Influence of earthworm invasion on soil microbial biomass
and activity in a northern hardwood forest
Xuyong Li
a, Melany C. Fisk
b,*, Timothy J. Fahey
b, Patrick J. Bohlen
caInstitute of Geography Science and Natural Resources Research, Chinese Academy of Sciences, Beijing 100101, People’s Republic of China bDepartment of Natural Resources, Cornell University, Ithaca, NY 14853, USA
cArchbold Biological Station, Lake Placid, FL 33852, USA
Received 26 June 2001; received in revised form 11 September 2002; accepted 19 September 2002
Abstract
Recent invasion and activity of exotic earthworms has profoundly altered the chemical and physical environment of surface soils in northern hardwood forests that previously had mor humus horizons. We investigated the influence of earthworm invasion on soil microbial biomass and activity in surface soils of Allegheny northern hardwood forests in central New York state. Earthworm activity in these sites had transformed surface soils with clear Oi, Oe, and Oa horizons (forest floor) overlying mineral soil, to more uniformly mixed organic-enriched A horizons. The highest concentrations of microbial biomass and activity occurred in the forest floor. Microbial biomass (assayed by chloroform fumigation – extraction) nearly doubled in surface (0 – 5 cm) mineral soils in response to earthworm activity, an effect that corresponded directly to redistribution of organic matter from forest floor into the mineral soil. Microbial activity in surface mineral soils was even more sensitive to the presence of earthworms than microbial biomass. For example, substrate-induced respiration (or maximum initial respiratory rate, MIRR) was 6.7-fold greater, basal respiration was 5-fold greater, and microbial respiration per unit microbial biomass (metabolic quotient,qCO2) was almost 3-fold greater in surface mineral soils where earthworms were present than in earthworm-free sites.
Of the activity indices, only MIRR was higher when expressed on an organic matter basis. Surface mineral soils where earthworms were present thus appear to retain a high proportion of the microbial biomass and activity found in mor organic horizons. Our findings suggest that earthworm activity stimulates the activity of soil microorganisms, probably by enhancing organic C availability via processing and mixing of litter. The relative pattern in microbial properties did not change over the growing season; however, there were some seasonal changes in the proportional differences between worm and no-worm soils. Our results indicate interactions among earthworms, organic matter, and soil microbial activity that should alter the carbon and nutrient balance of northern hardwood forest surface soils, relative to non-invaded soils.
q2002 Elsevier Science Ltd. All rights reserved.
Keywords:Earthworm invasion; Microbial biomass; Microbial activity; Maximum initial respiration response; Metabolic quotient; Northern hardwood forest; Soil organic matter
1. Introduction
The soil microbial community drives nutrient transform-ations and thus plays a major role in nutrient cycling of ecosystems. Under suitable environmental conditions, the extent of the turnover of organic compounds will be mainly controlled by both the quantity and activity of the microbial
biomass (Martens, 1995). A better understanding of the
dynamics of soil microbial biomass and activity and their responses to natural and human-caused disturbances is
essential to improving our ability to predict patterns of nutrient cycling in ecosystems and their response to natural or human-induced disturbances.
In the north temperate and boreal forests of North America, native earthworms are rare due to eradication by Pleistocene glaciations (Gates, 1976; Reynolds, 1995), but active invasion by exotic earthworms is occurring in many regions, including the hardwood forests of the northeastern United States (Burtelow et al., 1998). The dominant exotic earthworms in this region are European species (Lumbricus rubellus, an epi-endogeic species; L. terrestris, and anecic
species) and Asian species (Amynthas hawayanus, an
epi-endogeic species). Earthworms play a major role in altering development of the soil profile, especially near the soil
0038-0717/02/$ - see front matterq2002 Elsevier Science Ltd. All rights reserved. PII: S 0 0 3 8 - 0 7 1 7 ( 0 2 ) 0 0 2 1 0 - 9
www.elsevier.com/locate/soilbio
* Corresponding author. Address: Department of Biology, Appalachian State University, Boone, NC 28608, USA.
surface (Edwards and Bohlen, 1996). The invasion by exotic earthworms has been linked to significant changes in organic matter breakdown and nutrient dynamics (Langmaid, 1964; Scheu and Parkinson, 1994; Alban and Berry, 1994; Bohlen and Edwards, 1995; Steinberg et al., 1996; Scheu, 1997; Burtelow et al., 1998). These changes can be expected to have significant effects on soil microbial biomass and activity. However, in forest ecosystems, the effect of exotic earthworm invasion on soil microbial biomass and activity is not well understood.
The objective of the present study was to quantify the effect of earthworm invasion on the quantity and activity of microbial biomass in a northern hardwood forest ecosystem. We employed the standard technique of chloroform fumigation and extraction (FE) as a measure of microbial biomass (Vance et al., 1987) together with the microbial metabolic quotient (qCO2) (Anderson and Domsch, 1990)
and maximum initial respiratory response (MIRR) (Beck
et al., 1997) as indices of microbial activity. We hoped to demonstrate how earthworm invasions interact with spatial and temporal environmental variation to affect both microbial populations and their metabolic activity in forest soils. These studies can then help us to interpret related responses of soil nutrient availability and soil solution chemistry to earthworm invasion of northern hardwood ecosystems.
2. Materials and methods
2.1. Study sites
The study sites were located at Arnot Forest in central New York, situated in the northern Allegheny Plateau physiographic province. (428160N, 768280W). Annual
rain-fall is 100 cm and average summer and winter temperature are 22.08C and 24.08C, respectively. Soils are acidic Dystrochrepts with a well-developed organic horizon averaging about 4 cm thick and overlying an acidic (pH 4.5 – 5.0) mineral horizon. Soils in the study area are well drained and exhibit pit and mound microtopography. In each of three separate sites, paired sample plots
(20 m£20 m) were established in adjacent locations that
had either been invaded recently by exotic earthworms (worm plot) or still remained worm-free (no-worm plot). Worm and no-worm plots in two sites (Site 1 and Site 2)
were dominated by sugar maple (Acer saccharum),
bass-wood (Tilia americana), and white ash (Fraxinus
amer-icana). Worm and no-worm plots in the third site (Site 3)
were dominated by aspen (Populus tremuloides) and oak
(Quercus rubra). Further information about forest compo-sition and dynamics at Arnot Forest is available (Fain et al., 1994; Fahey, 1998).
Earthworm populations were assessed in all plots in April 1999 and June 2000 using a standard formalin extraction technique (Raw, 1959). Eight liters of 0.25% formalin was
applied to four (1999) or five (2000) 0.25 m2areas within each plot and earthworms emerging from the soil were collected into vials containing 4% formalin and returned to the lab for identification. Samples were taken from plots designated as earthworm plots as well as from plots designated as worm-free reference plots, but the very few number of individuals collected from worm-free plots were negligible.
2.2. Soil sampling
Our aim was to quantify effects of the presence of earthworms on microbial biomass and activity, and to test whether those effects are consistent seasonally. In mid-July we intensively sampled all three sites. To examine seasonal patterns we sampled in Site 1 on four dates through the snow-free season (May 17, July 17, September 5 and November 8, 2000). Each sample collection consisted of four replicate soil cores (5 cm diameter) collected at random locations in each plot.
Our sampling was designed to compare the surface horizons where biological activity is concentrated and earthworm effects are most noticeable. In no-worm plots, each soil core was separated into forest floor (average 4.7 cm depth) and 0 – 5 cm mineral soil (NW FF and NW 0 – 5, respectively). These were typical mor profiles and the forest floor consisted of well-developed Oe and Oa (humus) horizons beneath the fresh litter (Oi). The forest floor had been eliminated in the worm plots; no Oe or Oa horizons remained and the Oi was present until about mid-summer. The surface horizon in the worm plots was an organic-enriched mineral (A). Each soil core in the worm plots was separated into 0 – 5 and 5 – 10 cm mineral soil (W 0 – 5 and W 5 – 10, respectively).
Samples were homogenized by hand, taking care to minimize disruption of soil structure. Coarse fragments
(.2 mm) and fine roots were removed. Samples were
refrigerated at 2 – 48C for approximately 24 h before
processing and incubations.
2.3. Sample analyses
Soil organic matter content was estimated from the
ash-free dry mass (4508C, 4 h). Microbial biomass C was
determined using the chloroform fumigation – extraction (FE) procedure (Brookes et al., 1985; Vance et al., 1987). For each sample, one subsample was extracted with 0.5 M K2SO4in a ratio of 1:10 (w/v) for forest floor samples and
1:5 (wt:vol) for mineral soil samples. A second subsample was fumigated with chloroform for 5 days in a vacuum
desiccator, followed by extraction in 0.5 M K2SO4.
Subsamples of extracts were sealed in glass ampules for
oxidation of DOC (Menzel and Vaccaro, 1964) and CO2in
the ampule headspace was analyzed on a CO2coulometer
unfumigated soils using a correction factor (Kc) of 0.45
(Vance et al., 1987).
Microbial respiration and the maximum initial respirat-ory rate (MIRR;Martens, 1995; Beck et al., 1997) also were assayed using the substrate-induced respiration (SIR)
approach (Anderson and Domsch, 1978). For each sample,
two replicate subsamples (fresh weight equivalent of 5 g dry weight for forest floor, 10 g dry weight for mineral soil) were incubated in air-tight jars at 228C. Following a 12 h pre-incubation, one replicate of each sample received glucose solution at a rate of 10 mg C per g soil for the surface organic-rich horizons (no-worm forest floor and worm mineral 0 – 5 cm), or 5 mg C per g soil for the subsurface mineral horizons (no-worm 0 – 5 cm, worm 5 – 10 cm), in 3 ml deionized water. Preliminary incubations showed that these were optimum C concentrations for stimulating a maximum respiratory response over the first 6 – 8 h of incubation. The second replicate received an equivalent amount of deionized water (3 ml). Water addition had a proportionately larger effect on water content of mineral soils than forest floor, which had higher water content to begin with.
Jar headspace was sampled at 0 and 5 h and CO2
concentrations were analyzed by gas chromatography. Basal respiration rates were estimated as the rate of CO2
accumulation in jars receiving deionized water only; MIRR
was estimated as the rate of CO2 accumulation in jars
receiving glucose. Microbial qCO2 was determined by
dividing basal respiration (BR) (mg CO2– C per g dry soil
h21) by FE microbial biomass C (g Cmic per g dry soil)
(Anderson and Domsch, 1990).
2.4. Statistical analyses
Statistical analyses were performed using SPSS for Windows Release 10.00. Two-way ANOVA (GLM
multivariate) was used to test effects of earthworm presence (comparing 0 – 5 cm mineral soil in worm and no-worm plots) and sites for mid-July data, or earthworm presence and sampling dates for seasonal data in Site 1. The microbial biomass and activity indices were calculated and tested on both a unit soil dry mass and organic matter (OM) basis. Differences among means of specific treatments and different soil horizons were tested with Student—New-man – Keuls method. We also used SPSS to analyze the
correlations between FE microbial biomass, MIRR, qCO2
and organic matter content, soil moisture, and average monthly soil temperature.
3. Results
Earthworms were absent from our no-worm plots, and
averaged 158 individuals/m2in worm plots at the time of
our surveys (Table 1). Forest floor (Oi, Oe, and Oa horizons) depth averaged 4.7 cm in no-worm plots. Earthworm invasion had mixed the surface organic and mineral horizons, eliminating the Oe and Oa and resulting in a surface (0 – 5 cm) mineral soil horizon that was significantly enriched in organic matter (Table 2). Mineral soil water content also was higher in the presence of earthworms (Tables 2 and 3).
Microbial biomass and activity exhibited significant effects of earthworm presence for the 0 – 5 cm mineral soil
horizon in mid-July (Table 4). With the exception of
microbial biomass, differences caused by site location in 0 – 5 cm mineral soils were not significant, and there were no significant interactions between earthworm presence and site. Soil microbial biomass and activity also differed significantly between worm and no-worm soils in the seasonal analysis (Table 5). Sampling date was significant for BR and for the maximum initial respiratory response
Table 1
Earthworm population density and biomass estimated from samples collected from forest plots with worms in April 1999 and June 2000. Data are not presented for no-worm plots because abundance was negligible (,3 individuals/m2). Standard errors of the mean are in parentheses,n
¼3. There were no significant differences between sample dates in number or biomass of any earthworm species or all species combined (P.0.05). The ecological category of the earthworm provides our best estimate of the feeding behavior of the different earthworm species at our site, recognizing that there is overlap between categories under different ecological conditions and at different life stages
Species Ecological category/feeding behaviora Population density (no./m2) Biomass (g/m2)
1999 2000 1999 2000
L. rubellus Epi-endogeic 67 (26.2) 85 (38.4) 22 (7.4) 28 (11.9)
Lumbricus terrestris Anecic 10 (9.7) 4 (3.9) 9 (8.9) 8 (8.3)
Octolasion tyrteum Epi-endogeic 59 (29.5) 54 (30.1) 10 (4.6) 7 (3.4)
Aporrectodea tuberuculata Endogeic 4 (3.8) 14 (8.7) 2 (1.9) 4 (2.2)
Dendrobaena rubida Epigeic 0.3 (0.3) 1 (1.3) 0.02 (0.02) 0.3 (0.3)
Eisenia rosea Epigeic 2 (2.3) 0 0.4 (0.4) 0
Lumbricus immatures N/A 7 (7.3) 6 (5.5) 0.4 (0.4) 1 (1.1)
Total 150 (29.2) 165 (40.8) 43 (2.5) 48 (3.1)
(MIRR), but not for FE biomass (Table 5). Significant interactions in the seasonal analysis were found only for BR (Table 5).
Microbial biomass concentrations in surface horizons were significantly lower in the presence of worms (W 0 – 5) than in their absence (NW FF). Comparison of all soil horizons using the Newman – Keuls test showed that the means of FE microbial biomass decreased in the order: NW
FF.W 0 – 5.NW 0 – 5¼W 5 – 10. Means of FE/OM
differed between surface and subsurface horizons, in the
order: NW 0 – 5¼W 5 – 10.NW FF¼W 0 – 5 (Fig. 1);
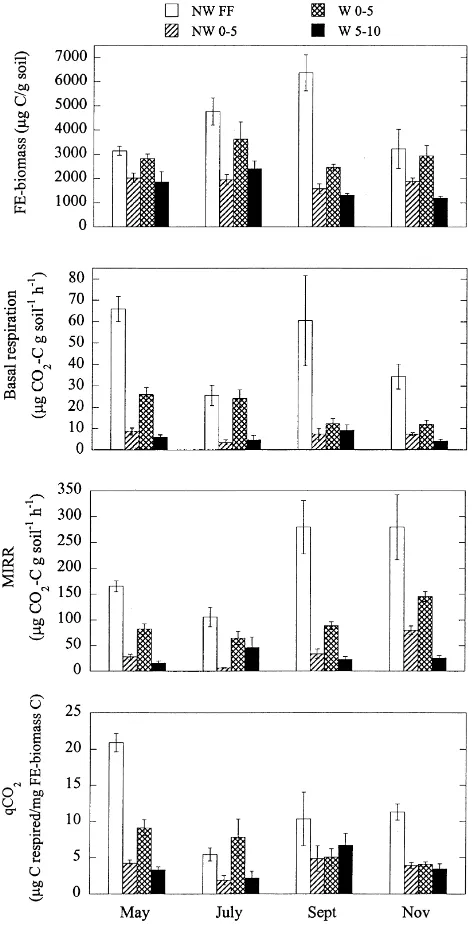
hence, the differences in FE-biomass between NW FF and W 0 – 5 appear to be associated primarily with the redistribution of soil organic matter by worms. Seasonal variation in FE microbial biomass was minimal for all but
NW FF, which peaked in September (Fig. 2).
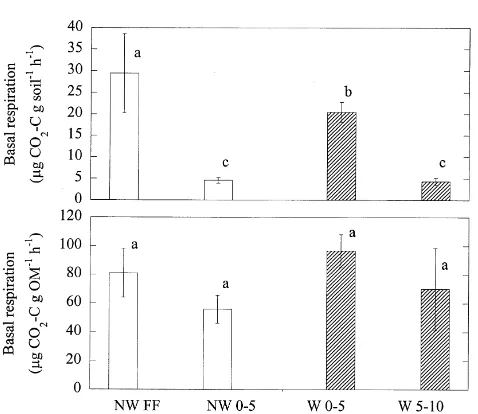
BR rates per g soil in mid-July were similarly higher in NW FF than W 0 – 5, and followed the same overall pattern among soil horizons as FE biomass (Fig. 3). This was true also for the respiratory potential of soil microorganisms per
g soil (MIRR), which in the surface mineral soil (0 – 5 cm) was almost 7 times higher in worm than no-worm plots in mid-July (Fig. 4). In fact, the worm effect was observed even at greater depths, as MIRR per g soil at 5 – 10 cm depth in worm plots was significantly higher than that of 0 – 5 cm depth in no-worm plots (Fig. 4). Basal respiration on an organic matter basis was more variable and did not differ
among horizons (Fig. 3), suggesting that much of the
earthworm effect on microbial respiratory activity was associated with redistribution of organic matter. MIRR was slightly more sensitive to earthworm effects, however, and MIRR/OM in W 0 – 5 cm was intermediate to NW FF and NW 0 – 5 (Fig. 4).
Both BR and MIRR exhibited the same relative pattern among soil horizons throughout the growing season (Fig. 2) but, unlike FE-biomass, differed significantly among
sampling dates (Table 5). MIRR increased overall in the
autumn and the proportional difference between worm and no-worm 0 – 5 cm mineral soils was slightly greater in May and July than in the autumn (Fig. 2). Basal respiration was notably high in worm relative to no-worm 0 – 5 cm mineral soils in May and July, but not September or November.
The metabolic quotient (qCO2) followed the same trend Table 2
Organic matter content (%) and gravimetric soil water content (%; dry weight basis) for soils sampled in mid-July, 2000. Incubation moisture was calculated based on the quantity of water added to soils prior to incubations. Standard errors of the mean are in parentheses,n¼3 sites
Organic
Forest floor 40 (8.7) 92 (28.0) 152 Mineral soil,
Gravimetric soil water content (%; dry weight basis) in soils collected from Site 1 in May, July, September, and November. Standard errors of the mean are in parentheses,n¼4 samples
17 May 17 July 5
Forest floor 145 127 118 142
Mineral soil, 0 – 5 cm 71 62 60 77
Worm
Mineral soil, 0 – 5 cm 86 94 78 103 Mineral soil, 5 – 10 cm 66 68 64 67
Table 4
Results (F-values and significance) of ANOVA testing effects of treatment (earthworms present or absent) and different sites on microbial biomass and activity in the surface mineral soil horizon (0 – 5 cm depth) of Arnot Forest soils (n¼24)
Dependent variablea Source
Worm treatment Site Treatment£site
FE 8.70** 3.61* 1.04
a FE: FE microbial biomass C per gram dry soil basis; FE/OM: microbial biomass C per gram organic matter basis; MIRR: MIRR respiration rate per gram dry soil basis; MIRR: MIRR respiration rate per gram organic matter basis;qCO2:qCO2value with FE microbial biomass C basis.
Table 5
Results of ANOVA (F-values and significance) testing effects of treatment (earthworms present or absent) and sampling date on the microbial biomass and activity in the surface mineral soil horizon (0 – 5 cm depth) of Arnot Forest soils (n¼32)
Dependent variable Source
Worm treatment Date Treatment£date
FE 22.14*** 1.79 0.69
MIRR 90.00*** 28.39*** 0.17
BR 44.07*** 4.17* 5.36**
as FE and MIRR, but surface horizons did not differ
significantly: NW FF¼W 0 – 5.NW 0 – 5¼W 5 – 10
(Fig. 5). In the surface mineral soil the presence of
earthworms increased qCO2 by almost 3-fold in
mid-summer. Seasonally, however, this effect was observed only in May and July and no differences were found in September and November (Fig. 2).
Earthworm invasion markedly increased organic matter as well as soil moisture content in the surface mineral soil (Tables 2 and 3), and these changes probably affected microbial biomass, MIRR andqCO2significantly. Overall,
these environmental factors were more strongly correlated with the activity than with the size of the soil microbial biomass. For example, the correlation coefficient between
field soil moisture and MIRR (R¼0.73, P,0.001) was
greater than that between field soil moisture and FE
microbial biomass (R¼0.60, P,0.001). Similarly, a
slightly stronger correlation was observed between soil
organic matter and MIRR (R¼0.90, P,0.001), than
between soil organic matter and FE microbial biomass (R¼0.77,P,0.001). No relationships between microbial biomass or activity and seasonal variation in soil tempera-ture were detected.
4. Discussion
When earthworms invade acidic forest soils with a mor humus layer, over a period of a few years they can mix the organic horizons and the mineral soil by their feeding activity (Langmaid, 1964; Alban and Berry, 1994).
Earth-worms vary in their feeding behaviors and hence the specific manner in which they impact the soil profile and organic matter distribution. In our study sites, the dominant species (L. rubellusandO tyrteum) are epi-endogeic, and are active in and process organic matter in both organic and surface
mineral soils. The anecic L terrestris, the next most
abundant species, likely transports deeper mineral soils to the surface and feeds on surface organic matter. Consistent with these ecological categories of earthworms, we observed a surface layer A horizon in earthworm plots that was intermediate in organic matter content to the forest floor and underlying mineral soil in reference, no-worm plots. Both surface and deeper-dwelling earthworms rapidly
Fig. 1. Chloroform fumigation extraction (FE) microbial biomass in worm and no-worm soils in three sites, July 2000. Bars are standard errors of the mean,n¼12. Different letters indicate significant differences according to the Student—Newman– Keuls method,P,0.05. NW FF: forest floor of no-worm plots; NW 0 – 5: 0 – 5 cm mineral soil horizon of no-worm plots; W 0 – 5: 0 – 5 cm mineral soil horizon of worm plots; W 5 – 10: 5 – 10 cm mineral soil horizon of worm plots.
Fig. 2. Seasonal patterns of FE microbial biomass C, BR, MIRR, andqCO2 in worm and no-worm soils of Site 1. Bars are standard errors of the mean,
process fresh litterfall and mineral soil is exposed at the surface during summer and early autumn, accelerating the decomposition and mineralization of litter. Earthworms also affect soil structure by forming burrows and casts, and
they produce organic compounds (Lee, 1985) that become
concentrated in their casts and along burrow walls (Edwards and Bohlen, 1996; Tiunov and Scheu, 1999). Through these mechanisms of organic matter redistribution and proces-sing, earthworms directly and indirectly affect the compo-sition, abundance and activity of soil microorganisms.
We observed a significant increase in FE microbial biomass in the surface mineral soil layer (0 – 5 cm) of a
northern hardwood forest ecosystem in response to the invasion of exotic earthworms, principallyL. rubellus,
L. terrestris, andOctolasion tyrtaeum. The increase in FE-biomass in worm plot surface mineral soils probably was associated with the increase in organic matter concentration of this soil layer, because FE/OM was similar between the surface horizons (worm 0 – 5 cm and no-worm forest floor). However, FE/OM actually was reduced in the 0 – 5 cm worm soils compared to 0 – 5 no-worm soils, suggesting that microbial biomass did not increase to the same extent as organic matter following earthworm invasion.
Other studies of earthworm effects on microbial biomass
in forests have reported inconsistent responses. Burtelow
et al. (1998) observed increased microbial biomass in temperate deciduous forest in New England, whereas
reductions were reported in beech in Europe (Wolters and
Joergensen, 1992) and pine forest in southwestern Alberta (McLean and Parkinson, 1997). The increase in FE microbial biomass per unit organic matter reported by
Burtelow et al. (1998) contrasts with our results and indicates a variable influence of exotic earthworms that may depend on soils, forest or worm species, and possibly length of time since invasion.Burtelow et al. (1998)studied
Asian species (Amynthas), in contrast to the European
Lumbricus andOctolasion spp. common in our sites. The annual life cycle and epi-endogeic feeding behavior of
Amynthas may distinguish its effects from those of the perrenial, anecic and epi-endogeic species common at our sites. Furthermore, experimental work in mesocosms has shown that differential effects of earthworms on microbial biomass and activity depend not only on the ecological categories of earthworms present, but also on the species composition within those categories (Scheu et al., 2002).
Scheu and Parkinson (1994) observed that European
earthworms of another genus (Dendrobaena) caused a
decrease in microbial biomass in the L/F layers but an increase in the H and Ah horizons of aspen forests. In their study, introduction of earthworms had changed the soil profile to a lesser extent, probably because earthworm introduction was more recent and possibly because the
epigeic Dendrobaena carries out less mixing between
organic and mineral horizons. Nevertheless, these results
Fig. 3. Basal respiration in worm and no-worm soils in three sites, July 2000. Bars are standard errors of the mean,n¼12. Different letters indicate significant differences according to the Student—Newman – Keuls method,
P,0.05. SeeFig. 1for explanation of legends.
Fig. 4. MIRR in worm and no-worm soils in three sites, July 2000. Bars are standard errors of the mean,n¼12. Different letters indicate significant differences according to the Student—Newman– Keuls method,P,0.05. SeeFig. 1for explanation of legends.
Fig. 5. Metabolic quotient in worm and no-worm soils in three sites, July 2000. Bars are standard errors of the mean,n¼12. Different letters indicate significant differences according to the Student—Newman – Keuls method,
concur with ours and suggest that soil microbial biomass is shifted deeper down in the soil profile as a result of earthworm redistribution of surface soil organic matter. For example, in our study FE microbial biomass was as high in the 5 – 10 cm soil depth in worm-invaded plots as in the 0 – 5 cm depth in the worm-free sites.
Microbial activity was enhanced in the presence of earthworms to a greater extent than could be explained by
changes in organic matter content alone. Barley and
Jennings (1959) first reported that earthworms increased soil respiration, and similar results have been reported by
Ross and Cairns (1982), Haimi and Huhta (1990) and Ruz-Jerez et al. (1992). Our results reinforce and extend these findings. In particular, we observed in the surface mineral soil of the worm-invaded plots an increase in the maximum initial respiratory response (MIRR), expressed on both a soil dry mass and an organic matter basis, and in the microbial metabolic quotient (qCO2). The MIRR is largely
determined by the respiration rate of soil microorganisms that are stimulated by glucose addition and is intended as an
index of the active soil microorganisms (Wardle and
Parkinson, 1990), contrasting with the FE which includes
the biomass of dormant soil microorganisms. The qCO2,
calculated as the ratio of basal microbial respiration/FE biomass, provides an index of the overall activity of the total microbial pool. The increases in MIRR/OM andqCO2in the
worm-invaded surface mineral soil suggest that earthworms enhance resource availability or ameliorate environmental conditions that limit the activity of the soil microflora in worm-free mineral soils.
The most likely explanation of this earthworm effect on microbial activity is the mixing of labile organic substrates into the upper mineral soil horizons. In support of this supposition is the observation that both MIRR/OM and
qCO2are highest in the forest floor horizon and intermediate
in the upper mineral soil of the worm-invaded plots and lowest in the mineral soil of the no-worm plots. We interpret this pattern as an indication that the overall quality of organic substrates is highest in the forest floor where fresh litter and fine root exudation and turnover provide a continuous supply of labile substrates for microbial metabolism. Conversely, much of the organic matter in the upper mineral soil of the no-worm plots is probably condensed, recalcitrant humic substances, not readily available to most soil heterotrophs. The upper mineral soil of the worm plots would contain a mixture of the organic matter categories. Metabolism of that organic matter also may differ due to the influence of earthworm mixing and
consumption on fungal communities (McLean and
Parkin-son, 2000).
Other effects of the earthworms on both soil resources and environmental conditions also could contribute to the patterns we observed. Earthworms produce labile substrates as secretion and excretion products (Lee, 1985), and water-soluble, low-molecular-weight compounds are assimilated by the rapidly multiplying microbial community in the
earthworm gut (Barois and Lavelle, 1986). Also,Binet et al. (1998) have proposed that microbial metabolism is stimulated by low concentrations of chemical mediators released by earthworms. The effects of earthworm activity on soil structure could improve environmental conditions such as moisture and aeration to favor microbial activity. Although we have no direct evidence for these possible effects, we did observe moderately high linear correlations
between soil water content and both MIRR/OM (R¼0.51)
andqCO2(R¼0.52). The moisture content of the 0 – 5 cm
mineral soil was consistently higher in the worm-invaded plots than the no-worm plots, probably as a result of forest floor interception of summer rains and possibly increased water-holding capacity associated with higher organic matter content in mineral soil of the worm-invaded plots. As a consequence of the insulating effects of the forest floor, soil temperature of the surface mineral soil was slightly lower during summer in no-worm plots (0.5 – 18C), whereas this pattern was reversed during the autumn. However, these differences did not correspond to patterns of microbial properties when incubated at uniform temperature in the laboratory.
Microbial responses to the presence of earthworms should depend on the stage of invasion, and our results suggest that microbial activity remains elevated beyond the
initial colonization period. Langmaid (1964) documented
the complete transformation of surface organic horizons of acidic podzols to well-mixed mineral soils in 3 years following earthworm invasion. The initial consumption of organic layers when earthworms first invade can support high populations that peak in as little as 4 years and decline
somewhat thereafter (Ligthart and Peek, 1997), and may
also cause a transient stimulation of microbial activity. For example, studies early in colonization history suggest that
mineralization of soil organic matter is enhanced (Alban
associated with environment and with earthworm effects on availability of annual organic matter inputs.
These effects on decomposer microorganisms are likely a combination of direct organism interactions and indirect effects due to transformations of the physical and chemical soil environment. This study is intended to demonstrate general differences in microbial activity in forest soils with and without earthworms, and we cannot separate these influences. Nevertheless, seasonal patterns do give some insight into direct and indirect effects. Seasonal analysis in Site 1 showed consistency over time in the relative patterns between treatments and among soil horizons; hence, the results reported here for mid-July probably are generally applicable throughout the growing season. The general consistency over time in microbial activity suggests an indirect influence of earthworms via soil organic matter distribution and availability and soil physical properties. Direct comparisons of earthworm and microbial activity over time are needed to verify this explanation. Subtle seasonal changes did suggest some differences in the timing of microbial activity between worm and no-worm plots. For instance, seasonal patterns ofqCO2and BR suggest that the
earthworm effect on the activity of microbial biomass is greater in spring and summer than in autumn, possibly as a result of earthworm mixing of the previous year’s litter early in the season. The seasonal pattern of microbial activity also differed between no-worm forest floor and worm 0 – 5 mineral soil. Our indices of microbial activity (BR, MIRR,
qCO2) were similar between forest floor and worm surface
mineral soils in July, but differed in the autumn months (Fig. 2). These patterns are suggestive of direct effects of earthworms on microbial activity; again, data comparing earthworm to microbial activity are needed to test this idea. In conclusion, we have demonstrated significantly greater microbial biomass and activity in surface mineral soils where earthworms are present. It is important to interpret this change in microbial activity in surface mineral horizons in the context of a changed soil profile that no longer has a surface forest floor horizon. Although organic matter concentration of surface mineral soils approached that of no-worm forest floor, slightly lower microbial
activity (indexed by MIRR/OM andqCO2) suggested that
mixing of organic matter into the mineral soil matrix has changed its availability for microbial utilization. The quantitative effect of this change in the nature of organic matter depends on changes in total soil pools. Nevertheless, it appears that the transport and change in availability of organic matter has the potential to affect nutrient avail-ability, especially if microbial immobilization of nutrients is inherently greater in the organic-rich forest floor. Concen-trations of inorganic P were higher in soil leachate in these worm plots (D. Pelletier 2001, MS Thesis, Cornell University), and labile P pools also were higher in surface mineral horizons (E. Suarez 2001, MS Thesis, Cornell University). These differences in availability of mineral nutrients may be related to the microbial responses to
earthworms in surface soils of these forests. Changes in the timing of microbial activity that appear to result from earthworm activity also may affect nutrient pools via changes in the timing of nutrient mineralization relative to that of plant nutrient uptake.
Acknowledgements
We thank Suzanne Wapner, Dana Briel, Kurt Smemo, Derek Pelletier, Maryann Welsch, Noel Gurwick and Beth Lawrence for help in the field and laboratory. This work would not have been possible without the project support and valuable discussions provided by Peter Groffman. This research was supported by a grant from the National Science Foundation of USA.
References
Alban, D.H., Berry, E., 1994. Effects of earthworm invasion on morphology, carbon, and nitrogen of a forest soil. Applied Soil Ecology 1, 243– 249.
Anderson, J.P.E., Domsch, K.H., 1978. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biology & Biochemistry 10, 215– 221.
Anderson, J.-H., Domsch, K.H., 1990. Application of eco-physiological quotients (qCO2and qD) on microbial biomasses from soils of different cropping histories. Soil Biology & Biochemistry 22, 251– 255. Barley, K.P., Jennings, A.C., 1959. Earthworms and soil fertility III. The
influence of earthworms on the availability of nitrogen. Australian Journal of Agricultural Research 10, 364– 370.
Barois, I., Lavelle, P., 1986. Changes in respiration rate and some physico-chemical properties of a tropical soil during transit throughPoutoscolex corethrurus(Glossoscdecidae Oligochaeta). Soil Biology & Biochem-istry 18, 539– 541.
Beck, T., Joergensen, R.G., Kandeler, E., Makeschin, F., Nuss, E., Oberholzer, H.R., Scheu, S., 1997. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biology & Biochemistry 29, 1023 – 1032.
Binet, F., Fayolle, L., Pussard, M., 1998. Significance of earthworms in stimulating soil microbial activity. Biology and Fertility of Soils 27, 79 – 84.
Bohlen, P.J., Edwards, C.A., 1995. Earthworm effects on N dynamics and soil respiration in microcosms receiving organic and inorganic nutrients. Soil Biology & Biochemistry 27, 341– 348.
Brookes, P.C., Landman, A., Pruden, G., Jenkinson, D.S., 1985. Chloro-form fumigation and the release of soil nitrogen in soil. Soil Biology & Biochemistry 17, 837– 842.
Burtelow, A.E., Bohlen, P.J., Groffman, P.M., 1998. Influence of exotic earthworm invasion on soil organic matter, microbial biomass and denitrification potential in forest soils of the northeastern United States. Applied Soil Ecology 9, 197– 202.
Edwards, C.A., Bohlen, P.J., 1996. Biology and Ecology of Earthworms, third ed., Chapman and Hall, London.
Fahey, T.J., 1998. Recent changes in an upland forest in south-central New York. Journal of the Torrey Botanical Society 125, 51 – 59.
Fain, J.J., Volk, T.A., Fahey, T.J., 1994. Fifty years of change in an upland forest in south-central New York: general patterns. Bulletin of the Torrey Botanical Club 121, 130 – 139.
processes in raw humus forest soil: a microcosm study. Biology and Fertility of Soils 10, 178– 183.
Huffman, E.W.D. Jr., 1977. Performance of a new carbon dioxide coulometer. Microchemistry Journal 22, 567– 573.
Langmaid, K.K., 1964. Some effects of earthworm invasion in virgin podzols. Canadian Journal of Soil Science 44, 34 – 37.
Lee, K.E., 1985. Earthworms: Their Ecology and Relationships with Soil and Land Use, Academic Press, Sydney, 411 pp.
Ligthart, T.N., Peek, G.J.C., 1997. Evolution of earthworm burrow systems after inoculation of lumbricid earthworms in a pasture in the Netherlands. Soil Biology & Biochemistry 29, 453– 462.
Martens, R., 1995. Current methods for measuring microbial biomass C in soil: potentials and limitations. Biology and Fertility of Soils 19, 87 – 99.
McLean, M.A., Parkinson, D., 1997. Changes in structure, organic matter and microbial activity in pine forest soil following the introduction of
Dendrobaena octaedra(Oligochaeta, Lumbricidae). Soil Biology & Biochemistry 29, 537 – 540.
McLean, M.A., Parkinson, D., 2000. Field evidence of the effects of the epigeic earthworm Dendrobaena octaedra on the microfungal community in pine forest floor. Soil Biology & Biochemistry 32, 351– 360.
Menzel, D.W., Vaccaro, R.F., 1964. The measurement of dissolved organic and particulate carbon in seawater. Limnology and Oceanography 9, 138– 142.
Raw, F., 1959. Estimating earthworm populations by using formalin. Nature 184, 1661.
Reynolds, J.W., 1995. The distribution of earthworms (Annelida, Oligochaeta) in North America. In: Mishra, P.C., Guru, B.C., Senapati, B.K., Behera, N. (Eds.), Advances in ecology and Environmental Sciences, Ashish Publishing House, New Delhi, pp. 133– 153. Ross, D.J., Cairns, A., 1982. Effects of earthworms and ryegrass on
respiratory and enzyme activities of soil. Soil Biology & Biochemistry 14, 583– 587.
Ruz-Jerez, B.E., Ball, P.R., Tillman, R.W., 1992. Laboratory assessment of nutrient release from a pasture soil receiving grass or clover residues, in the presence or absence ofLumbricus rubellusorEisenia fetida. Soil Biology & Biochemistry 24, 1529 – 1534.
Scheu, S., 1997. Effects of litter (beech and stinging nettle) and earthworms (Octolasion lacteum) on carbon and nutrient cycling in beech forest on a basalt-limestone gradients: a laboratory experiment. Biology and Fertility of Soils 24, 384 – 393.
Scheu, S., Parkinson, D., 1994. Effects of earthworms on nutrient dynamics, carbon turnover and microorganisms in soils from cool temperate forests of Canadian Rocky Mountains—laboratory studies. Applied Soil Ecology 1, 113– 125.
Scheu, S., Schlitt, N., Tiunov, A.V., Newington, J.E., Jones, T.H., 2002. Effects of the presence and community composition of earthworms on microbial community functioning. Community Ecology, Oecologia DOI 10.1007/s00422-002-1023-4.
Steinberg, D.A., Pouyat, R.V., Parmelee, R.W., Groffman, P.M., 1996. Earthworm and nitrogen mineralization rates along an urban – rural land use gradient. Soil Biology & Biochemistry 29, 427 – 430.
Tiunov, A.V., Scheu, S., 1999. Microbial respiration, biomass, biovolume and nutrient status in burrow walls of Lumbricus terrestris L. (Lumbricidae). Soil Biology & Biochemistry 31, 2039 – 2048. Vance, E.D., Brookes, P.C., Jenkinson, D.S., 1987. An extraction method
for measuring soil microbial biomass C. Soil Biology & Biochemistry 19, 703– 707.
Wardle, D.A., Parkinson, D., 1990. Comparison of physiological techniques for estimating the response of the soil microbial biomass to soil moisture. Soil Biology & Biochemistry 22, 817– 823. Wolters, V., Joergensen, R.G., 1992. Microbial carbon turnover in beech