related to the concentration of the chemicals, suggesting that these compounds
nega-Table 3
ATP content (ng per 108 cells) in Azotobacter
chroococ-cum cells in the presence of benzidine, 4-aminobiphenyl and 3-3′-diaminobenzidine in a dialysed-soil medium
Chemical (mg ml−1) Incubation
time (h)
24 48 72
Control 0 28.4±1.6 36.0±3.0 40.9±2.8
Benzidine 5 38.3±4.3 43.2±4.6 48.7±3.4 10 34.0±6.4 48.9±3.2 50.3±2.1
3-3′-Diamino 5 22.3±2.5 24.6±1.3 33.5±3.4
benzidine 10 18.3±2.5∗ 18.7±3.5∗ 20.3±1.5∗
25 11.1±0.3∗ 12.5±2.4∗ 13.5±1.8∗
50 9.6±2.1∗ 7.0±0.6∗ 2.7±0.7∗ ∗Significantly different from control (p
<0.001); values are
means±standard error of five replicates.
tively affect the biological activity ofAzotobacter in
agricultural soil.
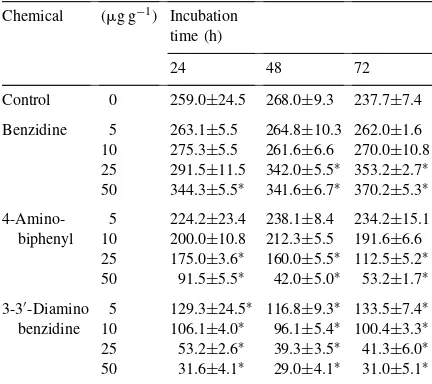
WhenA. chroococcumH23 was cultured in sterile
soil amended with 25 and 50mg ml−1 of benzidine,
strong stimulation of growth and nitrogenase activity was observed (Fig. 3 and Table 4). However, the
ad-dition of 4-aminobiphenyl or 3-3′-diaminobenzidine
caused an inhibitory effect on growth and nitrogenase
activity ofA. chroococcum(Fig. 3 and Table 4). Our
results show that these chemical compounds affect
biological activity ofA. chroococcumgrown in sterile
agricultural soil amended with 0.5% glucose.
4. Discussion
The data reported here indicate that benzidine and benzidine analogues affect microbial growth of
A. chroococcumin either a dialysed-soil medium with 0.5% glucose, nonsterile soil or sterile soil with 0.5% glucose. Since dinitrogen fixation is a growth-linked process, it was expected that as the chemicals influ-ence growth it would also affect nitrogenase activity.
In this sense, when A. chroococcum was cultured
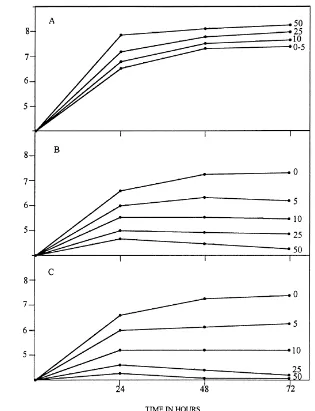
Fig. 2. Growth ofAzotobacterspp. in the presence of 0–50mg ml−1of (A) benzidine; (B) 4-aminobiphenyl and (C) 3-3′-diaminobenzidine
in nonsterile soil amended with 0.5% glucose. The experiment was carried out five times.
soil amended with benzidine, which enhanced the bacterial growth, a stimulatory effect on nitrogenase activity was observed at benzidine concentrations
ranging from 25 to 50mg ml−1 or 10 to 50mg g−1.
Under these culture conditions, the ATP content of
Azotobacterwas also increased, indicating that cellu-lar activity was higher than in control cells grown on unamended media.
The chemicals 4-aminobiphenyl and 3-3′
-diamino-benzidine tested in our study considerably
de-creased the growth and ATP level of Azotobacter
in dialysed-soil media, sterile agricultural soil or nonsterile agricultural soil. Our data indicate that
10–50mg ml−1 or 5–50mg g−1 of those compounds
Fig. 3. Growth of Azotobacter chroococcum in the presence of 0–50mg ml−1 of (A) benzidine; (B) 4-aminobiphenyl and (C)
3-3′-diaminobenzidine in sterile soil amended with 0.5% glucose. The experiment was carried out five times.
many azo compounds are reported to be poorly min-eralized, except for partial degradation into aromatic amines such as benzidine analogues, and accumulate in certain geographical areas (Chung and Stevens, 1993; Chung et al., 1997). Thus, it could be suggested that accumulation of these compounds in agricultural soils may result in a negative effect on the nitrogen economy of soils. The effect of 4-aminobiphenyl
and 3-3′-diaminobenzidine on growth and biological
activity of Azotobacter could affect the productivity
of the soil. Similar results are reported by Chung et al.
(1998) forA. vinelandiigrowth in chemically-defined
media in the presence or absence of a nitrogen source with different substrates (sucrose or glucose).
The tolerance of Azotobacter for benzidine
com-pared with the toxic effect due to 4-aminobiphenyl and
3-3′-diaminobenzidine could be of importance.
Table 4
Ethylene production (nmol h−1 per 108 cells) by Azotobacter
chroococcumin the presence of benzidine, 4-aminobiphenyl and 3-3′-diaminobenzidine in sterile agricultural soil amended with
0.5% glucose
Chemical (mg g−1) Incubation
time (h)
24 48 72
Control 0 259.0±24.5 268.0±9.3 237.7±7.4
Benzidine 5 263.1±5.5 264.8±10.3 262.0±1.6 10 275.3±5.5 261.6±6.6 270.0±10.8 25 291.5±11.5 342.0±5.5∗ 353.2±2.7∗
50 344.3±5.5∗ 341.6±6.7∗ 370.2±5.3∗
benzidine 10 106.1±4.0∗ 96.1±5.4∗ 100.4±3.3∗
25 53.2±2.6∗ 39.3±3.5∗ 41.3±6.0∗
50 31.6±4.1∗ 29.0±4.1∗ 31.0±5.1∗ ∗Significantly different from control (p
<0.001); values are
means±standard error of five replicates.
for testing the effects of agrochemicals on nitrogen fixation, have also been investigated for their positive activities in soil and rhizosphere as enhancers of plant growth (Martinez-Toledo et al., 1991). However, the degree to which the effects observed in laboratory tests
can be extrapolated toAzotobacterin the soil and
rhi-zosphere deserves more attention because a number of factors like climate, soil type, agricultural practice and the composition of the microbial community, also influence the effects of those compounds.
References
Chapman, A.G., Fall, L., Atkinson, D.E., 1971. Adenylate energy charge in Escherichia coli during growth and starvation. J. Bacteriol. 108, 1072–1086.
Chung, K.T., Stevens, S.E., 1993. Degradation of azo dyes by environmental microorganisms and helminths. Environ. Toxicol. Chem. 12, 2121–2132.
Chung, K.T., Stevens, S.E., Cerniglia, C.E., 1992. A review of the reduction of azo dyes by intestinal microflora. Crit. Rev. Microbiol. 18, 175–190.
Chung, K.T., Chen, S.C., Zhu, Y., Wong, T.Y., Stevens Jr., S.E., 1997. Toxic effects of some benzamines on the growth of
Azotobacter vinelandii and other bacteria. Environ. Toxicol. Chem. 16, 1366–1369.
Chung, K.T., Chen, S.C., Wong, T.Y., Wei, C.I., 1998. Effects of benzidine and benzidine analogues on growth of bacteria includingAzotobacter vinelandii. Environ. Toxicol. Chem. 17, 271–275.
Gaur, A., Bhattacherjee, J.W., 1991. Toxicity of selected chromium compounds in microbial bioassay system. Water Air Soil Pollut. 59, 193–197.
Hardy, R.W., Holstein, R.D., Jackson, E.K., Burris, R.C., 1968. The acetylene–ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol. 43, 1185– 1207.
Kriek, E., 1979. Aromatic amines and related compounds as carcinogenic hazards to man. In: Emmelot, E., Kriek, E.E. (Eds.), Environmental Carcinogenesis. Elsevier, Amsterdam, The Netherlands, pp. 143–164.
Martinez-Toledo, M.V., Gonzalez-Lopez, J., de la Rubia, T., Ramos-Cormenzana, A., 1985. Isolation and characterization of
Azotobacter chroococcumfrom the roots ofZea mays. FEMS Microbiol. Ecol. 31, 197–203.
Martinez-Toledo, M.V., Salmeron, V., Gonzalez-Lopez, J., 1990. Metolachlor and the biological activity of Azotobacter chroococcum. Soil Biol. Biochem. 22, 123–125.
Martinez-Toledo, M.V., Salmeron, V., Gonzalez-Lopez, J., 1991. Effect of simazine on the biological activity of Azotobacter chroococcum. Soil Sci. 151, 459–467.
Martinez-Toledo, M.V., Salmeron, V., Rodelas, B., Pozo, C., Gonzalez-Lopez, J., 1998. Effect of the fungicide captan on some functional groups of soil microflora. Appl. Soil Ecol. 7, 245–255.
Meyer, U., 1981. Biodegradation of synthetic organic colorants. FEMS Symp. 12, 371–385.
Michaels, G.B., Lewis, D.L., 1985. Sorption and toxicity of azo and triphenylmethane dyes to aquatic microbial populations. Environ. Toxicol. Chem. 4, 45–50.