www.elsevier.com/locate/ibmb
Molecular cloning of a female-specific cDNA with unique repeat
sequences from the fat body of the adult locust, Locusta
migratoria
Qili Feng
a, b, Subba R. Palli
b, Tim R. Ladd
b, Sardar S. Sohi
b, Arthur Retnakaran
b,
Kenneth G. Davey
a,*aDepartment of Biology, York University, 4700 Keele Street, North York, Ontario M3J 1P3, Canada
bGreat Lakes Forestry Centre, Canadian Forest Service, PO Box 490, 1219 Queen Street East, Sault Ste. Marie, Ontario P6A 5M7, Canada
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
A cDNA clone encoding a 25-kDa protein (25K) was isolated from a cDNA library made from RNA isolated from the adult fat body and ovaries of the locust, Locusta migratoria. The longest open reading frame of this cDNA clone encodes a 225-amino acid polypeptide, the N-terminal end of which was similar to the 21-kDa and 19-kDa juvenile hormone induced proteins identified in the locust hemolymph, but the C-terminal end was different. The C-terminal end of the 25K cDNA contained seven unique repeat elements of 10 amino acids each, most of which are polar residues. Expression of the 25K mRNA was tissue-, development- and sex-specific. A 1.2-kb mRNA was detected using the 25K cDNA as a probe only in the fat body of adult females. The mRNA started to appear at day 4 after the insect molted to the adult and rapidly increased by day 6. The mRNA was absent in the ovarian follicle cells and fat body of adult males. In vitro transcription and translation of the 25K cDNA produced a protein that migrated around 32 kDa on sodium dodecyl sulfate polyacrylamide gels. The 25K cDNA was expressed in a baculovirus expression system and the protein produced also migrated around 32 kDa. 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Hemolymph; Fat body; Follicle cell; Reproductive maturation; Repeat element; Baculovirus expression system
1. Introduction
Juvenile hormone (JH) plays an important role in the reproductive physiology of adult insects (Wyatt and Davey, 1996). During reproductive development in adult
Locusta migratoria, JH induces the synthesis of several
proteins, including vitellogenins (Vgs) (Dhadialla et al., 1987; Locke et al., 1987), a hexameric storage protein (Wyatt et al., 1992), a 19-kDa hemolymph protein (19K) (Kanost et al., 1988), and a 21-kDa hemolymph protein (21K) (Zhang et al., 1993). Most of these proteins are female-specific, synthesized in the fat body, transported in the hemolymph and finally taken up into the developing oocytes (Wyatt and Davey, 1996). The
syn-* Corresponding author. Tel.:+1-416-736-2100; fax: +1-416-736-5698.
E-mail address: [email protected] (K.G. Davey).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 5 5 - 2
thesis of these proteins is stimulated by an increase in JH titer, which occurs at about 1 week after adult ecdysis (Zhang et al., 1993).
mRNA. The N-terminal end of the deduced amino acid sequence of this cDNA shows similarity with the amino acid sequence of the 21-kDa and 19-kDa proteins ident-ified from the locust (Kanost et al., 1988; Zhang et al., 1993), but the amino acids at the C-terminal end are different. Expression of the mRNA for this protein is tissue-, development- and sex-specific.
2. Materials and methods
2.1. Animals
African migratory locusts (Locusta migratoria) were taken from a large, gregarious colony and reared under a 12-h light/12-h dark cycle, day temperature of 36°C, night temperature of 22°C, and 70% relative humidity. The sexes were separated at the fifth larval stage and the adults were fed for 16 days after emergence.
2.2. cDNA library construction and screening
A cDNA library was constructed using mRNA iso-lated from the locust vitellogenic ovaries and the fat bod-ies of mated females that had oviposited. The synthe-sized cDNAs were cloned into Uni-ZAP XR vectors at the EcoR I and Xho I sites using a cDNA synthesis/cloning kit from Stratagene (La Jolla, CA, USA), following the manufacturer’s instructions.
Screening of the cDNA library was performed using an immuno-screening kit from Stratagene using poly-clonal antibodies produced by immunizing a rabbit with partially purified 35-kDa JH membrane receptor (Kim et al., 1999). The phage was plated in E. coli XL1-Blue MRF9, transferred to a nitrocellulose filter, and then screened with the polyclonal antibodies at a dilution of 1:2000. Sheep anti-rabbit antibodies conjugated with alkaline phosphatase (Sigma Chemicals Company, USA) were used as the secondary antibody at a dilution of 1:5000.
2.3. Sequencing and analysis of sequence
The inserts of the cDNA clones that reacted positively to the antibodies were sequenced on both strands using Cy5 AutoRead Sequencing Kit and ALFexpress DNA Sequencer (Pharmacia Biotech Inc., Piscataway, NJ, USA). Sequence analysis was performed using the MacVector DNA Analysis Program (International Biotechnologies Inc., New Haven, CT, USA). The sequences were then compared to the sequences in the GenBank database at the National Center for Biotechnol-ogy Information using the BLAST network services (Altschul et al., 1990). Amino acid sequences were aligned using the Clustal Alignment Program (Higgins and Sharp, 1988).
2.4. Northern blot
Total RNA was extracted from the fat body and ovaries of adults aged 0, 2, 4, 6 and 8 days using the guanidinium thiocyanate–phenol–chloroform method (Chomczynski and Sacchi, 1987). Ten micrograms of total RNA samples were separated on a 1% formal-dehyde–agarose gel and transferred to a Hybond N nylon membrane. The Northern blot was hybridized with a cDNA probe labeled with [32P]dATP. Hybridization and
washes were conducted as described in Palli et al. (1998).
2.5. SDS–PAGE and Western blot
Proteins were extracted from cells infected with recombinant viruses expressing the cloned cDNA using homogenization buffer (20 mM Tris, 50 mM KCl, 300 mM sucrose, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 1
µg/ml leupeptin, 1µg/ml pepstatin, pH 7.5). The protein samples were denatured at 100°C for 5 min in a denatur-ing buffer (0.1 M Tris, pH 6.8, 2% SDS, 0.5%β -mercap-toethanol, 12% glycerol, and 0.002% bromphenol blue). SDS–PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) was performed on 12% polyacrylamide gels in Tris–glycine–SDS buffer (10 mM Tris, 50 mM glycine, 0.1% SDS, pH 8.0). Gels were stained with 0.5% Coomassie Blue R-250 for 1 h and then destained with destaining solution containing 7% acetic acid and 10% MeOH in H2O.
After electrophoresis, proteins were transferred from gels to Hybond C nylon membranes in transfer buffer (10% Tris–glycine–SDS buffer and 20% MeOH in H2O), and immunodetection was performed using the
primary and secondary antibodies as described in Sec-tion 2.2.
2.6. In vitro translation
In vitro translation of cDNA was performed using the TNT Coupled Reticulocyte Lysate System from Pro-mega (Madison, WI, USA). The reaction mixture con-tained 25 µl of rabbit reticulocytes lysate, 2 µl of 10×reaction buffer, 1 µl of T3 RNA polymerase, 0.02 mM amino acid mixture minus methionine, 0.3 mCi/ml [35
S]-methionine, 40 units RNase inhibitor, and 1µg of DNA template in a total volume of 50 µl. The reaction mixture was incubated at 30°C for 2 h. The translation products were resolved on an SDS–PAGE gel (12%) and [35S]-labeled protein products were detected using an
InstantImager Electronic Autoradiography System (Packard Instrument Company, Meriden, CT, USA).
2.7. Construction of recombinant baculovirus
Expression System from Life Technologies (Gaithersburg, MD, USA) according to the manufac-turer’s protocol. The cDNA insert was first cloned into the mini-Tn7 element of a pFASTBAC donor plasmid at the sites of EcoR I and Xho I. The recombinant plas-mid was then transformed into DH10BAC cells contain-ing helper plasmid and Autographa californica multicap-sid nucleopolyhedrovirus (AcMNPV) genomic DNA. The mini-Tn7 element carrying the cDNA insert was transposed into the AcMNPV genome, resulting in a recombinant virus. The recombinant baculovirus DNA was selected by disruption of the lacZ9 gene and the recombinants were confirmed by PCR followed by Southern blotting.
Spodoptera frugiperda cells (SF-21, Vaughn et al.,
1977) were cultured in Grace’s medium (Grace, 1962) in six-well plates at a concentration of 106 cells/well.
The cells were incubated for 5 h at 28°C with a transfec-tion mixture containing 300 ng of the recombinant DNA and 10µl of CellFectinreagent (Life Technologies).
After removal of the transfection mixture, the cells were cultured at 28°C for 4 days. The recombinant baculo-virus was harvested and the titer was determined. The virus was then used at an MOI of 0.04 to infect SF-21 cells cultured in 15-ml flasks. The infected cells were harvested at 0, 1, 6, 12, 24, 48, 72, and 96 h post-inocu-lation (h.p.i.) for analysis of mRNA and protein.
2.8. JH binding assay
SF-21 cells inoculated with the recombinant virus were homogenized in the homogenization buffer described in Section 2.5. The homogenate was centri-fuged at 1000g for 10 min to pellet cell debris. The supernatant was centrifuged again at 30 000g for 60 min to pellet cell membranes. The membrane pellet was sus-pended in 20 mM Tris–HCl buffer, pH 7.5. For specific binding, the binding mixture contained 50 mM Tris–HCl (pH 7.5), 0.1 mg/ml membrane protein, 15 nM [3H]-JH
III in a total volume of 200µl. For determination of non-specific binding, unlabeled JH III at 1.5 µM was added to the above mixture. The binding reaction was perfor-med at 28°C for 60 min. The mixture was centrifuged at 30 000g for 30 min to pellet the membranes. The pel-let was rinsed twice with 500 µl of 50 mM Tris–HCl binding buffer. The top portion of the tubes was cut off right above the membrane pellet and the bottom portion was dropped into 10 ml of scintillation cocktail and the radioactivity was counted.
3. Results
3.1. Cloning of 25K cDNA
A cDNA library was constructed using mRNA iso-lated from locust vitellogenic ovaries and fat bodies and
screened using the polyclonal antibodies raised against the 35-kDa JH III membrane receptor (Sevala et al., 1995). Ten positive clones were isolated after screening 3.2×104 recombinant λ phage plaques. Restriction
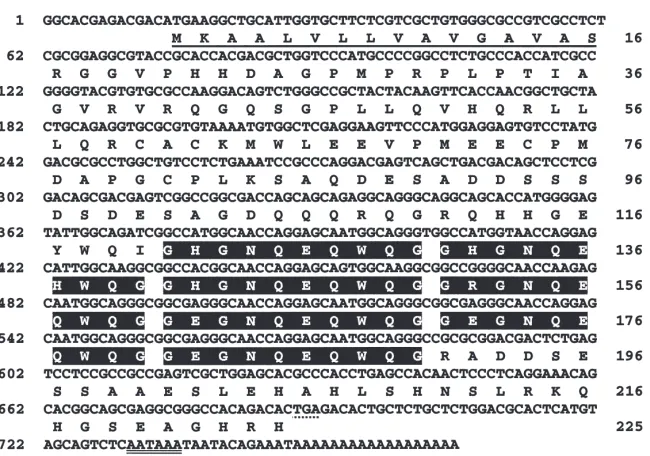
enzyme analysis and partial sequencing revealed that eight out of the 10 clones contained a 0.8-kb identical insert. Three of the eight clones containing an identical insert were sequenced completely on both strands. Sequence analysis showed that the three clones had an identical sequence of 766 nucleotides. The nucleotide sequence and deduced amino acid sequence are shown in Fig. 1. The longest open reading frame (ORF) begin-ning with ATG started at nucleotide 14 and ended at nucleotide 688. This ORF encodes a 225-amino acid protein with a predicted molecular mass of 24.688 kDa. A putative polyadenylation signal, AATAAA, was found between nucleotide 731 and 736, followed by a poly(A)18 sequence (Fig. 1). The deduced amino acid
sequence contained a putative signal peptide (1–16 amino acid residues), preceding a putative cleavage site between Ser-16 and Arg-17. Since this cDNA concep-tually translates into a 24.69-kDa protein, we named it 25K cDNA.
3.2. Sequence comparison
Comparison of the 25K cDNA sequence with the sequences in GenBank showed that the deduced amino acid sequence of 25K cDNA was similar to the amino acid sequence of the 21-kDa juvenile hormone-induced protein identified in the locust hemolymph (21K, Zhang et al., 1993). The amino acid identity was 42.3% between these two proteins. This 25K cDNA also shared an 11.6% amino acid identity with a 19-kDa protein (19K), which was also cloned from the locust (Kanost et al., 1988). The amino acid sequences of the N-ter-minal ends of these three proteins show considerable similarity, whereas the sequences from the amino acid residue 111 to the C-terminal end were different (Fig. 2A). The deduced amino acid sequence of the 25K cDNA contained seven repeat elements near the C-ter-minal end (Fig. 2B and C). Each repeat consists of 10 amino acids, nine of which are highly conserved. Seven of the nine highly conserved amino acids in the repeat elements were polar amino acids, which makes the con-served sequence highly hydrophilic (Fig. 2C). No such repeat elements were present in the deduced amino acid sequences of either 21K or 19K cDNA. A GenBank search revealed that no other protein identified so far had a similar repeat sequence.
3.3. Developmental expression of 25K mRNA
Fig. 1. Nucleotide and deduced amino acid sequences of 25K cDNA cloned from L. migratoria. The putative signal peptide is underlined. The stop codon TGA is marked with asterisks and the putative polyadenylation signal AATAAA is double-underlined. The seven repeat elements are shaded with black. The numbers on the left refer to nucleotides and those on the right refer to amino acid residues.
Fig. 3. Developmental expression of 25K mRNA in the fat bodies and ovarian follicle cells of adult locust. The top panel shows the Northern blot containing 10µg of total RNA hybridized with a 25K cDNA probe. The bottom panel shows ribosomal RNA stained with ethidium bromide, indicating equal loading of RNA.
adults and from the ovaries on days 0–8 after the insects had molted into adults. Expression of the mRNA for the 25K protein in these tissues was examined using North-ern blots hybridized with the 25K cDNA as a probe. A 1.2-kb mRNA was detected in the fat body of mated female adults (Fig. 3). The mRNA started to appear on day 4 after adult emergence. By day 6 the mRNA level increased in abundance and continued to increase up to day 8. This mRNA was absent in the fat body of adult males (day 8), and no mRNA was detected in the ovaries up to 8 days. These results indicated that the synthesis of 25K mRNA is tissue-, development- and sex-specific.
3.4. In vitro translation of 25K cDNA
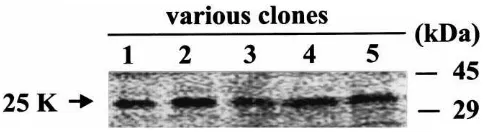
In vitro translation of the 25K cDNA was performed using a reticulocyte lysate system to characterize the peptide encoded by this cDNA. The translation product migrated at 32 kDa when resolved on SDS–PAGE (Fig. 4), instead of the predicted 25 kDa. We examined five independent clones and all of them produced the ident-ical protein with an apparent molecular mass of 32 kDa.
Fig. 4. In vitro translation of 25K cDNA in reticulocyte lysate system in the presence of [35S]-methionine. Lane C, control, pBluscript vector alone without any insert; Lanes 1–5, various clones with the 25K cDNA insert. The translation product was separated in a 12% SDS– PAGE gel.
3.5. Expression of 25K cDNA in a baculovirus expression system
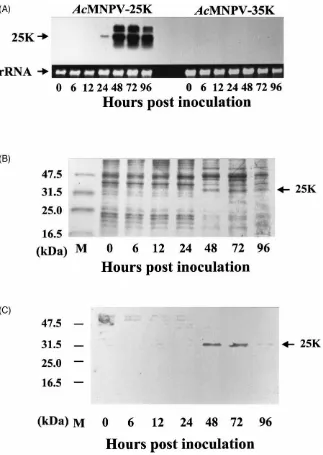
To express the 25K cDNA, a recombinant AcMNPV expressing the 25K cDNA (AcMNPV-25K) was con-structed and used to infect SF-21 cells. RNA and pro-teins were isolated from the infected cells at different times post-inoculation. Northern blots hybridized with a 25K cDNA probe showed that 1.2-kb mRNA started to appear at 24 h.p.i. in the cells inoculated with AcMNPV-25K (Fig. 5A, left). The mRNA level increased by 48 h.p.i. This mRNA was not detected in the control cells that were inoculated with another recombinant baculo-virus expressing a 35-kDa protein cDNA (AcMNPV-35K) (Fig. 5A, right).
Proteins in the cells inoculated with AcMNPV-25K were analyzed using SDS–PAGE followed by Western blotting. The expressed protein was detected in both the membrane fraction and the cytoplasm of the cells inocu-lated with the recombinant virus. The protein profile of SDS–PAGE showed a 32-kDa protein starting to appear at 48 h.p.i., 1 day after the appearance of the mRNA (Fig. 5B). The expressed protein was recognized immunologically by the antibodies used for screening the cDNA library (Fig. 5C). Thus, the 25K cDNA we cloned was expressed both in the baculovirus expression system and in the in vitro translation system as a protein with an apparent molecular mass of 32 kDa.
3.6. JH binding assay
Fig. 5. Expression of 25K cDNA in SF-21 cells inoculated with the recombinant baculovirus expressing the 25K cDNA (AcMNPV-25K). (A) mRNA expression in the SF-21 cells inoculated with AcMNPV-25K (left) or inoculated with a recombinant baculovirus carrying a 35-kDa protein cDNA as control (right). The top panel shows the Northern blot containing 10µg of total RNA hybridized with a 25K cDNA probe. The bottom panel shows ribosomal RNA stained with ethidium bromide, showing equal loading of RNA. (B) SDS–PAGE of the 25K protein expressed in SF-21 cells inoculated with AcMNPV-25K. Five micrograms of cell proteins were loaded in each lane. SDS–PAGE gel was stained with Coomsasie Blue R250. (C) Western blot was immunostained with the polyclonal antibodies (1:2000) produced against the JHMR, followed by sheep anti-rabbit antibodies conjugated with alkaline phosphatase (1:5000).
from the cells infected with AcMNPV-25K. The results showed no specific binding of [3H]-JH III to the
mem-brane protein (data not shown). This was consistent with the results of protease cleavage assay with Protease K and JH III that also showed that JH did not bind to the protein expressed from the in vitro translation system (data not shown).
4. Discussion
cDNA inserts. It is the most frequently recovered cDNA clone from the library screening with the antibodies. Transcription and translation of the cDNA clones pro-duced a protein that migrated at 32 kDa on SDS–PAGE. When this cDNA was expressed in a baculovirus system, it produced a protein that migrated at 32 kDa on SDS– PAGE and reacted with antibodies that were used to screen the library. The fact that this protein migrated at 32 kDa which is close to the size of the JHMR (35 kDa) and that the antibodies produced against JHMR recog-nized this protein indicated that this cDNA may be coding for JHMR. However, preliminary JH binding assays showed that the protein expressed from 25K cDNA did not bind JH specifically, indicating that this cDNA may not code for JHMR. Moreover, we did not detect mRNA for the protein in ovary, as we would expect if the protein were functioning as a receptor. Thus, it is likely that the protein was a contaminant in the original partial purification of the JHMR. In addition to the 35-kDa JHMR protein, the 25K protein may also have been present in the band used for antibody pro-duction because its apparent molecular mass is 32 kDa and the small difference in the molecular mass may not allow SDS–PAGE to separate these proteins well. Thus, the antiserum raised against this protein band may have contained the antibodies against both JHMR and 25K proteins.
The source of the contamination is at this stage a mat-ter for conjecture. We have not detected messenger RNA in the ovary for this protein, although it is present in female fat body. While it is possible that the original extracts of locust ovaries were contaminated with fat body, we regard this as very unlikely, since the prep-arations were very carefully dissected free of any other tissue. The original 35-kDa band from which the anti-bodies were derived came from a well-washed prep-aration of membranes sedimented at 30 000g (Sevala et al., 1995). If, as seems likely, the 25K protein is circulat-ing in the hemolymph, it may have been taken up either adventitiously or functionally into membrane bound ves-icles by the follicle cells. Follicle cells are known to sequester some molecules by endocytosis (Davey, in press). It will be of some interest to investigate the distri-bution of this protein using antibodies. Alternatively, the protein may be synthesized by ovarian tissues later in the gonotrophic cycle than 8 days post-emergence.
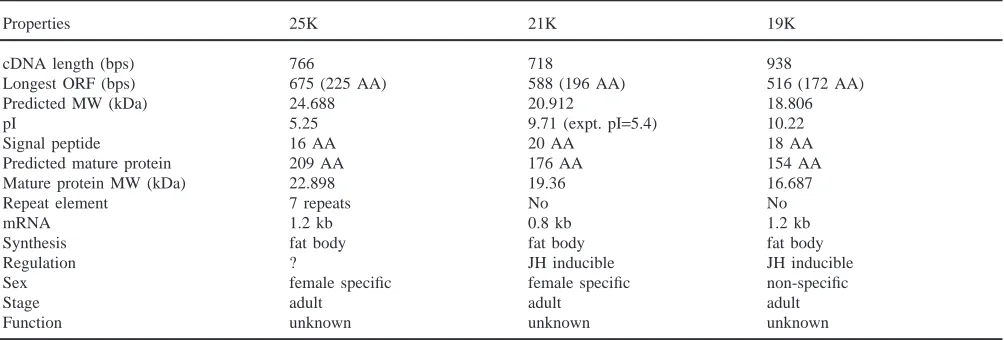
The 25K protein appears to belong to a protein group which includes the JH-inducible 21K and 19K proteins that were isolated from the locust hemolymph (Kanost et al., 1988; Zhang et al., 1993). These proteins share several properties as shown in Table 1. The mRNA for these proteins is detected only in the adult fat body. All of these proteins have a signal peptide, indicating that these are secretory proteins. The 21K and 19K proteins have been shown to be released into the hemolymph and transported to the ovary where they are taken up into the
developing oocytes. We do not have any evidence to suggest that the 25K protein is also present in the hemo-lymph and ovary. But the presence of a signal peptide in the deduced amino acid sequence of the 25K cDNA implies that this is a secretory protein. Developmental expression studies showed that the 25K mRNA first appeared at day 4 after adult emergence and then greatly increased by day 6. The 21K protein was first detected in the hemolymph on day 2 and reached a maximum level over days 15–20 after the insect molted into adult (Zhang et al., 1993). The time and rate of initial appear-ance and increase of the 21K protein in the hemolymph is similar to that of vitellogenins (Vgs) (Locke et al., 1987). The expression of the 19K protein started to increase rapidly by day 8 after adult emergence (Kanost et al., 1988). It is clear that these proteins are synthesized during the process of reproductive maturation and trans-ported to the ovaries. The presence of the 25K and 21K proteins is sex-linked and they are present only in adult females. The 19K protein is present in both male and female adults. Synthesis of 21K and 19K proteins is stimulated by the JH analog, methoprene (Kanost et al., 1988; Zhang et al., 1993; Zhang and Wyatt, 1996). It will be interesting to see if the 25K protein is also regu-lated by JH. Initial appearance of 25K mRNA is closely coordinated in time with that of Vgs, 21K and 19K, all of which are induced by JH, implying possible involve-ment of JH in the regulation of synthesis of the 25K pro-tein.
Another interesting finding of this study is that these three proteins are homologous at the N-terminal ends, indicating that the N-terminal ends may be involved in a similar function that all these three proteins perform. The C-terminal ends are different and may be respon-sible for specific functions for each protein. The biologi-cal role of these proteins is not known at this time. Besides these three proteins, two L. migratoria Vgs (Locke et al., 1987) are also female specific, produced in the fat body, regulated by JH, secreted into hemo-lymph, and taken up into oocytes. However, there is no similarity between the 25K protein and these Vgs.
One important feature of the 25K protein is the pres-ence of the unique repeat elements that consist of highly conserved amino acids that are hydrophilic. A GenBank search found no proteins with similar repeat elements. Lipid transport in insect hemolymph is achieved by lipo-proteins, or apolipophorins, that are synthesized in the fat body and serve to shuttle digested lipids from the midgut to growing and storage tissues (Prasad et al., 1986). In Manduca sexta, Apolipophorin-III is com-posed of tandem repeat sequences with length variability and potential amphiphilic helices (Cole et al., 1987). Vertebrate vitellogenin receptor, insect vitellogenin/yolk protein receptors and the low-density lipoprotein recep-tors all contain cysteine-rich repeats (|40 amino acids
Table 1
Comparison of three proteins isolated from Locusta migratoria
Properties 25K 21K 19K
cDNA length (bps) 766 718 938
Longest ORF (bps) 675 (225 AA) 588 (196 AA) 516 (172 AA)
Predicted MW (kDa) 24.688 20.912 18.806
pI 5.25 9.71 (expt. pI=5.4) 10.22
Signal peptide 16 AA 20 AA 18 AA
Predicted mature protein 209 AA 176 AA 154 AA
Mature protein MW (kDa) 22.898 19.36 16.687
Repeat element 7 repeats No No
mRNA 1.2 kb 0.8 kb 1.2 kb
Synthesis fat body fat body fat body
Regulation ? JH inducible JH inducible
Sex female specific female specific non-specific
Stage adult adult adult
Function unknown unknown unknown
one another (Sappington and Raikhel, 1998). However, because the repeat sequences in the 25K protein are hyd-rophilic, they are not likely to be the lipid-binding regions. In addition, the hydrophilic repeat sequences are also not likely to form transmembrane helices that are usually found in some membrane receptors. Knowing the possible role of the repeat sequences will be of interest and may facilitate understanding the possible function of this protein as well as other members in this group.
The 25K cDNA is 766 base pairs long and includes the start codon, ATG, the terminal signal, AATAAA, and a poly(A) trail. But the mRNA detected in Northern blots using the cDNA probe is 1.2 kb in length, indicat-ing that the cDNA clone is not full-length. The size dif-ference may be due to a missing untranslated region at the 59-end of the cDNA. Although the predicted molecu-lar mass of the deduced amino acid polypeptide encoded by the longest ORF of 25K cDNA is 25 kDa, the protein expressed in both the rabbit reticulocyte lysate trans-lation system and the baculovirus expression system showed an apparent molecular mass of 32 kDa. There is no putative N-linked glycosylation sites, such as Asp-X-Ser/Thr, in the deduced amino acid sequence. The sequence contains 16 serines, most of which are located in the regions flanking the repeat elements. These serines may serve for O-linked glycosylation sites. Glycosyl-ation at these sites may contribute to the difference in molecular mass of the deduced and expressed proteins. The members of both Vg and Vg receptor families in several insects contain putative O-linked glycosylation regions (Sappington and Raikhel, 1998), but they are dif-ferent structurally from those in this 25K protein. The biological role of this female protein, together with its unusual mobility, is currently under investigation.
Acknowledgements
This research was supported in part by a Natural Sciences and Engineering Research Council of Canada Research Grant, and a Collaborative Research and Development Grant from NSERC and Cyanamid Canada to K.G.D. and by the National Biotechnology Strategy Fund and the Science and Technology Opportunities Fund of the Canadian Forest Service to Biotechnology at GLFC.
References
Altschul, S.F., Warren, G., Webb, M., Myers, E.W., Lipman, D.J., 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403– 410.
Chomczynski, P., Sacchi, N., 1987. Single step method of RNA iso-lation by acid guanidinium thiocyanate–phenol–chloroform extrac-tion. Anal. Biochem. 162, 156–159.
Cole, K.D., Fernando-Warnakulasuriya, G.P., Boguski, M.S., Freeman, M., Gordon, J.I., Clark, W.A. et al., 1987. Primary structure and comparative sequence analysis of an insect apolipoprotein. Apoli-pophorin-III from Manduca sexta. J. Biol. Chem. 262, 11794– 11800.
Davey, K.G. Do thyroid hormones function in insects? Insect Biochem. Mol. Biol. 30, 877–884.
Davey, K.G., Sevala, V.L., Gordon, D.R.B., 1993. The action of juven-ile hormone and antigonadotropin on the follicle cells of Locusta
migratoria. Invert. Reprod. Dev. 24, 39–46.
Dhadialla, T.S., Cook, K.E., Wyatt, G.R., 1987. Vitellogenin mRNA in locust fat body: coordinate induction of two genes by a juvenile hormone analog. Dev. Biol. 123, 108–114.
Grace, T.D.C., 1962. Establishment of four strains of cells from insect tissues grown in vitro. Nature 195, 788–789.
Higgins, D.G., Sharp, P.M., 1988. CLUSTAL: a package for per-forming multiple sequence alignment on a microcomputer. Gene 73, 237–244.
G.R., 1988. Gene structure, cDNA sequence, and developmental regulation of a low molecular weight hemolymph protein from
Locusta migratoria. Arch. Insect Biochem. Physiol. 8, 203–217.
Kim, Y., Davari, E., Sevala, V., Davey, K.G., 1999. Functional binding of a vertebrate hormone, L-3,5,39-triiodothyronine (T3) to insect follicle cell membranes. Insect Mol. Biol. Biochem. 29, 943–950. Locke, J., White, B.N., Wyatt, G.R., 1987. Cloning and 59end nucleo-tide sequences of two juvenile hormone-inducible vitellogenin genes of the African migratory locust. DNA 6, 331–342. Palli, S.R., Ladd, T.R., Ricci, A.R., Primavera, M., Mungrue, I.N.,
Pang, A.S.D. et al., 1998. Synthesis of the same two proteins prior to larval diapause and pupation in the spruce budworm,
Choristone-ura fumiferana. J. Insect Physiol. 44, 509–524.
Prasad, S.V., Fernando-Warnakulasuriya, G.J., Sumida, M., Law, J.H., Wells, M.A., 1986. Lipoprotein biosynthesis in the larvae of the tobacco hornworm, Manduca sexta. J. Biol. Chem. 261, 17174– 17176.
Sappington, T.W., Raikhel, A.S., 1998. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 28, 277–300.
Sevala, V.L., Davey, K.G., Prestwich, G.D., 1995. Photoaffinity labe-ling and characterization of a juvenile hormone binding protein in the membranes of follicle cells of Locusta migratoria. Insect Biochem. Mol. Biol. 25, 267–273.
Vaughn, J.L., Goodwin, R.H., Tompkins, G.J., McCauley, P., 1977. The establishment of two cell lines from the insect Spodoptera
fru-giperda (Lepidoptera: Noctuidae). In Vitro 13, 213–217.
Wyatt, G.R., Kanost, M.R., Chin, B.C., Cook, K.E., Kawasoe, B.M., Zhang, J.Z., 1992. Juvenile hormone analog and injection effects on locust hemolymph protein synthesis. Arch. Insect Biochem. Physiol. 20, 167–180.
Wyatt, G.R., Davey, K.G., 1996. Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormone in adult insects. Adv. Insect Physiol. 26, 1–147.
Zhang, J., Wyatt, G.R., 1996. Cloning and upstream sequence of a juvenile hormone-regulated gene from the migratory locust. Gene 175, 193–197.