L
Journal of Experimental Marine Biology and Ecology 245 (2000) 57–68
www.elsevier.nl / locate / jembe
Acid lysis of macroalgae by marine herbivorous fishes:
effects of acid pH on cell wall porosity
*
W.L. Zemke-White , K.D. Clements, P.J. Harris
School of Biological Sciences, University of Auckland, Private Bag 90219, Auckland, New Zealand
Received 1 June 1999; received in revised form 3 September 1999; accepted 6 October 1999
Abstract
It has been demonstrated that treatment of algae at low pH values, similar to those found in the stomachs of herbivorous fishes, damages the algal cells, allowing digestive enzymes to enter the cells. However, the effects of the low pH treatment on the porosity of algal cell walls has not been examined. We tested the effects of low pH on the porosity of cells of four species of dietary algae,
Enteromorpha intestinalis and Ulva rigida (Chlorophyta) and Porphyra sp. and Polysiphonia strictissima (Rhodophyta) consumed by herbivorous fishes. The uptake of fluorescein
iso-thiocyanate (FITC)-conjugated dextrans of different molecular sizes was used to determine the cell-wall pore size in these algae. Secondly we tested whether acidic conditions increased the porosity of the algal cell walls by first immersing the algae in seawater adjusted to a low pH, then used the uptake of the FITC-dextrans into the acid treated cells to measure changes in cell-wall porosity. Limiting cell-wall pore diameter in E. intestinalis, U. rigida and P. strictissima was less than 8.8 nm, and in Porphyra sp. was less than 7.1 nm. The low pH treatment increased the porosity of the cell walls in all four algae. Porphyra sp. was the most resistant to this low pH treatment, followed by P. strictissima, then E. intestinalis and finally U. rigida. The cell-wall pore size of all algae increased to at least 13.5 nm after 20 min at pH 2.0, and after 60 min at either pH 2.5 or pH 3.0. These findings have important implications for the ability of marine herbivorous fish to digest these algae. Fish proteases range in molecular diameter from 4.2 to 5.4 nm and would therefore be able to pass through the cell walls of untreated algae in under 10 min.
a-Amylases have molecular diameters ranging from 6.1 to 6.5 nm, and would require up to 30 min to traverse the algal cell walls. The increase in algal cell-wall porosity as a result of exposure to low pH conditions in the stomachs of marine herbivorous fishes would allow molecules, similar in size to proteases anda-amylases, to enter the cells in under 5 min, and is therefore likely to be an important factor in the digestion of intracellular algal nutrients. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Acid lysis; Algae; Cell walls; Fluorescein isothiocyanate; Porosity
*Corresponding author. Tel.: 164-9-3737-599 ext. 8483; fax: 164-9-3737-414. E-mail address: l.zemke-white@auckland.ac.nz (W.L. Zemke-White)
1. Introduction
The porosity or lack of porosity of algal cell walls presents special problems for marine herbivorous fish that seek to utilise algae as a food source. It is generally believed that marine herbivorous fishes lack the endogenous enzymes necessary to break down the cell walls of marine macroalgae (Horn, 1998). Therefore, to gain access to the intracellular polymeric components (e.g., starch) of their dietary algae, fish enzymes capable of degrading these polymers must be capable of passing through the algal cell
walls. However, Zemke-White et al. (1999) have shown thata-amylases were incapable
of entering live algal cells without the algae first being treated in acidic conditions. Therefore, these fish must possess some mechanism either to break down, or increase, the porosity of algal cell walls in order to gain access to the intracellular nutrients of their dietary algae. Four methods have been proposed to overcome this problem (Horn, 1989): (i) acid lysis in a thin walled stomach, where the plant or algal cells are said to be broken down by acidic gastric conditions, (ii) trituration by pharyngeal jaws, (iii) trituration in a gizzard like stomach, and (iv) degradation by enzymes produced by the resident microflora. It has been pointed out more recently that these mechanisms are not
mutually exclusive (Choat and Clements, 1998). Approximately 150 species (|33%) of
marine herbivorous fish species ostensibly utilise acid lysis to gain access to intracellular algal nutrients (Zemke-White et al., 1999).
While the plant plasma membrane is the primary interface which regulates the passage of substances into and out of the cells (Lee, 1980), plant cell walls can also act as filters, restricting the entry or exit of ions or molecules from cells (Bacic et al., 1988). Consequently, the porosity of the cell wall can be an important factor in determining the communication both between individual cells and between cells and their external environment. While there have been a number of studies on the porosity of angiosperm cell walls (reviewed by Read and Bacic, 1996), a literature search revealed no such studies on the porosity of algal cell walls.
Acid lysis of marine macroalgae was first tested by Lobel (1981). We extended this work (Zemke-White et al., 1999) by showing that exposing these algae to the pH levels found within the stomachs of the fishes enabled digestive enzymes to enter rhodophyte and chlorophyte algal cells and hydrolyse the starch contained within. We demonstrated that the algal plasma membrane was lysed by the low pH conditions, but we did not examine the porosity of the algal cell walls.
Methods that have been used to determine the pore size of plant cell walls include: (i) microscopic visualisation of cell wall pores; (ii) a ‘‘solute exclusion’’ technique, in which cells are placed in hypertonic solutions of solutes of differing molecular sizes and plasmolysis or cytorrhysis is determined; and (iii) observation of the uptake of tracer molecules or particles (Read and Bacic, 1996). The last of these methods allows an examination of the abilities of different sized molecules to transport across the cell wall in close to physiological conditions, and can determine the limiting size of the pores. However, it does not resolve variations in pore sizes or reveal whether pores arise simply as gaps in the polymer lattice of the cell wall or are specifically constructed pores (Read and Bacic, 1996).
on the porosity of algal cell walls. We examined four marine macroalgae: the subtidal
Ulva rigida Adams (Chlorophyta: Ulvaceae) and Polysiphonia strictissima C. Agardh
(Rhodophyta: Rhodomelaceae), and the intertidal Enteromorpha intestinalis (Chloro-phyta: Ulvaceae) and Porphyra sp. Tag no. PYGRosP13 / 5 / 98, Museum of New Zealand, Te Papa (this genus is currently under taxomonic revision, pers. com., Glenys Knight, Museum of New Zealand, Te Papa, New Zealand). We determined the ability of the algal cells to take up fluorescein isothiocyanate (FITC) conjugated to dextrans of different molecular size to: (1) determine the porosity of algal cell walls in their native state, and (2) determine any changes in that porosity after immersion in seawater with a lowered pH.
2. Methods and materials
2.1. FITC dextran (FD) preparations
3
FDs with the molecular sizes 10K (K510 M ), 20K, 40K, 70K, 150K and 250Kr
were obtained from Sigma (St. Louis, MO, USA). As some commercial preparations of FD have been shown to contain unconjugated FITC (Cole et al., 1990), all FDs used in this study were purified by gel chromatography, using the method of Cole et al. (1990).
21
FD (50 mg ml ) in water was loaded onto Sephadex G-25-150 (Sigma) packed in a
prewashed 2531.5 cm glass column. The FD was allowed to settle onto the column and
21
was then eluted (using water) at a flow-rate of 100ml min . The FD was collected as
the first fluorescent band and freeze–dried. All of these procedures were carried out at 58C in low light. For the uptake experiments, each purified FD was dissolved (10 mg
21
ml ) in seawater (FD solution) that had been filtered through a 0.2-mm pore size filter.
We used thin-layer chromatography (TLC) to test for unconjugated FITC in both the FD preparations and in the algae from the uptake experiments. The purified FD and the supernatant from homogenised algae was chromatographed on Silica gel TLC plates (F-1500, Schleicher and Schuell, Germany) using chloroform–ethanol (3:1, v / v) as the solvent. Pure FITC (Sigma) was used as a standard in all experiments. The plates were allowed to dry and viewed in UV radiation.
2.2. Algal collection
The algae were collected from South Piha Beach (368589S, 1748289E), near Auckland, New Zealand. They were kept in a 20-l aquarium under natural light conditions, and the seawater changed daily for the duration of the experiments (six days maximum).
All four algae used in this study are commonly found in the diet of New Zealand’s marine herbivorous fishes. This was determined by examining the gut contents of four species of herbivorous fish from New Zealand: Girella tricuspidata (Quoy and Gaimard,
(Pomacen-tridae) (n519); Aplodactylus arctidens Richardson, 1839 (Aplodactylidae) (n528); ¨
and Kyphosus sydneyanus (Gunther, 1886) (Kyphosidae) (n530). These fish were
caught between August 1996 and August 1999 from various locations in the Hauraki Gulf, North-eastern New Zealand. These results were supplemented by data from a seasonal diet analysis of P. alboscapularis (McMurtry, 1999), and from Vial (1997), who examined the gut contents of 43 K. sydneyanus.
2.3. Uptake experiments
To determine the porosity of the cell walls of live algae, samples of the algae were placed in a plasmolysing solution (seawater containing mannitol, 0.75 M final con-centration) containing the FD. Mannitol has been used in similar experiments on angiosperms (Baron-Epel et al., 1988), and preliminary experiments indicated that a 0.75 M solution in seawater was required to plasmolyse the algae in this study. The algae were left in these solutions for 10–120 min. At the end of the allotted time the algal samples were removed and washed three times in filtered seawater containing mannitol (0.75 M). The samples were then mounted on slides and the coverslips sealed to the slide with nail varnish.
To test the effects of pH on the cell-wall porosity, algal samples were placed for 20 or 60 min in seawater filtered through a 0.2-mm pore size filter and adjusted to pH 2.0, 2.5 or 3.0 with HCl. These times of incubation were chosen as we wanted our findings to be comparable to previous work on acid lysis which used these incubation times (Lobel, 1981; Zemke-White et al., 1999). The algae were then removed and washed three times before being placed into the FD solution. After incubation for 5–120 min the algae were removed, rinsed, and mounted on slides as above. Controls were run for all of the FDs, where the algae were placed in each of the FD solutions without pH treatment. All treatments in the uptake experiments were replicated twice with different algal specimens.
2.4. Microscopy
The mounted samples were viewed with a Leica TCS 4D confocal laser scanning microscope system (CLSM). The fluorescence of the FD was collected using a FITC filter set (excitation 488 nm, emission approx. 525–545 nm). The chloroplasts within the algae autofluoresced strongly and this fluorescence was collected with a rhodamine filter
set (excitation 568 nm, emission.590 nm). The FITC and rhodomine images from each
optical section were then false coloured and combined using Adobe Photoshop 3.0 (Microsoft).
3. Results
3.1. Plasmolysis experiments
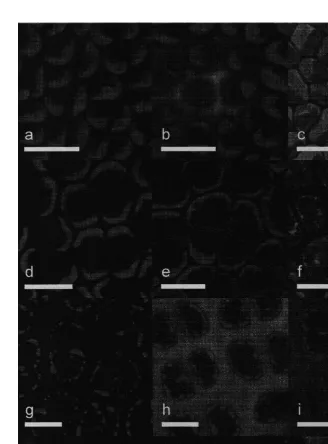
All algal species were plasmolysed by treating them with mannitol (0.75 M) in seawater. The FD equilibrated between the plasmolysing solution and either the cell wall, or the aqueous volume between the plasma membrane and the cell wall. The 10K FD was taken up within 10 min in all four of the algae tested, as indicated by the green fluorescence. This is shown in Fig. 1b, e, h and f, for Enteromorpha intestinalis, Ulva
rigida, Porphyra sp., and Polysiphonia strictissima, respectively. In P. strictissima, the 10K FD was taken up into the spaces between the cell wall and the plasma membrane created by the plasmolysis of the algal cells. Although a clear space between plasma membrane and cell wall was not apparent in E. intestinalis, U. rigida or Porphyra sp, the 10K FD was evident throughout the cell wall of these algae. The 20K FD was taken up by E. intestinalis, U. rigida and P. strictissima, although it took 30 min for significant amounts to be found either within the space between the cell wall and the plasma
membrane in P. strictissima or within the cell walls of E. intestinalis and U. rigida. All
larger FDs were excluded from these three algal species even after 2 h incubation. The 20K FD was not able to pass through the cell walls of the Porphyra sp. tested even after 2 h incubation in the plasmolysing solution.
3.2. pH treatments
Every low pH treatment had the effect of increasing the cell-wall porosity in all the algae in this study. After incubation in pH 2.0 for 60 min all four algal species took up the 250K FD within 5 min (Fig. 1c, f, i and l). For each species of alga, there was a gradient of effect on the cell-wall porosity depending on both the pH and treatment time (Table 1). An examination of all of the low pH treatments showed that, among the algal species, there was also a gradient of resistance to the effects on the cell-wall porosity due to low pH treatment. The rhodophytes were more resistant that the chlorophytes. Within the rhodophytes, the subtidal Porphyra sp. was more resistant than the intertidal
Polysiphonia strictissima. Within the chlorophytes, the subtidal Enteromorpha intes-tinalis was more resistant than the inter-tidal Ulva rigida.
The higher resistance of Porphyra sp. was apparent in all pH treatments except for 60 min at pH 2.0. After 20 min at pH 2.0, Enteromorpha intestinalis, Ulva rigida and
Polysiphonia strictissima took up the 250K FD in under 5 min, but 60 min was required
for Porphyra sp. to take up this FD. After 20 min at pH 2.5, a difference became
apparent between P. strictissima and the two chlorophytes: P. strictissima took up the
250K FD in under 60 min, but the two chlorophytes took this FD up in less than 30 min. After 20 min at pH 3.0, the difference between E. intestinalis and U. rigida became apparent: 60 was min required for the former to take up significant amounts of the 250K FD, but the latter required 30 min to take up this FD.
4. Discussion
Fig. 1. Confocal micrographs of Enteromorpha intestinalis (a, b, c), Ulva rigida (d, e, f), Porphyra sp. (g, h, i) and Polysiphonia strictissima (j, k, l). The red colour represents the autofluorescence of the algal chloroplasts; the green represents the FITC-conjugated dextran which was taken up by the algae. Untreated controls for each alga are shown in a, d, g and j. In b, e, h, and k the algae were plasmolysed for 10 min in seawater containing
21
mannitol (0.75 M) and 10K FD (10 mg ml ). Enteromorpha intestinalis, Ulva rigida and P. stictissima (c, f and l respectively) were immersed in seawater (adjusted to pH 2.0 with HCl) for 20 min before being placed in
21
Table 1
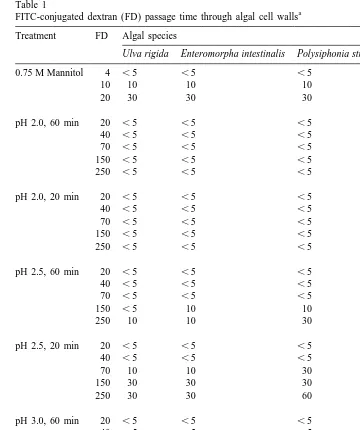
a FITC-conjugated dextran (FD) passage time through algal cell walls Treatment FD Algal species
Ulva rigida Enteromorpha intestinalis Polysiphonia strictissima Porphyra sp.
0.75 M Mannitol 4 ,5 ,5 ,5 10
In the 0.75 M mannitol treatment, algae was placed in seawater containing mannitol (0.75 M) and FD (10
21
mg ml ). In the low pH treatments, algae was placed in seawater with the pH adjusted by adding HCl. The
21
movement through cell walls as defined by Baron-Epel et al. (1988): unhindered]]]
movement, defined as movement through the cell walls in less than 10 min; hindered]]]
movement, defined as taking up to 2.5 h to move through the cell wall; and excluded,]]]
defined as not moving through the cell wall at all.
The limiting size of cell-wall pores can be determined by determining the diameter of the largest molecule that can pass through the cell wall. The diameter of a molecule is twice its Stokes radius as calculated from physical measurement (Squire, 1981).
However, the relationship between the Stokes radius and the molecular mass (M ) differsr
between classes types of molecules (e.g., dextrans and proteins), making it difficult to
compare them on the basis of M alone. Dextrans form a random coil, whereas proteinsr
are generally more tightly folded, and this physical configuration will reflect a different molecular diameter. Additionally, molecules can not be viewed as simple spheres, as some proteins are ellipsoidal (Peters, 1986). Ellipsoidal molecules may penetrate cells more rapidly than spherical ones by virtue of having at least one shortened dimension. The charge of a molecule may also affect its ability to pass through the cell wall. A molecule of a certain fixed charge may be repelled by molecules within the cell wall which carry the same charge (Read and Bacic, 1996).
Using the data from Carpita et al. (1979) and Peters (1986) (as well as references therein), Read and Bacic (1996) demonstrated that the molecular diameter of a given
type of molecule (protein or dextran) increases approximately as the cube of M .r
Dextrans of 20K have a diameter of 7.1 nm, whereas dextrans of 40K have a diameter of 9.2 nm. In the algae used in this study, the 10K (4.7 nm) FD exhibited unhindered passage across the cell walls. In Enteromorpha intestinalis, Ulva rigida and
Poly-siphonia strictissima, the 20K (7.1 nm) FD exhibited hindered transport. This FD was
excluded from Porphyra sp. The 40K (8.8) dextran was excluded from all four algal species. We thus conclude that the limiting pore size in the cell walls of untreated E.
intestinalis, U. rigida and P. strictissima was approximately 8 nm, whereas the pore size of untreated Porphyra sp. was approximately 7 nm. This result is comparable to the limiting pore sizes of angiosperm primary cell walls; values of limiting diameters of pores have been reported ranging from 4.5 nm in Nicotiana pollen tubes (O’Driscoll et al., 1993) to 8.6 nm in Hordeum suspension cells (Shedletzky et al., 1992).
Extrapolating from the published data on the molecular diameters of dextrans, the
diameters of the larger dextrans in this study were 70K511 nm, 150K512.1 nm and
250K513.5 nm. The effect of low pH on the algal cell walls was to increase the pore
diameters. Immersion in seawater at pH 2.0 for 60 min increased the cell-wall pore sizes of all four algal species to at least 13.5 nm. In the other low pH treatments, there was a range of unhindered and hindered passage as well exclusion of the FDs. It is only possible to infer a measurement of the limiting diameter of the pore when the smallest molecule to be excluded has been determined. Consequently, we could only determine the limiting pore size after low pH treatment in two cases. The 150K FD was excluded from Porphyra sp. after 20 min in pH 2.5, and the 150K FD was excluded from both
Porphyra sp. and Polysiphonia strictissima after 20 min in pH 3.0. These results indicate
In both the plasmolysis and low pH experiments, larger FDs took longer to pass through the cell walls than smaller ones. As Baron-Epel et al. (1988) point out, the differing times taken by the FDs to pass through the cell wall could either be the result of a few large transport channels or a slowed diffusion through a homogenous series of narrower channels. Although both explanations are possible in the cell walls of the algae in this study, we consider it more likely that the larger molecules are slowly diffusing through a more or less homogenous series of pores in the cell walls.
In the primary cell walls of angiosperms, the organisation of the pectic polysac-charides in the cell walls, rather than that of the cellulose, appears to determine the sieving properties of the cell walls (Baron-Epel et al., 1988). These pectic polysac-charides are negatively charged, are able to form gels (Bacic et al., 1988), and comprise a large proportion of the matrix phase of the cell wall in which the cellulose microfibrils are embedded. The cell walls of the algae used in this study also have a microfibrillar phase embedded in a matrix phase. In Enteromorpha intestinalis, Ulva rigida and
Polysiphonia strictissima, the microfibrillar phase is cellulose, but in Porphyra sp. it is
composed of a (1→3)-b-xylan (Preston, 1974; Bobin-Dubigeon et al., 1997). The
matrix of the cell walls also contains negatively charged gel-forming polysaccharides. In
P. strictissima and Porphyra sp. these are sulphated galactans; agar (Usov et al., 1983)
and porphyran (Brasch et al., 1981), respectively. Whereas in U. rigida, they are sulphated xyloglucuronorhamnans (ulvan) (Layhaye et al., 1996). Polysaccharides similar to ulvan also occur in the cell wall of E. intestinalis, which is a closely related genus (Percival and McDowell, 1981). Low pH treatment may cause changes in these polysaccharide gels which resulted in the observed increased porosity of the cell walls. In addition, the cell walls of Porphyra have been shown to have an outer layer, enriched
in an insoluble (1→4)-b-mannan (Preston, 1974). This may explain why this alga was
the most resistant to low pH treatments. Angiosperm primary cell walls exhibit no increase in porosity when treated in conditions as low as pH 3.5 (Baron-Epel et al., 1988); however, no study has examined the effects on angiosperm cell wall pores below pH 3.5. The increase in porosity as a result of pH treatment has important implications for the digestion of the algae after ingestion by marine herbivorous fishes.
Many marine herbivorous fish have acidic stomachs (Horn, 1998). The fish cited in this study have stomach pH levels ranging from 2.0060.15 to 2.8760.06 (Zemke-White et al., 1999). These fish therefore have the necessary stomach conditions to produce the changes in cell-wall porosity exhibited by the algae in this study. How do these changes in porosity relate to the ability of fish to digest algae?
Proteases and amylolytic enzymes are important digestive enzymes for algivorous fish (Horn, 1989). Proteases are essential for protein digestion (Stevens and Hume, 1995). Amylolytic enzymes are important for fish which ingest chlorophytes and rhodophytes (Sabapathy and Teo, 1993), as these algae contain starch and floridean starch, respectively (Percival and McDowell, 1967), which are important energy sources for the
fish. The a-amylase found in the intestinal lumen of fish (Sabapathy and Teo, 1993;
Kuz’mina and Gelman, 1997) begins the process of degrading starch to glucose by
hydrolysing starch to a-limit dextrans and other oligosaccharides, mainly maltose and
proteases trypsin and chymotrypsin are found in the intestinal lumen (Chakrabarti et al., ´ ´
1995; Jonas et al., 1983, Kapoor et al., 1975). Proteases and amylolytic enzymes must gain entry to the algal cells in order for intracellular protein and starch to be digested.
Although we could find no published data on the M of proteases specifically fromr
herbivorous fish, proteases have been isolated from number of other teleost species. These include pepsins of 27K and 33.9K (Sanchez-Chiang and Ponce, 1981), a trypsin of 24 K (Reeck et al., 1970), and chymotrypsins of 25.6K (Cohen et al. 1981). Although a-amylases have not been purified from any fishes, most known mammaliana-amylases are monomers of 50–57K (Karn and Malacinski, 1979). Using published data on protein molecular diameter (Baron-Epel et al., 1988), and assuming a spherical shape, the proteases range in molecular diameter from 4.2 to 5.4 nm (analogous to dextrans of 7 to
10K), while the a-amylases range between 6.1 and 6.5 nm (analogous to dextrans of
15–18K). Thus, for all of the algae in this study proteases should be able to pass through the cell walls unhindered (in under 10 min). For Enteromorpha intestinalis, Ulva rigida
and Polysiphonia strictissima,a-amylases would exhibit hindered transport, taking up to
30 min to pass through the cell walls. Only proteins of over 100K (8.2 nm) would be excluded from these algae, excepting those proteins that were ellipsoidal with at least one short dimension, as these may also able to pass through the cell-wall pores. Given thata-amylases range in molecular masses from 50 to 57K, which is analogous to FDs of 15–18K, and that the 20K FD was excluded from passing through the cell wall of untreated Porphyra sp., we conclude that proteins in this size range would either exhibit hindered transport, or be completely excluded.
These results demonstrate that once the plasma membrane is damaged, macro-molecules of the dimensions of many digestive enzymes could potentially pass into algal cells without the need for the effects of low pH such as occurs in the stomachs of many marine herbivorous fishes. However, the increase in the porosity of the cell walls as a result of low pH conditions would facilitate a more rapid transport of these enzymes into
the algal cells. Thea-amylases could take up to 30 min to pass through unaffected cell
walls. But after treatment for 20 min at pH 2.0 or pH 2.5, or after treatment for 60 min at pH 3.0, this time would be reduced to less than 5 min for Enteromorpha intestinalis,
Ulva rigida and Polysiphonia strictissima, and less than 10 min for Porphyra sp.
Therefore, low pH treatment would dramatically increase the ability of the fish to digest intracellular starch.
We conclude that the main effects of the ‘‘acid lysis’’ of chlorophyte and rhodophyte macroalgae by marine herbivorous fishes are (i) to lyse the plasma membrane, and (ii) to increase the porosity of the cell walls to facilitate more rapid amylolytic degradation of the starch in algal cells.
Acknowledgements
References
Bacic, A., Harris, P.J., Stone, B.A., 1988. Structure and function of plant cell walls. In: Preiss, J. (Ed.), The Biochemistry of Plants, Vol. 14, Academic Press, London.
Baron-Epel, O., Gharyal, P.K., Schindler, M., 1988. Pectins as mediators of wall porosity in soybean cells. Planta 175, 389–395.
Bobin-Dubigeon, C., Lahaye, M., Barry, J.L., 1997. Human colonic bacterial degradability of dietary fibres from sea-lettuce (Ulva sp.). J. Sci. Food Agric. 73, 149–159.
Brasch, D.J., Chang, H.M., Chuah, C.T., Melton, L.D., 1981. The galactan sulfate from the edible, red alga, Porphyra columbina. Carbohydr. Res. 97 (1), 113–126.
Carpita, N., Sabularse, D., Montezinos, D., Delmaer, D.P., 1979. Determination of the pore size of cell walls of living plants. Science 205, 1144–1147.
Cohen, T., Gertler, A., Birk, Y., 1981. Pancreatic proteolytic enzymes from carp (Cyprinus carpio): 1. Purification and physical properties of trypsin, chymotrypsin, elastase and carboxypeptidase B. Comp. Biochem. Physiol. B Comp. Biochem. 69 (3), 639–646.
Cole, L., Coleman, J., Evans, D., Hawes, C., 1990. Internalisation of fluoroscein isothiocyanate and fluoroscein isothiocyanate-dextran by suspension-cultured plant cells. J. Cell Sci. 96, 721–730.
Chakrabarti, I., Gani, Md.A., Chaki, K.K., Sur, R., Misra, K.K., 1995. Digestive enzymes in 11 freshwater teleost fish species in relation to food habit and niche segregation. Comp. Biochem. Physiol. A 112 (1), 167–177.
Choat, J.H., Clements, K.D., 1998. Vertebrate herbivores in marine and terrestrial environments: a nutritional ecology perspective. Annu. Rev. Ecol. Syst. 29, 375–403.
Horn, M.H., 1989. Biology of marine herbivorous fishes. Oceanogr. Mar. Biol. Annu. Rev. 27, 167–272. Horn, M.H., 1998. Feeding and digestion. In: Evans, D.H. (Ed.), The Physiology of Fishes, 2nd ed., CRC
Press, Boca Raton, FL, pp. 43–63.
´ ´ ´ ´
Jonas, E., Ragyanszki, M., Olah, J., Boross, L., 1983. Proteolytic digestive enzymes of carnivorous (Silurus glanis L.), herbivorous (Hypophthalmichthys molitrix Val.) and omnivorous (Cyprinus carpio L.) fishes. Aquaculture 30, 145–154.
Kapoor, B.G., Smit, H., Verighina, I.A., 1975. The alimentary canal and digestion in teleosts. In: Russel, F.R., Youge, M. (Eds.), Advances in Marine Biology, Vol. 13, Academic Press, London.
Karn, R.C., Malacinski, G.M., 1979. Comparative biochemistry, physiology and genetics of animal a -amylases. Adv. Comp. Physiol. Biochem. 7, 1–102.
´
Kuzmina, V.V., Gelman, A.G., 1997. Membrane-linked digestion in fish. Rev. Fish. Sci. 5, 99–129. Layhaye, M., Ray, B., Baumberger, S., Quemener, B., Axelos, M.A.V., 1996. Chemical characterisation and
gelling properties of cell wall polysaccharides from species of Ulva (Ulvales, Chlorophyta). Hydrobiologia 326 / 327, 473–480.
Lee, R.E., 1980. Phycology, Cambridge University Press, Cambridge.
Lobel, P.S., 1981. Trophic biology of herbivorous reef fishes: alimentary pH and digestive capabilities. J. Fish Biol. 19, 365–397.
Matheson, N.K., McCleary, B.V., 1985. Enzymes metabolising polysaccharides and their application to the analysis of structure and function of glycans. In: The Polysaccharides. G.O. Aspinall (Ed.) Academic Press. New York.
McMurtry, M., 1999. The feeding biology of the Black Angelfish Parma alboscapularis. Unpublished M.Sc. Thesis, University of Auckland, Auckland.
O’Driscoll, D., Read, S.M., Steer, M.W., 1993. Determination of cell-wall porosity by microscopy: walls of cultured cells and pollen tubes. Acta. Bot. Neerl. 42, 237–244.
Percival, E., McDowell, M.R., 1967. Chemistry and Enzymology of Marine Algal Polysaccharides, Academic Press, London.
Percival, E., McDowell, R.H., 1981. Algal cell walls – composition and biosynthesis. In: Tanner, W., Loewus, F.A. (Eds.), Encyclopedia of Plant Physiology New Series, Plant Carbohydrates II Extracellular Carbohy-drates, Vol. 13B, Springer-Verlag, Berlin, pp. 277–316.
Preston, R.D., 1974. The Physical Biology of Plant Cell Walls, Chapman and Hall, London.
Read, S.M., Bacic, A., 1996. Cell wall porosity and its determination. In: Linskins, H.F., Jackson, J.F. (Eds.), Modern Methods of Plant Analysis, Vol. 17, Springer-Verlag, Berlin, pp. 63–80.
Reeck, G.R., Winter, W.P., Neurath, H., 1970. Pancreatic enzymes of African lungfish – Protopterus aethiopicus. Biochemistry 9, 1398–1403.
Sabapathy, U., Teo, L.H., 1993. A quantitative study of some digestive enzymes in the rabbitfish, Siganus canaliculatus and the sea bass, Lates calcarifer. J. Fish Biol. 42, 595–602.
Sanchez-Chiang, L., Ponce, O., 1981. Gastricsinogens and gastricsins from Merluccius gayi, purification and properties. Comp. Biochem. Physiol. B 68, 251–257.
Shedletzky, E., Shmuel, M., Trainin, T., Kalman, S., Delmer, D., 1992. Cell wall structure in cells adapted to growth in the cellulose-synthesis inhibitor 2,6-dichlorobenzonitrile. Plant Physiol. 100, 120–130. Squire, P.G., 1981. Calculation of hydrodynamic parameters of random coil polymers from size exclusion
chromatography and comparison with parameters by conventional methods. J. Chromatogr. 210, 433–442. Stevens, C.E., Hume, I.D., 1995. Comparative Physiology of the Vertebrate Digestive System, 2nd ed.,
Cambridge University Press, New York.
Usov, A.I., Ivanova, E.G., Shashkov, A.S., 1983. Polysaccharides of algae: 33. Isolation and carbon-13-NMR spectral study of some new gel-forming polysaccharides from Japan Sea red seaweeds. Bot. Mar. 26 (6), 285–294.
Vial, T.H., 1997. The comparative feeding biology of two temperate water herbivorous fish, Silver Drummer, Kyphosus sydneyanus, and Parore, Girella tricuspidata. Unpublished M.Sc. Thesis, University of Auckland, Auckland.