Controlling gene expression in transgenics
Daniel R Gallie
The repertoire ofcis-regulatory elements has increased to a level of sophistication that offers considerable spatial and temporal control over transgene expression. Recent advances made with transgenes have revealed that the control of their expression is also influenced by factors that range from transgene copy number and arrangement to nuclear architecture and chromosomal location. These factors must now be included with the standard considerations of transcriptional and translational enhancers of gene expression during transgene design.
Addresses
Department of Biochemistry, University of California Riverside, CA 92521-0129, USA; e-mail: [email protected] Current Opinion in Plant Biology1998,1:166–172 http://biomednet.com/elecref/1369526600100166 Current Biology Ltd ISSN 1369-5266
Abbreviations
CP coat protein dsRNA double-stranded RNA MAR matrix attachment region PVX potato virus X
RdRp RNA-dependent RNA polymerase TEV tobacco etch virus
UAR upstream activator region
Introduction
The first phase of transgenic technology in the 1980’s focused on the isolation of genes and developing gene transfer protocols. Once these initial goals had been reached, the emphasis changed from simple gene transfer to one of ever increasing control over the spatial or tem-poral control of gene expression. Transgenic technology is also being used to control endogenous gene expression as a means to generate functional gene knockouts. This review will highlight some of the more intriguing and innovative paths that transgenic technology has traveled in recent months.
The influence of MARs and insertion site on
transgene expression
Matrix attachment regions (MARs) are AT-rich (>70%) chromosomal DNA regions attached to the nuclear matrix that often flank actively expressed genes [1–3] and affect expression through influencing chromatin structure. MARs are 1 kb long and are separated by loop domains ranging from 5–200 kb [4]. A MAR flanking a transgene can increase expression 4–140-fold in plants (reviewed in [5•]), perhaps by maintaining the chromosomal region containing the transgene in an open configuration to facilitate communication between an enhancer and a promoter [6].
Analysis of insertion sites following direct gene transfer in rice revealed massive rearrangements of genomic DNA at the site and in flanking regions of the transgene [7]. In contrast,Agrobacterium-mediated T-DNA insertion typically does not involve genomic rearrangements and usually only a small number of T-DNA insertions (low copy number) are made. The insertions also exhibit a less complex expression pattern than those made using direct gene transfer [8], although an analysis of transgene stability in Nicotiana tabacumandN. plumbaginifolia indicated that
Agrobacterium-introduced T-DNA loci were destabilized duringin vitroculture [9]. Stable transgene expression was observed when a simple T-DNA insertion was flanked on one side by a MAR in gene-rich regions that are close to the telomere [10•]. In contrast, low or unstable expression was associated with rearranged, multiple or incomplete copies of the T-DNA flanked by prokaryotic vector sequences that insert into paracentromeric or intercalary locations within a chromosome (i.e. regions distant from telomeres) [10•]. Although stably expressed loci may not completely escape the repression of expression associated with gene silencing [10•], MARs may reduce its severity [5•].
Gene silencing
Awareness of gene silencing arose only after the advent of transgenic plant production on a wide scale. Since then, much work has been done to elucidate what now appears to be several mechanisms that lead to gene silencing. Silencing is divided into two categories: homology-depen-dent gene silencing and post-transcriptional gene silencing (or co-suppression). The former arises when a host senses the presence of numerous copies of identical or highly related genes and responds by methylating each copy of the gene, thus resulting in their transcriptional repression. This may be a manifestation of a defense mechanism that evolved in plants in response to transposable elements as a means to repress their further introgression throughout the genome [11]. See the review by Matzke and Matzke (this issue, pp 142–148) for a more detailed discussion of this type of silencing.
Co-suppression requires transgene expression [12•] and increases with the transcriptional state and copy number of the transgene [13•,14••]. For example, when a gene encoding chalcone synthase (Chs) was inserted under the control of a 35S promoter containing multiple copies of the 35S upstream activator region (UAR) more co-suppression was observed than when only one copy of the UAR was used [14••]. Nonsense codon-mediated destabilization of
or an indirect effect, resulting from synthesis of transgene antisense RNA by endogenous RNA-dependent RNA polymerase (RdRp) activity or aberrant transgene RNA (i.e. unspliced, truncated, poly(A)–RNA, double-stranded
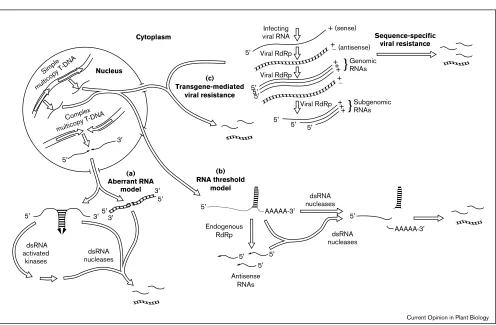
(ds) RNA or modified RNA). Although these results were obtained with simple transgene insertions, different results were observed for complex transgene arrangements, such as inverted repeats. In the latter case, a higher frequency of co-suppression requiring a lower level of transcription was observed [14••,15,16•], data which strongly support an aberrant RNA model (Figure 1).
Earlier work demonstrating that a promoterless transgene can trigger co-suppression suggested that transcription
may not be required to initiate gene silencing [17]. Further work revealed that this silencing resulted from multimeric transgene/T-DNA loci arranged as inverted repeats in which silencing sequences are proximal to the center were more effective than when the repeats were distal to the center [18•]. Read through from upstream genes in complex transgene loci proceeding through polyadenylation sequences which inefficiently terminate transcription [19•] may account for some cases of co-suppression.
DNA methylation may also play a role in the production of aberrant RNAs. Cytosine methylation within the 3′ flanking region of a gene was found to correlate with
Figure 1
}
Nucleus
5'
5'
3'
3'
5' 3'
dsRNA activated kinases
+ (sense)
_ +
+
+
}
GenomicRNAsSubgenomic RNAs
Sequence-specific viral resistance Cytoplasm
AAAAA-3' 5'
Endogenous RdRp
Antisense RNAs
dsRNA nucleases Simple
multicopy T-DNA
5'
3'
Complex multicopy T-DNA
(a) Aberrant RNA
model
dsRNA nucleases
(b) RNA threshold
model (c) Transgene-mediated
viral resistance
dsRNA nucleases
5'
AAAAA-3' Viral RdRp
Viral RdRp
Viral RdRp
5' 5'
5'
+ +
_
+ ++ Infecting
viral RNA
5' 5' 5' 5'
5' 5' 5'
Current Opinion in Plant Biology
(antisense)
co-suppression and the accumulation of unproductive RNAs [20,21]. Co-suppression may be influenced by environmental conditions [22,23] and appears to be elicited more easily by certain sequences, for example, nontranslatable viral genes (N or NSM) of tomato spotted
wilt virus [24•], the GUS 3′ coding region [21], or the 3′flanking region of neomycin phosphotransferase II (nptII) [20], suggesting that specific cleavage sites or secondary structure may be necessary for efficient co-suppression. Moreover, co-suppression often resets following meiotic division and does not reappear until a few weeks after germination [22,25•,26,27], perhaps as a result of a change in the amount or activity of the machinery required for the perception or implementation of the co-suppressed state during seed development. Expression is not reactivated, however, when plantlets regenerate through mitotic division, that is to say, in vitro from co-suppressed leaf explants [25•].
Although aberrant or antisense RNAs produced as a consequence of high transgene expression may initially induce co-suppression, how these RNAs continue to exert a direct effect once the level of sense RNA, upon which they may depend for their continued production, has dropped following the onset of co-suppression remains an outstanding question. Modification (e.g. methylation of the coding or 3′ flanking regions) of the silenced gene may increase aberrant RNA production, although the reactivation of expression of post-transcriptional gene silencing following meiotic division is quite unlike the persistence of homology-dependent gene silencing (i.e. methylation of the promoter and 5′flanking region result-ing in transcriptional repression) followresult-ing meiotic division and transgene segregation, suggesting that methylation may not play a significant role in post-transcriptional gene silencing. It is more likely that the detection of aberrant RNAs, for example through intramolecular or intermolecular basepairing, may result in cellular changes that sensitize the cell to aberrant RNA in order that the co-suppressed state can persist following the reduction of the original signal.
A diffusible signal from tobacco stock co-suppressed with a nitrate reductase (Nia2) transgene triggered co-suppres-sion in a grafted, non-suppressedNiatransgene scion even when the two were separated by 30 cm of stock of a nontarget wild-type tobacco [28••]. This systemic acquired silencing required active transcription from the transgene in the target scion, was specific to the transgene in the target, was observed when the stock and scion (recipient of the graft) were both transgenic for either nitrite reductase (Nii) orgus, and did not affect wild-type scions [28••].
Replicating viruses can also trigger co-suppression [29•] (Figure 1). The presence of a viral transgene can result in transgene-mediated resistance to the virus, even if the infecting virus contains only a 60 nucleotide region of homology with the viral transgene [30•]. Two types of
RNA-mediated resistance to viral pathogens have been described, ‘immunity’ and ‘recovery’, and are probably highly related. Tobacco containing four or six copies of a nontranslatable tobacco etch viral (TEV) coat protein (CP) gene triggered co-suppression of the viral coat protein transgene in these immune plants prior to TEV infection, although 20–28 days of growth following germination was required to manifest both co-suppression and immunity [31••], observations that suggest that perception and/or implementation of the co-suppression mechanism may be under developmental control. The one or two copies of the TEV CP transgene present in the plants exhibiting a recovery phenotype were insufficient to trigger co-sup-pression prior to TEV infection and the plants remained susceptible until replicating TEV triggered co-suppression and viral inhibition in new leaves [31••]. Transgene RNA degradation intermediates in co-suppressed tissue were present on polysomes as poly(A)– RNAs with intact 5′
ends and polyadenylated 3′ fragments [31••], suggest-ing a cytoplasmic location for co-suppression. Similar poly(A)–RNAs were observed withChstransgene mRNA
[32•] although polysome association was not examined. Transgene-mediated viral resistance (Figure 1) may be a manifestation of a natural virus defense mechanism: only infected and adjacent Nicotiana leaves inoculated with nepovirus exhibited viral symptoms whereas leaves that subsequently developed had reduced viral levels and were resistant to further infection with similar viruses [33••].
One prediction from viral-mediated co-suppression is that wild-type viral RNAs, in which newly synthesized viral RNAs are packaged by the CP, might serve as weaker inducers of co-suppression than would CP null mutants that no longer encapsidate the viral RNA. Such results were obtained with a CP-deficient potato virus X (PVX) transgenic construct in which the CP gene was replaced by thegusgene, resulting in a greater difference between the highest to lowest levels of GUS expression in transgenic plants compared to transgenics in which GUS expression was driven by a 35S promoter [34•]. These observations suggest that co-suppression was triggered in some plants perhaps as a consequence of a higher level of unpackaged viral RNA accumulating earlier in the infection cycle.
A post-transcriptional silencing mechanism in Neurospora crassa that is transgene-mediated is known as quelling. Quelling defective (qde) mutants [35••] fail to quell transgenes suggesting that the qde gene product is not transgene specific. As high levels of transgene expression do not cause quelling,qdegenes may function by sensing aberrant RNAs or by targeting and degrading the native RNA [35••].
Cis
-acting regulatory elements
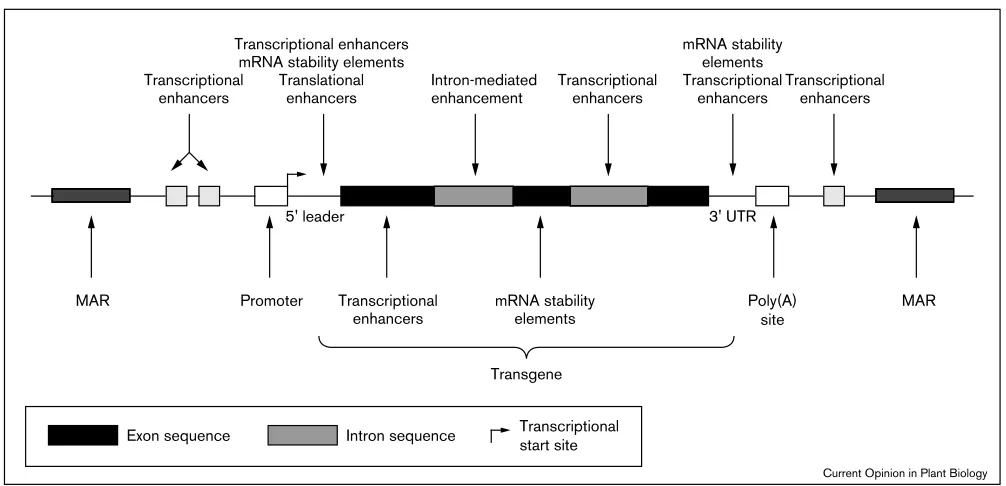
available for tailoring transgene expression to specific needs. Cis-regulatory elements are increasingly turning up in locations other than the 5′ flanking region of a gene (Figure 2). Although an intron often increases expression post-transcriptionally, introns can also contain other regulatory information. For example, a 3.8 kb intragenic region containing the large second intron of
AGAMOUS (AG) is required for the correct spatial and temporal expression of AG in Arabidopsis flowers [43••]. TheMe1gene fromFlaveria bidentisrequires an apparent enhancer-like element in the 3′ flanking region for high-level expression in leaves [44], whereas the spinach
PsaD gene requires its intron for correct plastid- and light-dependent regulation [45].
The 5′-leader and coding region of the pea plastocyanin (PetE) gene is required for proper light- mediated regulation [46] and is similar to that observed for pea ferredoxin (Fed-1) mRNA [47]. In contrast, the 5′ leader of the spinach PetE gene is required for full promoter activity and is not involved in light-dependent regulation [48]. A light-responsive element has also been reported within the coding region of the tobacco psaDb gene [49]. These examples, in addition to previously reported
cases, illustrate the diversity of locations and functions of
cis-acting regulatory elements.
Transgenic dominant mutants as an
alternative to gene knockout
Although gene knockouts are easily performed in yeast and are possible in mice, plants have not yet proven to be amenable to targeted chromosomal deletions. Antisense or co-suppressed approaches are currently the most widely used, species-independent means to repress specific gene expression. To these must now be added the use of dominant transgene mutants. The introduction of the
Arabidopsis ethylene receptor mutant, etr1-1, previously shown to inhibit ethylene perception in Arabidopsis[50], into tomato and petunia resulted in significant delays in fruit ripening, flower senescence and abscission, demon-strating the interspecies effectiveness of this dominant mutant [51••].
The floury2 maize seed, characterized by a soft, starchy endosperm, reduced levels of zein seed storage protein, and an elevated lysine content are a consequence of an alanine-to-valine substitution in the signal peptide of the 24 kDa precursor to a 22 kDa zein that prevents correct
Figure 2
Transcriptional enhancers
Transcriptional enhancers mRNA stability elements
Translational enhancers
Transcriptional enhancers
Transcriptional enhancers
Transcriptional enhancers
MAR
MAR Poly(A)
site mRNA stability
elements Transcriptional
enhancers
mRNA stability elements Intron-mediated enhancement
Promoter
5' leader 3' UTR
Exon sequence Transcriptional
start site Intron sequence
Transgene
Current Opinion in Plant Biology
processing and results in the inappropriate accumulation of the mutant zein protein in the endoplasmic reticulum. Introduction of the mutant transgene into wild-type maize resulted in the floury2 phenotype [52]. This dominant mutant approach may prove to be useful in altering seed storage protein characteristics not only in maize but in other cereals.
Conclusions
From this brief overview of some recent studies in transgene expression, it is clear that a wealth of regulatory mechanisms exists in plants and many may yet be discovered. Those interested in transgene design must now be aware of the many types and locations ofcis-acting regulatory elements as well as how and where integration may affect transgene expression. In contrast to the complex and, in some instances, contradictory observations made in earlier work, reports within the past year have begun to reveal that several different mechanisms may be contributing to gene silencing and that these may be manifestations of defense strategies that have evolved in response to transposons and viral invasion. The presence of such natural defense mechanisms raises questions of how expression from multigene families escapes the same repression observed during co-suppression. Members of many multigene families, however, are expressed in a tissue-specific manner so that not all are actively expressed in the way that multicopy transgenes are. Expression from multigene family members that are scattered throughout the genome does argue against a DNA-pairing-mediated means of producing aberrant RNAs that might function as the trigger for post-transcriptional gene silencing.
Several predictions can be made from the evidence suggesting that co-suppression requires the participation of dsRNA nuclease activity. Viral transgenic plants infected by a coat protein deficient, homologous virus might be expected to ‘recover’ faster, with a greater frequency, or to a greater extent than with the wild-type virus. The possible role of dsRNA in triggering co-suppression and the diffusible signal observed in grafting experiments with Nia2, Nii, and gus transgenic plants raises the possibility of dsRNA detection, dsRNA induction of protein activities, and the propagation of the signal throughout the plant. dsRNA-activated proteins have been well characterized in virally infected animal cells, in which interferon serves as the induction signal for uninfected cells [53]. A dsRNA-activated kinase has been identified in barley [54]; it appears to be the plant homolog of the mammalian PKR (double-stranded-RNA-dependent kinase), a dsRNA-activated kinase that phosphorylates and represses the activity of the eukaryotic initiation factor 2, resulting in translational repression. The 2′,5′ -oligoad-enylate synthase, another mammalian dsRNA-activated and interferon-induced enzyme, generates 2′-5′-linked oligoadenylates that specifically activate RNase L [53]. A dsRNA nuclease associated with ribosomes has been recently identified in rye [55]. Examination of
dsRNA-ac-tivated cellular factors in plants exhibiting co-suppression or systemic acquired silencing may reveal how these phenomena may be implemented. Ironically, the recent discovery of co-suppression (quelling) in fungi and the isolation of quelling-deficient mutants inNeurospora[35••] may represent the most promising developments in unraveling the decade-long quest to uncover the basis of co-suppression in plants.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest •• of outstanding interest
1. Schoffl F, Schroder G, Kliem M, Rieping M:An SAR sequence containing 395 bp DNA fragment mediates enhanced, gene-dosage-correlated expression of a chimaeric heat shock gene in transgenic tobacco plants.Transgenic Res1993,2:93-100. 2. Van der Geest AHM, Hall GE, Spiker S, Hall TC:Theβ-phaseolin
gene is flanked by matrix attachment regions.Plant J1994, 6:413-423.
3. Chinn AM, Comai L:The heat shock cognate 80 gene of tomato is flanked by matrix attachment regions.Plant Mol Biol1996, 32:959-968.
4. Pienta KJ, Getzenberg RH, Coffey DS:Cell structure and DNA organization.Crit Rev Euk Gene Express1991,1:355-385. •
5. Spiker S, Thompson WF:Nuclear matrix attachment regions and transgene expression in plants.Plant Physiol1996,110 :15-21.
A thorough review that describes the effect and function of nuclear matrix attachment regions in plants and relates this information to how similar se-quences function in animals and yeast.
6. Van der Geest AHM, Hall TC:Theβ-phaseolin 5′matrix attachment region acts as an enhancer facilitator.Plant Mol Biol1996,33:553-557.
7. Takano M, Egawa H, Ikeda J-E, Wakasa K:The structures of integration sites in transgenic rice.Plant J1997,11:353-361. 8. Pawlowski WP, Somers DA:Transgene inheritance in plants
genetically engineered by microprojectile bombardment.Mol Biotech1996,6:17-30.
9. Risseeuw E, Franke-van Dijk MEI, Hooykaas PJJ:Gene targeting and instability ofAgrobacteriumT-DNA loci in the plant genome.Plant J1997,11:717-728.
•
10. Iglesias VA, Moscone EA, Papp I, Neuhuber F, Michalowski S, Phelan T, Spiker S, Matzke M, Matzke AJM:Molecular and cytogenetic analyses of stably and unstably expressed transgene loci in tobacco.Plant Cell1997,9:1251-1264. A study investigating the effect of the chromosomal position of the insertion site on gene silencing. The authors show that stably expressed transgene loci integrate as simple T-DNA arrangements near AT-rich regions that bind to nuclear matricesin vitrowhich may also function as matrix attachment re-gionsin vivo. Stably expressed transgene loci also integrated into gene-rich regions near the telomeres of chromosomes. Unstably expressed loci were those that had undergone rearrangements and had integrated into regions distant from telomeres.
11. Bennetzen JL:TheMutatortransposable element system of maize.Curr Top Microbiol Immunol1996,24:195-229. •
12. English JJ, Davenport GF, Elmayan T, Vaucheret H, Baulcombe DC: Requirement of sense transcription for homology-dependent virus resistance andtrans-inactivation.Plant J1997,12 :597-603.
This is an analysis of the requirement for active transgene transcription in transgene-mediated viral resistance. The authors show that the presence of a transcriptionally silenced transgene locus was incapable of mediating re-sistance to an infecting virus engineered to contain a copy of the transgene. •
A carefully controlled analysis which shows a positive correlation between the copy number of a transgene or host gene and the frequency or onset of co-suppression. Data are also presented that demonstrate that the frequency or onset of co-suppression is reduced in the absence of transcription from the host gene and is abolished if transcription from the transgene is impeded. ••
14. Que Q, Wang H-Y, English JJ, Jorgensen RA:The frequency and degree of cosuppression by sense chalcone synthase transgenes are dependent on transgene promoter strength and are reduced by premature nonsense codons in the transgene coding sequence.Plant Cell1997,9:1357-1368. This thorough analysis correlates transgene RNA level with the frequency and degree of co-suppression. An increase in the number of enhancers in-troduced upstream of a 35S promoter resulted in increased co-suppression. In a separate approach, the authors used the presence of nonsense codons to reduce the steady state level of transgene mRNA. When present early in a transcript, nonsense codons destabilize mRNA. Introduction of premature stop codons resulted in not only reduced transgene RNA levels but also a reduced level of co-suppression.
15. Cluster PD, O’Dell M, Metzlaff M, Flavell RB:Details of T-DNA structural organization from a transgenic Petunia population exhibiting co-suppression.Plant Mol Biol1996,32:1197-1203.
•
16. Jorgensen RA, Cluster PD, English J, Que Q, Napoli CA:
Chalcone synthase cosuppression phenotypes in petunia flowers: comparison of sense vs. antisense constructs and single-copy vs. complex T-DNA sequences.Plant Mol Biol
1996,31:957-973.
The authors present a thorough analysis of co-suppression of chalcone syn-thase expression in petunia. They demonstrate that co-suppression is de-termined by the repetitiveness and organization pattern of the transgene and that the genomic sequence surrounding the integration site has little influence on the co-suppression. Analysis of transgenics carrying either a single copy or multiple dispersed copies of the transgene suggested that co-suppression correlates with transgene dosage.
17. Van Blokland R, Van der Geest N, Mol JNM, Kooter JM:
Transgene-mediated suppression of chalcone synthase expression inPetunia hybridaresults from an increase in RNA turnover.Plant J1994,6:861-877.
•
18. Stam M, de Bruin R, Kenter S, van der Hoorn RAL, van Blokland R, Mol JNM, Kooter JM:Post-transcriptional silencing of chalcone synthase inPetuniaby inverted transgene repeats. Plant J1997,12:63-82.
A follow up analysis of promoterless transgene silencing in which co-sup-pression was correlated with T-DNA rearrangements following integration. The authors demonstrate that insertion of inverted transgene repeats is a potent trigger of co-suppression, particularly when the inverted repeats are proximal to the center of the inserted DNA.
•
19. Thompson AJ, Myatt SC:Tetracycline-dependent activation of an upstream promoter reveals transcriptional interference between tandem genes within T-DNA in tomato.Plant Mol Biol
1997,34:687-692.
This paper demonstrates that some polyadenylation regulatory regions that are in common use may not result in the efficient termination of transcription. This can lead to transcriptional interference between tandem genes within a T-DNA. The authors also point out that such interference could occur with other T-DNA constructs near promoters subject to regulation. Such transcriptional read through might contribute to triggering co-suppression for those T-DNAs that integrate in complex rearrangements.
20. Van Houdt H, Ingelbrecht I, Van Montagu M, Depicker A: Post-transcriptional silencing of a neomycin phosphotransferase II transgene correlates with the accumulation of unproductive RNAs and with increased cytosine methylation of 3′flanking regions.Plant J1997,12:379-392.
21. English JJ, Mueller E, Baulcombe DC:Suppression of virus accumulation in transgenic plants exhibiting silencing of nuclear genes.Plant Cell1996,8:179-188.
22. Palauqui J-C, Elmayan T, Dorlhac de Borne F, Cr ´et ´e JJP, Charles C, Vaucheret H:Frequencies, timing, and spatial patterns of co-suppression of nitrate reductase and nitrite reductase in transgenic tobacco plants.Plant Physiol1996,112:1447-1456. 23. Cerutti H, Johnson AM, Gillham NW, Boynton JE:Epigenetic
silencing of a foreign gene in nuclear transformants of Chlamydomonas.Plant Cell1997,9:925-945.
•
24. Prins M, de Oliveira Resende R, Anker C, van Schepen A, de Haan P, Goldbach R:Engineered RNA-mediated resistance to tomato spotted wilt virus is sequence specific.Mol Plant-Microbe Inter1996,9:416-418.
This work characterizes transgene-mediated plant resistance to viruses of a negative sense virus. The authors show that the transgene probably targets the corresponding viral mRNA and not the replicating viral genome.
•
25. Balandin T, Castresana C:Silencing of aβ-1,3-glucanase transgene is overcome during seed formation.Plant Mol Biol
1997,34:125-137
The authors demonstrate that the silenced state of a co-suppressed trans-gene is maintained throughout vegetative growth and floral development but transgene expression is restored in developing seeds. In contrast, expres-sion was not restored in plantlets regeneratedin vitrofrom leaf explants, suggesting that gene silencing is reversed following meiotic but not mitotic cell division.
26. Hart CM, Fisher B, Neuhaus J-M, Meins F:Regulated inactivation of homologous gene expression in transgenicNicotiana sylvestrisplants containing a defense-related tobacco chitinase gene.Mol Gen Genet1992,235:179-188.
27. Boerjan W, Bauw G, Van Montagu M, Inze D:Distinct phenotypes generated by overexpression and suppression of S-adenosyl-L-methionine synthetase reveal developmental patterns of gene silencing in tobacco.Plant Cell1994,6 :1401-1414.
••
28. Palauqui J-C, Elmayan T, Pollien J-M, Vaucheret H:Systemic acquired silencing: transgene- specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions.EMBO J1997,16:4738-4745.
Data are presented demonstrating that co-suppression may involve a dif-fusible signal. Stock that exhibitedNia2-transgene-mediated co-suppression triggered co-suppression in a grafted, nonsuppressedNia2transgene scion even when the two were separated by 30 cm of stem of a nontarget wild-type tobacco. Systemic acquired silencing required active transcription from the transgene in the target and was specific to the transgene in the target scion. Transmission was unidirectional from stock to scion.
•
29. Baulcombe DC:Mechanisms of pathogen-derived resistance to viruses in transgenic plants.Plant Cell1996,8:1833-1844. An excellent, recent review of the different types of pathogen-derived resis-tance to viruses, protection mediated by viral proteins, sense- and antisense-mediated resistance, and the evidence supporting the various models.
•
30. Sijen T, Wellink J, Hiriart J-B, van Kammen A:RNA-mediated virus resistance: role of repeated transgenes and delineation of targeted regions.Plant Cell1996,8:2277-2294.
The authors show that an infecting virus containing as little as a 60 nucleotide region of homology with a transgene is sufficient to trigger transgene-mediated viral resistance. The arrangement of the transgene in the genome and perhaps the extent to which the transgene’s coding region is methylated may be important in determining the strength of the resistance. As similar fates of the recombinant viral genomes were observed, whether sequences of a sense or antisense orientation relative to the transgene were used, a similar mechanism may be used in both cases.
••
31. Tanzer MM, Thompson WF, Law MD, Wernsman EA, Uknes S:
Characterization of post-transcriptionally suppressed transgene expression that confers resistance to tobacco etch virus infection in tobacco.Plant Cell1997,9:1411-1423. The authors show that the observed ‘immunity’ and ‘recovery’ resulting from transgene-mediated viral resistance are probably manifestations of the same phenomenon. Tobacco containing four or six copies of a nontranslatable tobacco etch viral (TEV) coat protein (CP) gene triggered co-suppression in immune plants prior to TEV infection, although 20–28 days of growth following germination was required to manifest both co-suppression and immunity as the silenced transgene underwent meiotic resetting. Prior to the onset of the silenced state, these plants were susceptible to TEV infection. The presence of only one or two copies of the TEV CP transgene was insuf-ficient to trigger co-suppression prior to TEV infection and such transgenics were susceptible until the multiplying TEV triggered co-suppression. This co-suppression, in turn, led to suppression of TEV replication in new leaf growth resulting in the ‘recovery’ observation. Transgene RNA degradation intermediates were present on polysomes as poly(A)–RNAs with intact 5′
ends and polyadenylated 3′fragments in co-suppressed tissue, suggesting that the RNAs were targeted for destruction following their transport to the cytoplasm and their recruitment onto polysomes.
•
32. Metzlaff M, O’Dell M, Cluster PD, Flavell RB:RNA-mediated RNA degradation and chalcone synthase A silencing in petunia.Cell
1997,88:845-854.
Fragments chsAmRNA were examined in silenced petunia flowers. Both poly(A)+and poly(A)–mRNA fragments were observed. A region within the
3′end ofchsAmRNA was more resistant to degradation than was the re-mainder of the mRNA, suggesting that degradation may be initiated through endonucleolytic cleavage.
••
33. Ratcliff F, Harrison BD, Baulcombe DC:A similarity between viral defense and gene silencing in plants.Science1997,276 :1558-1560.
had been introduced into the second infecting viral genome, demonstrating that virus resistance is sequence specific. This type of virus-mediated re-sistance may induce a rere-sistance mechanism similar to that observed with transgene-induced co-suppression.
•
34. Angell SM, Baulcombe DC:Consistent gene silencing in transgenic plants expressing a replicating potato virus X RNA. EMBO J1997,16:3675-3684.
This work demonstrates that active viral replication is required to trigger gene silencing. No gene silencing was observed in transgenic plants expressing a potato virus X (PVX) that is unable to replicate. Plants with aPVXtransgene carrying a copy of thegusgene (PVX/GUS) in place of the coat protein gene (CP) exhibited a greater difference between the highest and lowest levels ofGUSexpression than did plants with a PVX transgene carrying a copy of thegusgene in addition to the coat protein gene, suggesting that lack of encapsidation of thePVX/GUSRNA functioned as a more potent trigger of co-suppression.
••
35. Cogoni C, Macino G:Isolation of quelling-defective (qde) mutants impaired in post-transcriptional transgene-induced gene silencing inNeurospora crassa.Proc Natl Acad Sci USA 1997,94:10233-10238.
A genetic approach to determining the genetic basis for co-suppression, referred to as quelling in fungi. The results with quelling defective (qde) mutants suggest that theqdegene product is not transgene specific during quelling. Quelling was not caused by high levels of transgene expression, suggesting thatqdegene function may be to sense the production of aber-rant sense RNA and to target or degrade the native RNA.
36. Hong HP, Ross JHE, Gerster JL, Rigas S, Datla RSS, Hatzopoulos P, Scoles G, Keller W, Murphy DJ, Robert LS:Promoter sequences from two differentBrassica napustapetal oleosin-like genes direct tapetal expression ofβ-glucuronidase in transgenicBrassicaplants.Plant Mol Biol1997,34:549-555. 37. Van der Geest AHM, Hall TC:A 68 bp element of theβ
-phaseolin promoter functions as a seed-specific enhancer. Plant Mol Biol1996,32:579-588.
38. Beaudoin N, Rothstein SJ:Developmental regulation of two tomato lipoxygenase promoters in transgenic tobacco and tomato.Plant Mol Biol1997,33:835-846.
39. Fordham-Skelton AP, Lilley C, Urwin PE, Robinson NJ:GUS expression inArabidopsisdirected by 5′regions of the pea metallothionein-like genePsMTA.Plant Mol Biol1997,34
:659-668.
40. Shah J, Klessig DF:Identification of salicylic acid-responsive element in the promoter of the tobacco pathogenesis-related β-1,3-glucanase gene,PR-2d.Plant J1996,10:1089-1101. 41. Rouster J, Leah R, Mundy J, Cameron-Mills V:Identification of
a methyl jasmonate-responsive region in the promoter of a lipoxygenase 1 gene expressed in barley grain.Plant J1997, 11:513-523.
•
42. Aoyama T, Chua N-H:A glucocorticoid-mediated transcriptional induction system in transgenic plants.Plant J1997,11 :605-612.
A description of a tightly controlled, transcriptional induction system for use in plants. A chimeric transcription factor containing the yeast GAL4 DNA-binding domain, the herpes viral protein VP16trans-activating domain, and the rat glucocorticoid receptor domain functions as the regulatory factor that is activated by dexamethasone, a strong synthetic glucocorticoid. A 35S promoter containing six tandem copies of the GAL4 upstream acting se-quence is used to drive regulated expression from the transgene of interest. Induction levels correlated with the concentration of dexamethasone used and maximum expression was obtained within four hours of induction. Three to four days were required after dexamethasone was removed to return to pre-induction levels of expression.
•
43. Sieburth LE, Meyerowitz EM:Molecular dissection of the
AGAMOUScontrol region shows thatciselements for spatial
regulation are located intragenically.Plant Cell1997,9 :355-365.
An important demonstration that transcriptional regulatory information is not confined to the 5′and 3′flanking regions of a gene but can also be found within the transcribed region. A 3.8 kb intragenic sequence within AGA-MOUS(AG) was found to be necessary for its proper spatial and tem-poral expression during flower development. This intragenic sequence was required to respond to the negative regulators ofAG(apetala2,leunigand curly leaf) and to the positive regulator,LEAFY.
44. Marshall JS, Stubbs JD, Chitty JA, Surin B, Taylor WC:
Expression of the C4Me1gene fromFlaveria bidentisrequires an interaction between 5′and 3′sequences.Plant Cell1997, 9:1515-1525.
45. Bolle C, Herrmann RG, Oelm ¨uller R:Intron sequences are involved in the plastid- and light-dependent expression of the spinachPsaDgene.Plant J1996,10:919-924.
46. Helliwell CA, Webster CI, Gray JC:Light-regulated expression of the pea plastocyanin gene is mediated by elements within the transcribed region of the gene.Plant J1997,12:499-506. 47. Dickey LF, Nguyen T-T, Allen GC, Thompson WF:Light
modulation of ferredoxin mRNA abundance requires an open reading frame.Plant Cell1994,6:1171-1176.
48. Bolle C, Herrmann RG, Oelm ¨uller R:Different sequences for 5′-untranslated leaders of nuclear genes for plastid proteins affect the expression of theβ-glucuronidase gene.Plant Mol Biol1996,32:861-868.
49. Yamamoto Y, Konda Y, Kato A, Tsuji H, Obokata J: Light-responsive elements of the tobacco PSI-D gene are located both upstream and within the transcribed region.Plant J1997, 12:255-265.
50. Bleecker AB, Estelle MA, Somerville C, Kende H:Insensitivity to ethylene conferred by a dominant mutation inArabidopsis thaliana.Science1988,241:1086-1089.
••
51. Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ:A dominant mutant receptor from
Arabidopsisconfers ethylene insensitivity in heterologous plants.Nat Biotechnol1997,15:444-447.
In this work, an introducedArabidopsis etr1-1mutant receptor gene con-fers dominant ethylene insensitivity on tomato and petunia as it had in Ara-bidopsis. Expression frometr1-1resulted in delayed fruit ripening, flower senescence, and flower abscission. The effect was specifically on reducing ethylene perception and not on reducing ethylene synthesis, as ethylene evolution following pollination inetr1-1transgenic petunia exceeded that of the control.
52. Coleman CE, Clore AM, Ranch JP, Higgins R, Lopes MA, Larkins BA:Expression of a mutantα-zein creates thefloury2
phenotype in transgenic maize.Proc Natl Acad Sci USA1997, 94:7094-7097.
53. Mathews MB, Sonenberg N, Hershey JWB:Interactions between viruses and the cellular machinery for protein synthesis.In Translational Control. Edited by Mathews MB, Sonenberg N, Hershey JWB. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1996:505-548.
54. Langland JO, Langland LA, Browning KS, Roth DA:
Phosphorylation of plant eukaryotic initiation factor-2 by the plant-encoded double-stranded RNA-dependent protein kinase, pPKR, and inhibition of protein synthesisin vitro.J Biol Chem 1996,271:4539-4544.