Enzymatic activities associated with decomposition of particulate organic
matter in two shallow ponds
S. Alvarez*, M.C. Guerrero
Ecology Department, Universidad Autonoma de Madrid, 28049 Madrid, Spain
Accepted 4 May 2000
Abstract
Particulate organic matter (POM) is an important source of energy in aquatic systems, where decomposition rates have traditionally been studied using the litter-bag technique. However, this method has limitations for estimating in situ decomposition rates, especially for fine particles. In this study the litter-bag technique is combined with measurements of microbial enzyme activities to estimate POM mass loss rates in situ. Litter-bags containing benthic POM in two size ranges, coarse (.1 mm) and fine (0.063–0.5 mm), were placed in two contrasting ponds (Toro and Oro) located in the Don˜ana area in southern Spain. Litter-bags were collected over a period of a year and analysed for mass loss and the activities of five extracellular enzymes (b-glucosidase,b-xylosidase,b-N-acetylglucosamidase, phenol oxidase, alkaline phosphatase). In situ sediment POM samples were also collected, sorted in the same size ranges, and assayed for the same enzyme activities. Using separate regression models relating mass loss to average cumulative enzyme activity, the mean turnover times for in situ coarse particulate organic matter (CPOM) and fine particulate organic matter (FPOM) were 149 and 2778 days in Toro pond and 382 and 4347 days in Oro pond, respectively. This compared with turnover times of 578 and 2109 days for Toro and 778 and 5296 days in Oro pond for POM confined in bags. Integrating enzyme measurements and litter-bag techniques provides in situ organic matter turnover rates and may allow the assessment of global controlling factors (e.g. flooding regime, temperature and POM quality) in the decomposition process in temporally and spatially heterogeneous environments.q2000 Elsevier Science Ltd. All rights reserved.
Keywords: Decomposition; Enzyme activities; Particulate organic matter; Enzyme decomposition models; Don˜ana
1. Introduction
Decomposition is a fundamental ecosystem process comparable in importance to primary production (Moorhead et al., 1996). It is also a composite phenomenon that can be viewed at several levels of resolution (Swift et al., 1979). At all levels, microbial decomposition plays a critical role in the macronutrient cycles and energy flows of aquatic ecosystems (Wetzel, 1992) but it has been difficult to link microbiological variables with ecosystem processes (Parnas, 1975; Howard and Howard, 1993).
Enzyme assays, used primarily to collect descriptive information about soils, have become useful techniques for monitoring microbial activity and uncovering the mechanisms that underlie microbial processes (Sinsabaugh et al., 2000). Recently, new types of models have been developed to examine organic matter processing using the activities of extracellular enzymes. These models have been used in the analysis of dissolved organic materials in aquatic
environments (Chro´st, 1991), mass loss of plant litter in terrestrial systems (Sinsabaugh et al., 1992; Sinsabaugh and Moorhead, 1994), processing of particulate and dissolved organic carbon (POC, DOC) in streams (Sinsa-baugh et al., 1994a; Sinsa(Sinsa-baugh and Findlay, 1995; Findlay et al., 1998) and processing of particulate organic matter (POM) in wetlands (Jackson et al., 1995).
Non-living natural organic matter (NOM) comprises in most aquatic environments the largest organic matter frac-tion and can be subdivided into dissolved organic matter (DOM) and POM (Thurman, 1985). POM processing has traditionally been studied using the litter-bag technique, which has some inherent problems (Boulton and Boon, 1991), both for large-mesh and, especially, for fine-mesh bags, where the mesh can act as a filter restricting exchanges with the surrounding system.
The decomposition of POM into soluble compounds is mediated by extracellular enzymes (Burns, 1982; Chro´st, 1991). As microbial breakdown of detritus is directly linked to the activity of these enzymes, statistical models that link mass loss rates of decomposing litter to the activities of selected microbial enzymes have been
0038-0717/00/$ - see front matterq2000 Elsevier Science Ltd. All rights reserved. PII: S 0 0 3 8 - 0 7 1 7 ( 0 0 ) 0 0 1 7 0 - X
www.elsevier.com/locate/soilbio
developed (Sinsabaugh et al., 1992, 1994b, 2000). These models may help circumvent some of the methodological constraints that have hindered studies of in situ decomposi-tion rates, especially for fine particles.
The purpose of this study was to investigate POM proces-sing in two contrasting ponds. Enzymatic decomposition models (EDM) were applied to obtain estimates of in situ processing rates. These models further our understanding of the decomposition process at the enzyme level, and help document the extent to which the process varies among systems. At the same time, the study provides some of the first results about decomposition processes at the microbial community level in aquatic systems in Spain, under contrasting flooding regimes and weather conditions.
2. Materials and methods
2.1. Study sites
The study was conducted from March 1998 to April 1999
at Pond del Charco del Toro and Pond del Rio Oro (Toro and Oro ponds), located in the southwest coast of Spain. Both are shallow ponds (maximum depth 115 and 120 cm for Toro and Oro, respectively) with similar morphometric and size features, and both are located on the ‘eolian littoral mantle’ (Montes et al., 1998). Oro pond is located in a phytostabilised sand area, whereas Toro pond is located along the northeast margin of an active dune system. Both have a temporary flooding regime with a tendency towards drying at the end of the warm season (July, August). These ponds are fed by a shallow water table and rainfall that occurs predominantly in the spring and autumn. Toro pond is located in Don˜ana National Park, while Oro pond is located in Don˜ana Natural Park. Don˜ana ponds are impor-tant aquatic environments because local differences in groundwater, soils, or vegetation can produce water of vari-able quality (Sacks et al., 1992; Serrano and Toja, 1995). Until now, such heterogeneity has been studied using struc-tural measures such as zooplankton (Galindo et al., 1994), or phytoplankton and aquatic vegetation (Lo´pez et al., 1991) but not in terms of the functioning of the system (i.e. measuring rates of different processes).
Despite their similarities, Toro and Oro ponds have important differences. Toro pond is surrounded by a pine (Pinus pinea) forest and has a littoral belt ofJuncus mari-timus,Scirpus holoschoenus, andS.lacustris. Oro pond is surrounded by a eucalyptus (Eucalyptus globulus and E. camaldulensis) plantation, and some of the trees are within the basin of the pond. These plantations were very abundant during 1960s, and still persist in important areas of the region. Macrophyte vegetation, although composed of the same species, is more disturbed and much less dense in Oro pond than in Toro pond. These differences result in impor-tant differences in the inputs and the quality of organic matter that enters the system, and have an influence over the physico-chemical characteristics of the water.
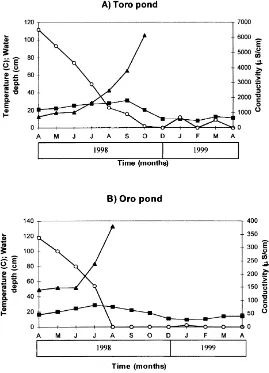
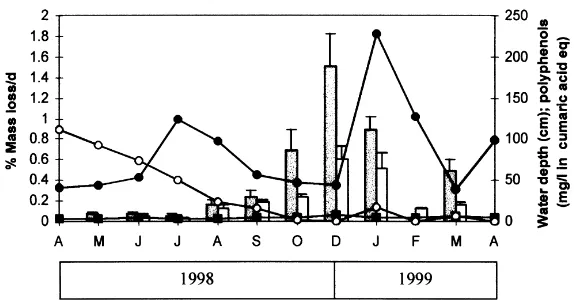
The area of study is dominated by a Mediterranean climate with relatively mild winters (mean temperatures of 98C during December and January) and very dry and hot summers (with maximum temperatures.308C). Annual rainfall is variable. The 1996–1997 hydrological cycle was extremely wet, and ponds reached their maximum depth values, but the 1997–1998 and 1998–1999 hydrological cycles were dry, with almost no rains, and water levels decreased continuously, except for some increases during 1999 spring season (Fig. 1).
2.2. Experimental design
Temperature, pH and conductivity (340-B WTW conduc-tivity meter) were measured in situ. Surface water levels were measured using 2-m long metal rods fixed to the bottom of the ponds. Polyphenolic concentrations in water and sediments were measured using a modified procedure for the Folin–Ciocalteu method (Box, 1983). Particulate organic matter inputs to the ponds were measured using litter-traps. Vertical traps consisted of a square structure (0.45 m2) elevated 0.5 m above ground level with a woven plastic mesh. Lateral traps consisted of a ground level 0.16 m2steel frame with a mesh like those used for vertical traps with its opening parallel to the shore and anchored to stakes. Five traps were placed at each sample site. Collected material was dried (758C, 48 h) and weighed.
POM for litter-bag preparation was collected from the study sites in winter 1997. The material was wet-sieved using 20-cm diameter sieves with mesh sizes of 1, 0.5 and 0.063 mm and dried at 758C for 48 h. Material.1 mm was designated as coarse particulate organic matter (CPOM) and material between 0.063–0.5 mm as fine particulate organic matter (FPOM). Only these two fractions were considered, as medium particulate organic matter (MPOM 0.5–1 cm) has shown erratic behaviour that prevented the development of enzymatic models in previous studies (Sinsabaugh et al., 1994a; Jackson et al., 1995). Also, the different size ranges of litter should be viewed as separate points along a decom-position continuum rather than discrete classes.
The dried CPOM was placed into 1-mm mesh fibreglass screening litter-bags, and FPOM was placed into litter-bags
of 0.06-mm mesh. Each CPOM bag received 3 g dry mass (DM) equivalent to 2.5 g OM in Toro and 2.6 g OM in Oro (organic mass as determined by loss on ignition at 5508C) and each FPOM bag received 10 g DM equivalent to 1.3 g OM in Toro and 0.5 g OM in Oro. In each pond, in the area of maximum initial depth, 45 bags of each size class were placed along three independent transects and weighed to the bottom. Three independent replicates of each size range were collected after 7 days to assess rapid leaching of solu-ble material. Subsequent collections were made at one-month intervals until the end of study except for November 1998. Collected litter-bags were sealed in plastic bags and transported on ice to the laboratory.
On each sampling site, in situ POM samples were also collected from the same area where bags were placed. Sedi-ment cores were collected using a 20 cm2corer to remove the top 5 cm of the substrate. On each sampling date four cores were taken from each site. In situ POM samples were also sealed within bags and transported on ice back to the laboratory.
2.3. Measurement of mass loss and enzyme activities
Each collected bag was analysed for mass loss and enzyme activities. The material in the bag was rinsed in slow running tap water and collected in a 0.063-mm mesh sieve. Macro-invertebrates present were removed and the recovered POM was divided into three subsamples in three pre-weighed aluminium pans. One subsample (consisting of 70% of recovered material) was dried at 758C for 48 h, re-weighed, ashed at 5508C for 3 h, and weighed again to determine moisture and organic matter content. The other two subsamples were suspended in 100 ml of 50 mM pH 5.0 acetate buffer and 50 mM pH 8.5 tris buffer, respectively. These suspensions were homo-genised (Euroturrax T20 dispersion unit).
Each core (in situ POM samples) was also wet sieved into the same two fractions as the litter-bag POM. Each fraction was divided into three subsamples as for the confined samples.
Each POM suspension, both from in situ — sediment core — and confined litter-bag samples, was assayed for the activity of five extracellular enzymes involved, respec-tively, in the degradation of cellulose and hemicellulose — b-1,4-glucosidase (EC 3.2.1.21) and b-xylosidase (EC 3.2.2.37); chitin — b-N-acetylglucosaminidase (NAGase, EC 3.2.1.30); polyphenolic substances — phenol oxidase (EC 1.10.3.2 and 1.14.18.1); and acquisition of N and P — b-N-acetylglucosaminidase (NAGase, EC 3.2.1.30) and alkaline phosphatase (EC 3.1.3.1). The substrates for b -glucosidase, b-xylosidase, b-NAGase and alkaline phos-phatase were pNP-b-d-glucopyranoside, pNP-b
-xylopira-noside, pNP-b-N-acetylglucosamidine and pNP-phosphate, respectively. The substrate for phenol oxidase was l
-3,4-dihydroxyphenylalanine (l-DOPA). All substrates were
Assays for b-glucosidase, b-xylosidase,b-NAGase and phenol oxidase enzymes were conducted at 208C in pH 5.0 acetate buffer, 50 mM, under previously determined substrate saturating conditions. For alkaline phosphatase, assays were conducted in pH 8.5 tris buffer 50 mM under previously determined substrate saturating conditions. In all
cases there were three analytical replicates and duplicate controls. A more detailed description of the procedures used can be found in Sinsabaugh et al. (1994a). Activities for all enzymes were analysed monthly, except for phenol oxidase, which was analysed every two months.
2.4. Data analysis
Data from the litter-bag samples were analysed to develop enzyme models establishing relationships between cumulative mass loss and cumulative enzyme activities (Jackson et al., 1995). The first step in the analysis is to integrate the enzyme activities over time by multiplying the mean activity over each interval by the length of the interval. The values obtained are expressed as activity-day, where activities for all enzymes are expressed in mmol h21g21OM. Cumulative activity was calculated as the activity-day summed over all previous sample intervals. Simple linear regressions are performed relating % initial mass loss as a function of cumulative activity-day for each enzyme and each size class and pond.
Organic matter losses between treatments were compared usingt-test P,0:05:Enzyme activities among treatments
were compared using Kruskal–Wallis tests, and Mann– Whitney tests were applied for a posteriori pairwise compar-isons, if appropriate. Relationships between different enzyme activities were determined by calculating Spear-man’s correlation coefficients (Zar, 1996).
3. Results
3.1. Breakdown rates
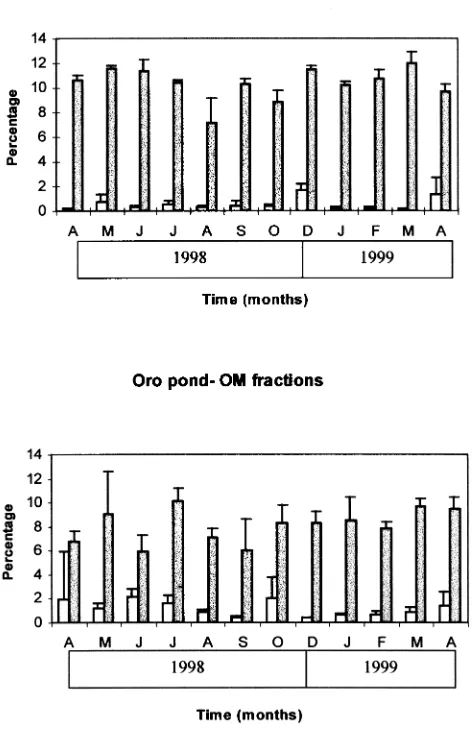
The physico-chemical characteristics of the two ponds differed considerably (Table 1). Toro had higher alkalinity, pH and nutrient concentrations than Oro throughout the studied period. The results in Fig. 2 show a similarity between the two ponds in the percentages of total coarse and fine particulate organic matter (CPOM, FPOM) over the period of study. The percentage of total OM is similar between ponds with average values of 26% in Toro and 20% in Oro. However, these similarities are in terms of Table 1
Physico-chemical characteristics of Toro and Oro ponds. Values are means of all sampling dates, with ranges given in parentheses. TNtotal nitrogen; TPtotal phosphorous; SRPsoluble reactive phosphorous
Characteristic Toro Oro
pH 7.28 (8.4–6.37) 6.4 (6.91–6.03)
Alkalinity (meq l21) 3.33 (4.66–1.646) 0.75 (0.961–0.48)
Conductivity (ms cm21) 2713 (6130–731) 211 (380–139)
Temperature 19.2 (31.5–10) 18.1 (29–10.5)
TN (mg l21) 6.02 (18.05–1.62) 2.85 (4.20–1.28)
Nitrate (mg l21) 4 (20–0) 0
Ammonium (mg l21) 1108 (4250–0) 96.5 (149–52)
TP (mg l21) 0.71 (2.99–0.06) 0.0975 (0.26–0.02)
SRP (mg l21) 9.72 (15.22–1.2) 1.405 (5.62–0)
quantity but not quality. The coarse material in Toro pond was a heterogeneous mixture of roots of submerged macro-phytes, portions ofJuncusspp., andScirpusspp., seeds, and pellets from different herbivores (deer, cattle). In Oro pond the coarse material consisted mainly of a mixture of leaf portions, wood fragments, seeds, stems, and bark of
Euca-lyptus spp. Arthropod exuviae were also present. In both
ponds the FPOM size fraction comprised fine amorphous particles.
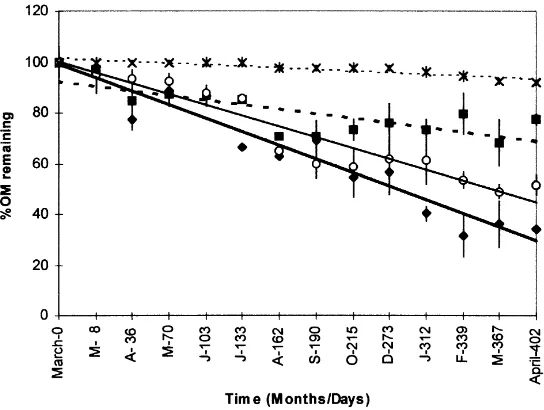
Mass loss of organic matter was linear for the two size fractions in both ponds during the studied period (Fig. 3). Zero-order rate constants were calculated from the linear regression of % OM remaining vs. time (Table 2). Regres-sions were statistically significant P,0:01;n14:The
highest rate of mass loss, (0.164% day21) was observed in the coarse fraction at Toro pond. The lowest rate (0.019% d21) was for the FPOM fraction in Oro pond.
There were no significant differences in OM mass loss rates for CPOM between the ponds but there were signifi-cant differences in OM loss rates for FPOM P,0:001:In
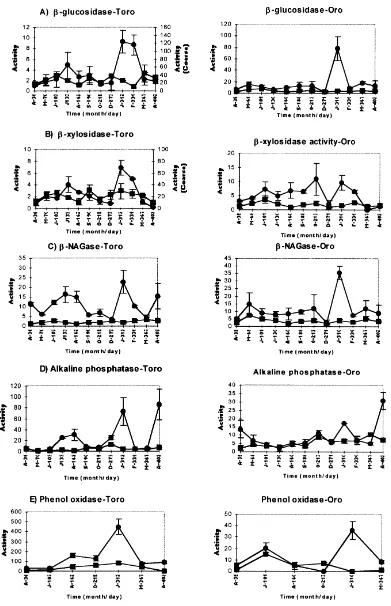
general, activity patterns of enzymes associated with confined POM differed from those associated with in situ samples (Figs. 4 and 5).
3.2. Enzyme activities
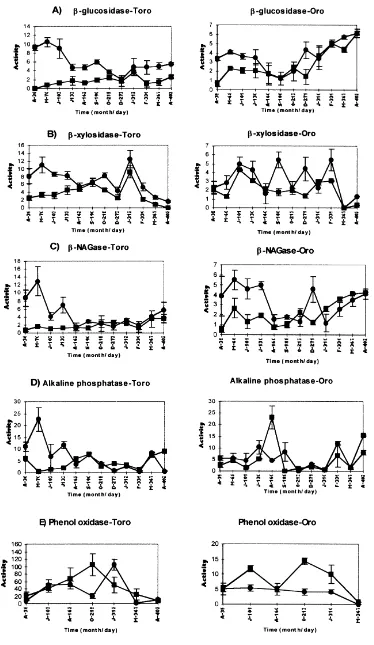
Enzyme activities on the confined material in CPOM for Toro pond decreased with time (Fig. 4). Enzyme FPOM activity values in Toro pond were relatively constant but for Oro pond more erratic (Fig. 4). Phenol oxidase values in Toro were 10-fold greater than in Oro, for both CPOM and FPOM fractions (Fig. 4E).
In situ enzyme activities for the CPOM fraction in Toro pond showed two main activity peaks (Fig. 5), one at 133– 162 days (July, August), and the higher one at 312 days (January) coinciding with a consecutive flooding and drying period (Fig.1). FPOM patterns remained constant over the study period. For CPOM material in Oro the pattern is less clear, but a decrease between days 101 and 160, when the pond dried up, can be observed, and then a sharp peak on day 310 when the pond became flooded (Fig. 1). In general, activities were higher in Toro than in Oro pond for CPOM and maximum values were reached on day 310 (124, 70, 23 and 456mmol h21g21OM forb-glucosidase,b-xylosidase, b-NAGase and phenol oxidase, respectively). Phenol oxidase activities were again 10-fold higher in Toro than in Oro pond for both CPOM and FPOM fractions (Fig. 5E). Statistically there were no differences between Toro and Oro ponds forb-glucosidase,b-NAGase and alkaline phos-phatase activities for both CPOM, FPOM and confined and in situ samples (Mann–Whitney U-test, P.0:1 for all
treatments). b-xylosidase, and, especially, phenol oxidase activities did show statistical differences between ponds P,0:01 and P,0:001; respectively). Phenol oxidase
activities were over 10 times higher in Toro than in Oro pond.
Within each pond, in situb-glucosidase andb-NAGase activities were significantly P,0:001 higher than
confined samples. b-xylosidase and phenol oxidase did Fig. 3. Organic matter (OM) vs. time for POM size fractions. CPOM (.1 mm); FPOM (0.063–0.5 mm). Toro CPOM (V); Toro FPOM (B); Oro CPOM (W); Oro FPOM (×); Toro CPOM lineal fitting ( —— ); Toro FPOM lineal fitting (· · ·); Oro CPOM lineal fitting (—); Oro FPOM linear fitting (· · ·).
Table 2
Linear regression statistics for relative mass loss with time for (POM). (CPOM).1 mm, (FPOM) 0.063–0.5 mm.n14 for each size fraction and site
Site Size fraction Slope r2 P
Toro CPOM 20.164 0.92 ,0.001
FPOM 20.041 0.42 ,0.05
Oro CPOM 20.118 0.71 ,0.001
not show differences between treatments P.0:1 in both
cases). Between coarse and fine fractionsb-glucosidase,b -xylosidase and b-NAGase showed significant differences P,0:001 for all treatments) being higher in coarse than
in fine fractions. Phenol oxidase activity was not signifi-cantly different between fractions P.0:1:
In Toro pond, b-glucosidase activity was significantly correlated with b-xylosidase r0:72; P,0:001; b
-NAGase r0:84; P,0:001 and alkaline phosphatase
r0:42;P,0:05in both CPOM and FPOM fractions,
in situ and in confined material. Significant correlations were also found between b-NAGase and b-xylosidase
activities r0:52;P,0:001 andb-NAGase and
alka-line phosphatase activities r0:54; P,0:001: Phenol
oxidase activity did not show any significant correlation with any other enzyme, exceptb-xylosidase r0:56;P,
0:01:
In Oro pond, b-glucosidase activity showed significant correlations in both CPOM and FPOM fractions and in situ and confined material withb-xylosidase r0:30;P,
0:05;b-NAGase r0:89;P,0:001and alkaline
phos-phatase activities r0:37;P,0:01:Significant
correla-tions were also found betweenb-NAGase andb-xylosidase r0:35; P,0:05 and b-NAGase and alkaline
phosphatase activities r0:37; P,0:001: Phenol
oxidase activity did not show any significant correlation with any other enzyme, except with b-xylosidase r 0:47;P,0:05:
b-Glucosidase and b-NAGase activities were similar with higher values in CPOM fraction and in in situ samples, and with no significant differences between the ponds.b -xylosidase presented a slightly different pattern, with statis-tically higher values in Toro pond, and with no apparent differences between in situ and confined samples, although it showed more activity on the CPOM fraction. There were no differences between phenol oxidase activities in CPOM and FPOM fractions and in situ or confined samples, but there were significant differences between the ponds, with values much lower in Oro than in Toro pond. Alkaline phosphatase showed the least consistent pattern and did not appear to be related to particle size or site.
3.3. Enzyme decomposition models
Linear regressions were calculated between cumulative enzyme activity-day and % OM remaining for each combi-nation of enzyme size fraction and site in confined samples. All regressions were significant P,0:001:The slope of
the relative organic mass remaining vs. cumulative activity-day can be viewed as the apparent enzyme efficiency (AEE) of a particular enzyme in the degradation process. This slope is analogous to a zero-order rate constant with units of activity-day (act-day21) (Sinsabaugh et al., 1994b).
Following the procedures of Sinsabaugh and co-workers (Sinsabaugh et al., 1994a; Jackson et al., 1995) a simple index model to incorporate all the enzyme data into one pool without the bias associated with the different ranges of activity was developed. The enzyme data were stan-darised to a 0–1 scale by dividing the activity recorded for a specific enzyme on a sampling date by the maximum value obtained for that enzyme during the study. Average enzyme activity for each sampling date was calculated by summing the relative activities of each enzyme and dividing the total by five (the number of enzymes assayed). This mean activity was integrated over time to obtain estimates of cumulative activity-day. Linear regressions of % organic mass remaining vs. average cumulative activity-day were performed. The slope obtained is the global AEE for each size fraction. For more details on the calculations see Jack-son et al. (1995).
Apparent enzyme efficiencies for CPOM and FPOM were 21.87 and20.085 act-day21for Toro pond and20.805 and 20.0525 act-day21 for Oro pond, respectively r20:99;
P,0:001;n11 for all cases).
The models generated for confined CPOM and FPOM from the litter-bag study were used to estimate mass loss rates for in situ POM. The enzyme activity data for each in situ sample (cores) were transformed to a relative scale as described for the confined samples, and the resulting values were averaged to calculate mean relative activity.
Instanta-neous mass loss rates were calculated by multiplying this value by the apparent enzymatic efficiency for that size class: 21.87 and 20.085 act-day21 for Toro pond and 20.805 and 20.0525 act-day21 for Oro pond for CPOM and FPOM, respectively (Fig. 6). By this calculation mass loss rates for in situ POM were higher for CPOM and FPOM in Toro pond in relation to Oro. Rates of FPOM decomposi-tion were much lower than CPOM loss rates. CPOM rates fluctuated throughout the sampling period. In Toro two clear peaks can be distinguished. The first coincided with the summer season and higher temperatures. The second peak came in January, when the pond became flooded again for a few days (Fig. 6A). In Oro pond, a decrease in mass loss rates can be seen in July, a few weeks before the pond dried up, which also coincided with the maximum inputs of euca-lyptus leaves and fruits and minimum water depth. Polyphe-nol concentrations also peaked at this time (Fig. 6B). Mass loss rates increased sharply in January, which coincided with a rainy period and, as in Toro, a brief period of flood-ing. Over the sampling period in situ organic mass loss rates averaged20.667 and20.036% day21in Toro and20.261 and20.023% day21in Oro for CPOM and FPOM, respec-tively. Based on these estimates the processing rates for in situ CPOM and FPOM, were 149 and 2778 days in Toro pond and 382 and 4347 days in Oro, respectively, compared with values of 578 and 2109 days in Toro and 778 and 5296 days in Oro pond determined by confined POM in bags.
4. Discussion
Microbial enzyme activities associated with confined POM did not follow the same trends as those associated with in situ POM. In situ activities were generally higher than confined ones. Similar observations were made by Sinsabaugh et al. (1994a), who attributed the differences to the mesh size acting as a filter between the confined material and the environment. Activities for CPOM are within the range of those observed in other aquatic systems (Sinsabaugh et al., 1992, 1994a). However, activities for FPOM fraction were lower than those reported by others (Sinsabaugh and Findlay, 1995; Sinsabaugh et al., 1994a; Jackson et al., 1995). In both ponds activities were similar. The only exception was phenol oxidase, with much lower values in Oro (almost negligible activity) than Toro and other previously studied sites (Sinsabaugh et al., 1994a; Jackson et al., 1995). b-Glucosidase,b-xylosidase, andb -NAGase indicated a litter quality-dependent behaviour, with no differences between ponds, which is expected given their roles in degradation (Eriksson and Wood, 1985). Phenol oxidase, unlike previous studies (Sinsabaugh et al., 1992) seemed to be site-related and not substrate-related. Phosphatase activity showed a more erratic pattern, not depending on site or particle size as has been observed before (Sinsabaugh and Moorhead, 1994). N and P acquir-ing enzymes (b-NAGase and phosphatase) increase their activities when the ponds dry and sediments shift to more aerobic conditions.
When all the enzymes were used to calculate global AEEs and in situ decomposition rates for each fraction, differences between size fractions and sites were found. CPOM proces-sing rates were over 10 times higher than FPOM in Toro and Oro ponds. The AEEs, and in situ organic mass loss rates, found for CPOM were 21.87 act-day21; 20.667% day21 and 20.805 act-day21; 20.261% day21 for Toro and Oro pond, respectively. These values, especially for Toro pond, were much higher than previously described (Jackson et al., 1995) for a wetland system (20.58 act-day21
, 20.2% day21) and, for Toro pond, lie in the range of values found in a fourth-order woodland stream (Sinsabaugh et al., 1994a) (21.82 act-day21;20.78% day21). The explanation for such values could be the high temperatures at the sample sites since there is strong relationship between AEE and litter exposure temperature (Sinsabaugh et al., 1994b). In Toro pond, higher pH, alkalinity and nutrient concentrations seem to favour higher decomposition rates.
In contrast with the high AEEs values for CPOM, AEEs and in situ processing rates for FPOM were extremely low (20.085 act-day21;20.036% day21and20.0525 act-day21; 20.023% day21for Toro and Oro, respectively, compared with values of 20.26 act-day21; 20.044% day21 and 20.70 act-day21; 0.54% day21 for a coastal wetland and a woodland stream, respectively). Again, rates were higher in Toro than in Oro pond. However, values for both ponds were extremely low in spite of high temperatures. It seems that the degradation of FPOM, with lower processing rates, is retarded. This is also consistent with the standing stock size
distribution (Fig. 2). Several authors (Serrano, 1992; Serrano and Guisande, 1990) have reported high polyphenolic concentrations in these systems and analysed their inhibitory effects on the primary producers community. There are also several reports of the inhibitory effect of polyphenolic and humic compounds on decomposition, which typically affect enzymes by enhancing their stability and reducing their activ-ity (Burns, 1983; Wetzel, 1991). This effect is more evident in Oro pond in both the FPOM fraction (where phenol oxidase and thus oxidation of humic substances seems to be very depressed) and CPOM fraction, with an important decrease in CPOM global AEE rate coinciding with maxi-mum deposition of eucalyptus leaves and fruits and minimaxi-mum water level, all leading to higher polyphenolic concentrations (Fig. 6B) (Pozo et al., 1997).
As temperature was always relatively high, an important controlling factor in decomposition rates seems to be rain-fall events. The area has a mediterranean type climate with an average rainfall of 580 mm concentrated in autumn and early spring. After the ponds had dried up they became flooded again due to early spring rains and AEEs increased sharply in both systems (Fig. 6). Reasons for this increase can be found in the fact that sediments (especially for Toro pond) became anaerobic before the pond dried and were re-oxygenated during the dried period. In addition, episodes of flooding and drought could reduce the concentration of inhi-bitory polyphenolic substances via photolytic cleavage. Data from desert ecosystems suggest that photochemical degradation of exposed litter is significant in hot, dry envir-onments (Schaefer et al., 1985). It has been reported that the soil organic content and the concentration of polyphenols decreased on the slopes of those ponds that had remained dry for longer (Serrano, 1992) and, in our experiments, a decrease in polyphenolic concentrations occured after dry periods in both water columns and sediments (Fig. 6). Drought and flooding fluctuations seem to be crucial in the detrital pathway functioning of these systems with short-lived but intense biological processes (Serrano and Toja, 1995).
EDMs allow the determination of instantaneous organic matter turnover rates in temporally and spatially heteroge-neous environments and seem to be particularly suitable in integrative studies. In the present study global controlling factors such as flooding regime and temperature, and a FPOM processing limitation were revealed using the EDM approach at an ecosystem level. The effects of euca-lyptus plantations (with differences in the type and timing of supply of litter to the system with respect to autochthonous vegetation) over decomposition processes were also described and can serve as a management tool when deci-sions concerning introduction or conservation of such vege-tation have to be taken.
Acknowledgements
(HID97-0321-C02-01). Sergio Alvarez was supported by a personal grant from the Ministry of Science and Education. We are grateful to C. Coleto for performing the nutrient analysis and to the personnel at the Don˜ana Biological Station (EDB) and Don˜ana Natural Park for their help in the field work. Many thanks also to Dr R. L. Sinsabaugh and two anonymous reviewers for their helpful comments and great improvements of the English text.
References
Box, J.D., 1983. Investigation of the Folin–Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Research 17, 511–525.
Boulton, A.J., Boon, P.I., 1991. A review of methodology used to measure leaf litter decomposition in lotic environments: time to turn over an old leaf? Australian Journal of Marine and Freshwater Research 42, 1–43. Burns, R.G., 1982. Carbon mineralization by mixed cultures. In: Bull, A.T., Slater, J.H. (Eds.). Microbial Interactions and Communities, Academic Press, London, pp. 475–543.
Burns, R.G., 1983. Extracellular enzyme-substrate interactions in soil. In: Slater, J.H., Whittenbury, R., Whittenberry, J.W.T. (Eds.). Microbes in their Natural Environments, Cambridge University Press, Cambridge, pp. 249–298.
Canhoto, C., Grac¸a, M.A.S., 1996. Decomposition ofEucalyptus globulus leaves and three native leaf species (Alnus glutinosa,Castanea sativa andQuercus faginea) in a Portuguese low order stream. Hydrobiologia 333, 79–85.
Chamier, A.C., 1992. Water chemistry. In: Ba¨rlocher, F. (Ed.). The Ecology of Aquatic Hyphomycetes, Springer-Verlag, Berlin, pp. 152–172. Chro´st, R.J. (Ed.), 1991. Microbial Enzymes in Aquatic Environments
Springer, New York.
Eriksson, K., Wood, T.M., 1985. Biodegradation of cellulose. In: Higuchi, T. (Ed.). Biosynthesis and Biodegradation of Wood Components, Academic Press, Orlando, pp. 469–503.
Findlay, S., Sinsabaugh, R.L., Fisher, D.T., Franchini, P., 1998. Sources of dissolved organic carbon supporting planktonic bacterial production in the tidal freshwater Hudson River. Ecosystems 1, 227–239.
Galindo, D., Mazuelos, N., Mata, J.A., Serrano, L., 1994. Microcrustacean and rotifer diversity relating to water temporality in different ponds in the Don˜ana National Park (SW Spain). Verhandlungen International Verein Limnologie 25, 1350–1356.
Howard, D.M., Howard, P.J.A., 1993. Relationship between CO2evolution, moisture content and temperature for a range of soil types. Soil Biology & Biochemistry 25, 1537–1546.
Jackson, C.R., Foreman, C.M., Sinsabaugh, R.L., 1995. Microbial enzyme activities as indicators of organic matter processing rates in a Lake Eire coastal wetland. Freshwater Biology 34, 329–342.
Jenkins, C.C., Suberkropp, K., 1995. The influence of water chemistry on the enzymatic degradation of leaves in streams. Freshwater Biology 33, 245–253.
Kok, C.J., Van der Velde, G., 1991. The influence of selected water quality parameters on the decay rate and exoenzymatic activity of detritus of Nymphaea albaL. floating leaf blades in laboratory experiments. Oeco-logia 88, 311–316.
Lo´pez, T., Toja, J., Gabellone, N.A., 1991. Limnological comparison of two peridunar ponds in the Don˜ana National Park (Spain). Archiv fu¨r Hydrobiologie 120, 357–378.
Montes, C., Borja, F., Bravo, M.A., Moreira, J.M., 1998. Don˜ana. Una Aproximacio´n Ecosiste´mica. Consejerı´a de Medio Ambiente. Junta de Andalucı´a.
Moorhead, D.L., Sinsabaugh, R.L., Linkins, A.E., Reynolds, J.F., 1996. Decomposition processes: modelling approaches and applications. The Science of the Total Environment 183, 137–149.
Parnas, H., 1975. Model for decomposition of organic material by micro-organisms. Soil Biology & Biochemistry 7, 161–169.
Pozo, J., Gonzalez, E., Diez, J.R., Molinero, J., Elo´segui, A., 1997. Inputs of particulate organic matter to streams with different riparian vegetation. Journal of North American Benthological Society 16, 602–611. Rybcyzck, J.M., Garson, G., Day, J.W., 1996. Nutrient enrichment and
decomposition in wetland ecosystems: models, analysis and efects. Current Topics in Wetland Biogeochemistry 2, 52–72.
Schaefer, D., Steinberger, Y., Whitford, W.G., 1985. The failure of nitrogen and lignin control of decomposition in a North American desert. Oeco-logia 65, 382–386.
Sacks, L.A., Herman, J.S., Konikow, L.F., Vela, A.L., 1992. Seasonal dynamics of groundwater-lake interactions at Don˜ana National Park, Spain. Journal of Hydrology 136, 123–154.
Serrano, L., 1992. Leaching from vegetation of soluble polyphenolic compounds and their abundance in temporary ponds in the Don˜ana National Park (SW Spain). Hydrobiologia 229, 43–50.
Serrano, L., Guisande, C., 1990. Effect of phenolic compounds on phyto-plankton. Verhandlungen International Verein Limnologie 24, 282– 288.
Serrano, L., Toja, J., 1995. Limnological description of four temporary ponds in the Don˜ana National Park (SW Spain). Archiv fu¨r Hydrobio-logie 133, 497–516.
Sinsabaugh, R.L., Carreiro, M.M., Alvarez, S., 2000. Enzyme and micro-bial dynamics of litter decomposition. In: Burns, R.G., Dick, R.P. (Eds.). Enzymes in the Environment, Marcel Dekker, New York (in press). Sinsabaugh, R.L., Moorhead, D.L., 1994. Resource allocation to extracel-lular enzyme production: a model for nitrogen and phosphorous control of litter decomposition. Soil Biology & Biochemistry 26, 1305–1311. Sinsabaugh, R.L., Findlay, S., 1995. Microbial production, enzyme activity and carbon turnover in surface sediments of the Hudson River estuary. Microbial Ecology 30, 127–141.
Sinsabaugh, R.L., Antibus, R.K., Linkins, A.E., McClaugherty, C.A., Rayburn, L., Repert, D., Weiland, T., 1992. Wood decomposition over a first-order watershed: mass loss as a function of lignocellulase activity. Soil Biology & Biochemistry 24, 743–749.
Sinsabaugh, R.L., Osgood, M.P., Findlay, S., 1994a. Enzymatic models for estimating decomposition rates of particulate detritus. Journal of the North American Benthological Society 13, 160–169.
Sinsabaugh, R.L., Moorhead, D.L., Linkins, A.E., 1994b. The enzymatic basis of plant litter decomposition: emergence of an ecological process. Applied Soil Ecology 1, 97–111.
Swift, M.J., Heal, O.W., Anderson, J.M., 1979. Decomposition in terrestrial ecosystems. Studies in Ecology, 5. University of California Press, Berkeley, CA.
Thurman, M. (Ed.), 1985. Organic Geochemistry of Natural Waters Nijh-off/Junk, Boston.
Wetzel, R.G., 1991. Extracellular enzymatic interactions: storage, redistri-bution, and interspecific communication. In: Chro´st, R.J. (Ed.). Micro-bial Enzymes in Aquatic Environments, Springer, New York, pp. 6–28. Wetzel, R.G., 1992. Wetlands as metabolic gates. Journal of Great Lakes
Research 18, 529–532.