The regulation of carbon partitioning between source and sink tissues in higher plants is not only important for plant growth and development, but insight into the underlying regulatory mechanism is also a prerequisite to modulating assimilate partitioning in transgenic plants. Hexoses, as well as sucrose, have been recognised as important signal molecules in source–sink regulation. Components of the underlying signal transduction pathways have been identified and parallels, as well as distinct differences, to known pathways in yeast and animals have become apparent. There is accumulating evidence for crosstalk, modulation and integration between signalling pathways responding to phytohormones, phosphate, light, sugars, and biotic and abiotic stress-related stimuli. These complex interactions at the signal transduction levels and co-ordinated regulation of gene expression seem to play a central role in source–sink regulation.
Addresses
Lehrstuhl für Zellbiologie und Pflanzenphysiologie, Universitaet Regensburg,Universitaetsstrasse 31, D-93053 Regensburg, Germany; e-mail: [email protected]
Current Opinion in Plant Biology1999, 2:198–206 http://biomednet.com/elecref/1369526600200198 © Elsevier Science Ltd ISSN 1369-5266
Introduction
Higher plants develop from an embryo which depends on heterotrophic metabolism of storage products. During the growth and differentiation of a plant, photosyntheti-cally active source tissues (mature leaves) develop. They export carbohydrates to photosynthetically less active or inactive sink tissues such as roots, fruits or tubers, which are characterised by a net import of sugars; however, this physiological mosaic is not static. The plant life cycle is accompanied by source–sink transitions as well as changes with respect to the sink strength of individual organs and the number of sink organs competing for a common pool of carbohydrates. In addition, exogenous factors such as abiotic stress or pathogen infection may also influence carbohydrate partitioning. Thus, complex mechanisms have to be assumed which integrate the expression of enzymes involved in carbohydrate produc-tion in source tissues with utilisaproduc-tion in sink tissues. These regulatory mechanisms ultimately determine the pattern of carbon allocation between the different plant organs and regulate source–sink transitions. The regula-tion of carbohydrate partiregula-tioning has attracted a lot of attention in the past few years. Insight into these mech-anisms is not only important for understanding plant growth and development, but it is also a prerequisite for the genetic manipulation of source–sink relations in transgenic plants to increase crop yield.
This review will focus on two aspects of source–sink regu-lation which are of particular importance: metabolic regulation by sugars, and the effect of stress-related exoge-nous stimuli. It has been recognised in the past ten years that sugars not only function as substrate to sustain the heterotrophic growth of sink tissues, but are also important signalling molecules that regulate both source and sink metabolism. In addition, both abiotic and biotic stress-related stimuli have profound effects on plant growth and development and are responsible for considerable loss of crop yield. An increasing number of studies are focused on the elucidation of the molecular mechanisms that link the effect of diverse stress stimuli to source–sink regulation.
Source–sink regulation by sugars
Feedback inhibition of photosynthesis as a result of decreased sink demand is a long known phenomenon. Different experimental approaches have shown that sug-ars play a key role in this regulatory mechanism by repressing the expression of photosynthetic genes [1]. The specific inhibitory effect of sugars on photosynthe-sis or on the expression of photosynthetic genes is further supported by recent studies which included some monocotyledonous species [2–4], whereas most previous studies concentrated on dicotyledonous plants. This feedback inhibition suggested that assimilates function as link between source and sink tissues. Indeed, sugars were shown to induce transcription of a number of sink specific enzymes involved in sucrose breakdown and metabolism and storage product synthesis [1,5•,6,7]. The co-ordinated and inverse regulation of mRNA levels of a photosynthetic enzyme and a sink specific enzyme in the same experiment has been demonstrated in glu-cose treated photoautotrophic cultures of Chenopodium rubrum. The induction of the mRNA for the sink specif-ic extracellular invertase by glucose showed the same time course and concentration dependence as the repres-sion of the mRNA for the small subunit of the ribulosebisphosphate-carboxylase [8•].
So far, most studies on source–sink regulation by sugars have concentrated on the effect of exogenously added sugars to protoplasts, suspension culture cells, and isolat-ed tissues, or the analysis of transgenic plants with modulated carbohydrate metabolism. An alternative experimental approach was followed by Abdin et al. [9•] who supplied exogenous sucrose into stems of soybean plants continuously for almost their entire life cycle using a modified pressurised injection technique. This sucrose supplementation suppressed photosynthesis and had positive effects on plant growth.
The elucidation of the underlying signal transduction pathways of source–sink-regulation is currently the major
Source–sink regulation by sugar and stress
focus of attention. A number of studies have shown that hexoses and sucrose can elicit sugar responses [1]. The studies involving sucrose, however, did not address the question as to whether sucrose itself or the readily pro-duced hexoses were the actual inducer. Recent studies on the nature of sugar signal molecules have shown that, in addition to hexose signalling, sucrose-specific pathways may be differentiated. Chiou and Bush [10•] have shown that sucrose specifically reduces the steady state mRNA level of a proton–sucrose symporter thought to be involved in phloem loading. Sucrose-specific signalling pathways were also shown to be responsible for the repression of the Arabidopsis ATBZ bZIP transcription factor [11•] and for the modulation of phytochrome signalling [12•]. Studies on starch synthesis in slices of potato tubers [13] and on seed development in transgenic Vicia narbonensis [14•] sup-port previous suggestions that sucrose specifically induces differentiation and storage product synthesis. Hexose sug-ars are thought to be important primarily to regulate growth and mitotic activity [15].
Several studies imply a possible role of hexokinase in sugar signalling and Jang et al. [16•] have even proposed that hexokinase functions as a sugar sensor in higher plants [16•]. However, there is still a matter of debate about the role of hexokinase in sugar sensing and sig-nalling and it has been proposed that hexokinase dependent and independent pathways are present in higher plants [17–19]. Some of the conflicting sugges-tions stem from the differential effect of non-phosphorylatable glucose-analogues in the individ-ual experiment system analysed. In some laboratories it has been found that 6-desoxyglucose or 3-O-methylglu-cose, that are not substrates for hexokinase, are able to trigger gene regulation, whereas in other laboratories these compounds are inactive and only the phosphorylat-able glucose analogue 2-desoxyglucose is phosphorylat-able to elicit the same effects as glucose [17,18,19]. The fact that the phosphorylatable glucose analogue is able to substitute glucose is interpreted as a direct interaction with hexoki-nase and a regulatory role of this enzyme in the system analysed. The observed differential effect of non-phos-phorylatable glucose analogues on gene regulation, however, may be completely independent of a direct interaction of the different sugar compounds with hexok-inase. This may rather reflect species specific differences in the specificity of an extracellular or membrane bound sugar binding protein. The function of such an extracel-lular sugar receptor would be in agreement with all experimental data and does not even exclude a role of a specific hexokinase isoenzyme in the corresponding intracellular signalling pathway, which is suggested both by transgenic approaches [16•] and inhibitor studies [20]. Although, so far, no direct experimental evidence exists for this hypothesis, extracellular sugar recognition in plants would be backed by the study of Herbers and Sonnewald and co-workers [21]. Possible paradigms for the extracellular sugar sensing molecules are extracellular
sugar binding proteins in bacteria [22] and plasmamem-brane bound sugar transporters in yeast [23].
Different screens have yielded a number of Arabidopsis mutants that are affected in sugar sensing and signalling and in feedback inhibition of photosynthesis [19,24•,25•,26]. So far, the corresponding target gene has been identified only from the highly pleiotropic mutant prl1(pleiotropic regulatory locus-1) that causes sugar hyper-sensitivity [25•]. The prl protein was shown to be imported into the nucleus and to interact with a nuclear import receptor protein. Also the other mutants isolated to date are also characterised by pleiotropic phenotypes, and this indicates an interaction between different signalling path-ways that will be discussed further below.
Transgenic plants overexpressing a heterologous invertase from yeast, or other enzymes to modulate carbohydrate metabolism, have greatly stimulated progress in under-standing source–sink regulation [27]. Sugars accumulate to unphysiologically high levels in these plants, however, and the target genes are regulated by stress related stimuli and thus are also expected to be regulated by osmotic stress resulting from sugar accumulation (see discussion below). It may be difficult, therefore, to differentiate between sugar and stress responses. These problems, inherent to the use of constitutive promoters, have been circumvented by the use of an ethanol inducible promoter [28•].
SNF-1-related protein kinases was shown to result in loss of sugar inducibility of sucrose synthase [34•]. These stud-ies indicate that proteins homologous to the SNF-1 kinase of yeast may have a function in sugar signalling of higher plants. However, future studies will be required to prove the speculations that plant SNF-1 related proteins are global regulators of carbon metabolism in plants [35] and that they even integrate cytokinin, light, glucose and brassinosteroid signalling [36].
By functional dissection of sugar regulated promoters it has been possible to identify cis-acting regulatory sequences required for sugar regulated gene expression [37,38,39•,40]. These results will be important to elucidate whether positively and negatively regulated promoters share the same regulatory sequences and thus are regulat-ed by the same transcription factors. Furthermore, the identified regulatory sequences may be a valuable tool to isolate such regulatory proteins.
Source–sink regulation by stress
Stress related stimuli may be both of abiotic and biotic ori-gin. Despite the dramatic effect of stress related stimuli on yield in agriculture, only few studies have focused on the regulation of enzyme activities and transcriptional regula-tion of genes in response to these stimuli. Therefore, it is difficult to compare the mostly unrelated studies on source-sink regulation by stress related stimuli and this section lists the recent advances sorted by the nature of the stimuli. Future work will require to focus on the effect of different stimuli on specific target proteins or genes to be able to better compare the results of different studies. Water has long been recognised as a crucial abiotic deter-minant of carbon allocation [41] and drought is a major factor limiting plant distribution and productivity. Water stress induces large alterations in source–sink relations due to a modification of growth priorities and to a reduction of the performance of photosynthetic organs. With respect to the effect of water deficiency on whole plants, source lim-itations such as a reduced photosynthetic capacity, resulting in a decreased export of assimilates seem to be responsible for decreased crop load [42]. Variations in stomatal conductance resulting in decreased water loss due to transpiration were found to be important in stress toler-ant cultivars [43,44]. A few studies have addressed the regulation of source and sink specific enzymes in response to drought. It has been shown that as water deficit is increased in discs of potato tubers, there is a progressive inhibition of starch synthesis [45]. This was also observed in peach seedlings where it appears to be caused by the inhibition of photosynthesis [46]. Different studies demonstrated an effect of water stress on soluble acid invertase that is thought to mobilise sucrose stored in the vacuole. It has been shown that induction of male sterility in wheat by meiotic stage water deficit is preceded by a decline in vacuolar invertase activity [47]. In contrast, mild water stress in maize leaves produced an early and large stimulation of vacuolar invertase activity which was tightly
related to the mRNA concentration for a specific vacuolar invertase gene [48,49,50•]. The conflicting data on the reg-ulation of vacuolar invertase may reflect tissue specific responses as well as differences in the time point, duration time and severity of the applied water stress which need to be addressed in future studies. In parallel to the analyses of the regulation of mRNA for vacuolar invertase in response to water stress, the genetic analysis of quantita-tive traits associated with DNA markers in segregating near isogenic lines has been applied to the drought response fo maize leaves [50•]. Several quantitative trait loci (QTLs) were detected for invertase activity in control plants and in stress conditions, respectively. The finding that only two QTLs were located close to the correspond-ing invertase gene locus indicates that other genes are also likely to be involved in the invertase stress response and some of them were found to form ‘stress clusters’.
Salinity is another important environmental factor result-ing in suboptimal growth. Despite the far-reachresult-ing impact in agronomic terms, however, only limited information about the effect on plant metabolism and source–sink rela-tions are available. It has been shown that salt stress not only alters photosynthesis and carbohydrate levels, at least transiently, but also alters the types of carbohydrates that are synthesised and exported by the source tissues [51•,52]. The proposal that alterations in the type of car-boyhdrate transported in response to salt stress may act as a (novel) sugar signalling system for acclimation responses in the sink tissues [51•] is an interesting extension of the concept of sugar signalling in plants but still lacks any experimental evidence.
An important biotic stress for plants is the infection by viruses. It has been shown that viral infection or expression of different viral movement proteins in transgenic plants influences photosynthesis, carbohydrate accumulation and assimilate partitioning (reviewed in [53]).
was shown to result in the same co-ordinated regulation of source–sink relations and defence responses as elicitor treatment of suspension culture cells. These studies involving both tissue culture cells as well as plants demon-strate that different stress related stimuli result in the same regulatory pattern of mRNAs for enzymes involved source and sink metabolism and defence reactions. This indicates that defence responses are tightly linked to the upregula-tion of sink metabolism to satisfy the energy requirements of the activation of the cascade of defence reaction.
Under natural conditions plants are simultaneously affect-ed by a variety of both biotic and abiotic stress relataffect-ed stimuli and other environmental factors. The presence of one stress may change plant responses to other stresses, thus creating additive or synergistic interactions. This fact is neglected when usually only the effect of a single stress or other environmental factor is analysed. Three recent studies provide some preliminary results about such natu-rally occurring interactions or multistress situation on source–sink relations. By combining elevated levels of the
air pollutant ozone and mild drought, the ozone-induced responses of all parameters, such as the disequilibrium of the carbon transfer between roots and shoots, were signifi-cantly amplified [54]. Elevated levels of CO2were shown to enhance the growth inhibitory effect of powdery mildew infection in barley [55] and to ameliorate ozone effects on biomass and leaf area in soybean [56•].
Fluorescence imaging has been suggested only recently as a diagnostic tool to analyse plant stress responses [57]. The relationship between laser-induced chlorophyll fluorescence and photosynthesis in drought and ozone stressed plants has been evaluated with respect to quantitative interpretation of the measurements and further practical applications of this method [58]. The available data indicate that this powerful noninvasive technique, which allows the measurement of both the photosynthetic activity as well as the sink status of the tissues analysed, could complement and extend molecu-lar studies on the co-ordinated regulation of sink and source specific genes. In addition, this method may help to compare the effect of different types of stress related stimuli. Figure 1
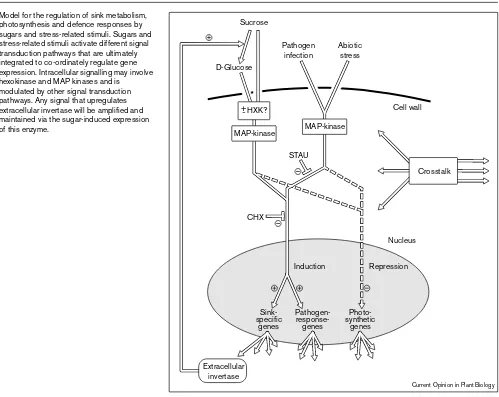
Model for the regulation of sink metabolism, photosynthesis and defence responses by sugars and stress-related stimuli. Sugars and stress-related stimuli activate different signal transduction pathways that are ultimately integrated to co-ordinately regulate gene expression. Intracellular signalling may involve hexokinase and MAP kinases and is modulated by other signal transduction pathways. Any signal that upregulates extracellular invertase will be amplified and maintained via the sugar-induced expression of this enzyme.
Cell wall
Sink-specific
genes
Pathogen- response-genes
Photo-synthetic
genes
Induction Repression D-Glucose
Nucleus CHX
STAU Sucrose
Pathogen infection
Abiotic stress
Extracellular invertase
Crosstalk MAP-kinase
MAP-kinase HXK?
Signalling pathways: crosstalk and integration
Interaction between different phytohormones is well established and best exemplified by the effect of auxin/cytokinin ratio on plant morphogenesis [59]. There are several studies that demonstrate interactions between sugar and phytohormone signalling pathways which further support the significance of sugar signalling in plant growth and development. Modulation of sugar responses by phytohormones or vice versa, or cross talk between the underlying signalling pathways has been determined for gibberellins [60•], ethylene [24•], auxin [61] and cytokinin [25•]. Inorganic ions such as phos-phate and nitrogen were also shown to modify sugar-mediated gene regulation in a gene specific man-ner [62,63]. Interactions between the environmental stimulus light and sugar has been reported by Dijkwel et al. [12•]. These studies demonstrate that sugar regula-tion in higher plants is linked to the effect of a number of other signals and that complex interactions exist between the underlying signal transduction pathways.Based on the sugar inducibility of a number of defence related enzymes it has been proposed that regulation of defence related enzymes in response to pathogen infection is indirectly mediated via the initial induction of an extra-cellular invertase and the resulting increased sugar concentration [64]. The study of Ehness et al. [8•], howev-er, shows that sugar and stress related stimuli independently activate different signalling pathways which are ultimately integrated to regulate source and sink metabolism and activate defence responses as depicted in
Figure 1. Thus, sugars may function as an extracellular indicator for pathogen infection, as suggested before [64], but the corresponding genes are under dual control and directly regulated both by the metabolic stimulus glucose as well as in response to stress related stimuli. Based on the pleiotropic phenotype of the prl1 mutation in Arabidopsis [25•], a connection between glucose and stress signalling has also been proposed [36].
Invertase: a key enzyme in source–sink
regulation
Supplying carbohydrates to sink tissues via an apoplastic pathway involves the release of the transport sugar sucrose into the apoplast by a sucrose exporter, cleavage of the disaccharide by an extracellular invertase and uptake of the hexose monomers by monosaccharide transporters (Figure 2). The regulation of extracellular invertase by a number of different stimuli indicates that extracellular invertase is not only important for supply-ing carbohydrates to sink tissues — experimental data suggest that this sucrose cleaving enzyme also plays a crucial role to mediate source–sink regulation in response to a variety of stimuli via sugar regulation of invertase expression. Extracellular invertase may be con-sidered as a central modulator of assimilate partitioning and defence response based on the following four differ-ent functions: firstly, supplying carbohydrates to sink tissues; secondly, regulation of source–sink transitions; thirdly, amplification of signals that regulate source–sink relations; and finally, integration of signals that regulate source–sink relations and defence responses.
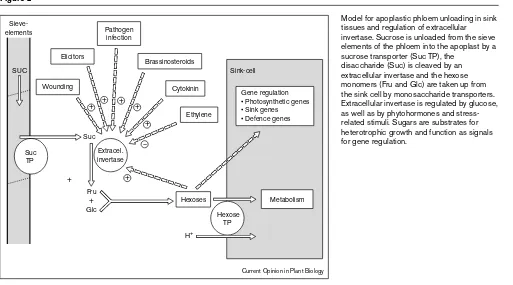
Figure 2
Model for apoplastic phloem unloading in sink tissues and regulation of extracellular invertase. Sucrose is unloaded from the sieve elements of the phloem into the apoplast by a sucrose transporter (Suc TP), the
disaccharide (Suc) is cleaved by an extracellular invertase and the hexose monomers (Fru and Glc) are taken up from the sink cell by monosaccharide transporters. Extracellular invertase is regulated by glucose, as well as by phytohormones and stress-related stimuli. Sugars are substrates for heterotrophic growth and function as signals for gene regulation.
Metabolism Gene regulation • Photosynthetic genes • Sink genes • Defence genes
Hexoses
H+
Hexose TP
Sink-cell
Wounding Cytokinin
Ethylene SUC
Extracel. invertase Suc
Sieve-elements
+ Fru
Glc + Elicitors
Pathogen infection
Brassinosteroids
Suc TP
+ + + +
+
+ –
Supplying carbohydrates to sink tissues
A number of studies demonstrate an essential function of extracellular invertase for phloem unloading, carbohydrate partitioning and growth of sink tissues (reviewed in [65,66•])
Regulation of source–sink transitions
The fast upregulation of extracellular invertase expression after the induction of metabolism in autotrophic cultures of tomato and Chenopodium rubrum [5•,67], and the induc-tion of sink metabolism in source leaves of transgenic plants by overexpression of a yeast invertase [27] indicate a role of extracellular sucrose cleavage in regulating source–sink-transitions and establishing sink metabolism.
Amplification of signals that regulate source–sink relations
As unloading of sucrose from the phloem into the apoplast follows the concentration gradient, and hexose transport into the sink cells is mediated by high affinity monosac-charide transporters, extracellular invertase with a high Km-value in the millimolar range is expected to be the lim-iting step for phloem unloading and thus a potential target for regulation. Indeed, extracellular invertases were shown to be specifically expressed under conditions that require a high carbohydrate supply and upregulated by a number of stimuli that affect source–sink relations (Figure 2). Extracellular invertase was shown to be specifically expressed in sink tissues. The corresponding gene was induced by growth stimulating phytohormones such as cytokinin and brassinosteroids ([68•], M Goetz and T Roitsch, unpublished observations), as well as elicitors, pathogen infection and wounding that lead to the require-ment for additional energy to elicit defence responses [8•,69]. In contrast, ethylene, that is associated with fruit ripening and thus terminates fruit growth, represses the expression of extracellular invertase [70]. Extracellular invertases from different species were also shown to be transcriptionally induced by sugars [5•,67,71] and a higher extracellular invertase activity will increase the sugar con-centration; therefore, any signal that upregulates extracellular invertase will be amplified and maintained by the positive sugar feedback circuit (Figures 1 and 2).
Integration of signals that regulate source–sink relations and defence responses
Since extracellular invertase is regulated by a number of different stimuli that influence source–sink relations, the corresponding signals are integrated via this enzyme. Elevated hexose levels brought about by an upregulated extracellular invertase expression have been suggested to be important in defence responses and systemic acquired resistance [21].
Conclusions and perspectives
Although there is accumulating evidence that stress relat-ed stimuli are important exogenous factors that regulate source–sink relations, it is still not known how these exogenous signals are sensed. Only scattered information
is available about the intracellular transduction of these signals and about the molecular and cellular mechanisms that contribute to the physiological responses. To further elucidate the effect of plant stress on source–sink regula-tion, it will be important to consider naturally occurring multistress situations. That is, to say, it will be important to determine possible additive, synergistic and compensating effects between different stress related stimuli and other environmental factors.
Sugars have been identified as important signal molecules that regulate source–sink relations. There is accumulating evidence that several independent sugar signal transduc-tion pathways, both specific for hexoses and sucrose, operate in parallel. The identified components of the underlying signal transduction pathways suggest homolo-gies as well as distinct differences to well characterised signal transduction pathways in yeast and mammals. Interactions and crosstalk between sugar and hormones, phosphate and light signal transduction pathways are evi-dent. There is experimental evidence that sugar and stress related signal transduction pathways are integrated to reg-ulate defence reactions as well as source–sink relations.
To elucidate the mechanisms that regulate source–sink relations, complementing experimental approaches are required. Protoplasts and suspension culture cells are suit-able experimental systems to study signal transduction, and photoautotrophic cultures have proven to be particu-larly suitable. Photosynthesis is usually repressed in heterotrophic cultures that also require a sugar depletion period which may cause artefacts. Studies involving whole plants will be required to assess source–sink relations, and controlled manipulation of pathways using regulated pro-moters seems to be very promising.
Parallels of plant signal transduction pathways to well char-acterised pathways in yeast will possibly allow cloning by complementation of yeast mutants, but the isolation and analysis of plant mutants will be necessary to identify com-ponents unique to plants. The analysis of signal transduction and response mutants was indispensable for the rapid and significant progress in the field of phytohor-mone signal transduction and the regulation of source–sink relations. To dissect the moleuclar basis for the apparent interaction and crosstalk between sugar and other signal transduction pathways it will be necessary to determine branch points, such as common second messengers or reg-ulatory proteins, by identifying corresponding upstream and downstream mutants; such mutants would be expect-ed to be characterisexpect-ed by either pleiotropic or more specific phenotypes, respectively.
and understand the complex regulation of processes taking place at the whole plant level and thus may be also a valuable tool to study the regulation of source–sink relations.
Understanding the complex interactions between different signals at the level of signal transduction pathways is like-ly to be the key for understanding source–sink regulation at the molecular level. Future studies should also consider possible interactions between carbohydrate, nitrogen and phosphate metabolism.
One driving force to study source–sink regulation was the projected practical application to manipulate assimilate partitioning in transgenic plants and to increase the parti-tioning of fixed carbon into harvestable sinks — however, the numerous approaches taken by many laboratories to modify carbon fluxes through modifying individual enzy-matic steps have been largely unsuccessful [73]. This insight and the available literature on source–sink regula-tion indicate that plants may display an enormous and underestimated metabolic flexibility and crosstalk between different signal transduction pathways. The chal-lenge remains, therefore, to unravel the underlying sophisticated network of highly flexible regulatory circuits to get insight into the fascinating biology of source–sink regulation. This will allow predictable genetic engineering of plants via manipulating signal transduction pathways rather than specific enzymatic reactions.
Acknowledgements
I would like to gratefully acknowledge the help of Rainer Ehneß, Markus Hofmann, Georgine Stühler and Doris Urbaneck for their help during the course of the preparation of this review and thank Marc Götz and Anja Stadler for critically reading of the manuscript.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Koch KE: Carbohydrate-modulated gene expression in plants.
Annu Rev Plant Physiol Plant Mol Biol 1996, 47:509-540. 2. Morcuende R, Pérez P, Martínez-Carrasco R: Short-term feedback
inhibition of photosynthesis in wheat leaves supplied with sucrose and glycerol at two temperatures. Photosynthetica1997, 33:179-188.
3. Felitti SA, Gonzalez DH: Carbohydrates modulate the expression of the sunflower cytochrome cgene at the mRNA level.Planta
1998, 206:410-415.
4. Winder TL, Sun J, Okita TW, Edwards GE: Evidence of the occurrence of feedback inhibition of photosynthesis in rice. Plant Cell Physiol1998, 39:813-820.
5. Godt DE, Roitsch T: Regulation and tissue-specific distribution of • mRNAs for three extracellular invertase isoenzymes of tomato
suggests and important function in establishing and maintaining sink metabolism. Plant Physiol1997, 115:273-282.
This study demonstrates that extracellular invertases of tomato are encoded by four differentially expressed and regulated genes. Specific isogenes are specifically expressed in sink tissue and/or induced in response to signals that require a high carbohydrate supply such as growth stimulating phyto-hormones, elicitors and wounding.
6. Ehness R, Roitsch T: Differential effect of D-glucose on the level of mRNAs for three invertase isoenzymes of Chenopodium rubrum.
J Plant Physiol 1997, 150:514-519.
7. Ismail I, De Bellis L, Alpi A, Smith SM: Expression of glyoxylate cycle genes in cucumber roots responds to sugar supply and can be activated by shading or defoliation of the shoot. Plant Mol Biol
1997, 35:633-640.
8. Ehness R, Ecker M, Godt DE, Roitsch T: Glucose and stress • independently regulate source and sink metabolism and defense
mechanisms via signal transduction pathways involving protein phosphorylation.Plant Cell 1997, 9:1825-1841.
The effect of sugar and stress-related signals on photosynthesis, sink metab-olism and defense response was studied in photoautotrophic cultures of
Chenopodium rubrumby analysing the regulation of mRNAs for represen-tative enzymes. All stimuli resulted in the induction of the genes encoding the sink specific extracellular invertase and the defense responsive PAL, where-as the photosynthetic gene RbcSwas repressed. The differential effect of the kinase inhibitor staurosporine demonstrated that glucose and stress independently activate different intracellular signalling pathways that are ulti-mately integrated to co-ordinately regulate source–sink relations and defense mechanisms. Both glucose and stress-related stimuli triggered the rapid and transient activation of protein kinases that specifically phosphory-late the MAP kinase substrate myelin basic protein.
9. Abdin OA, Zhou X, Coulman BE, Cloutier D, Faris MA, Smith DL: • Effect of sucrose supplementation by stem injection on the
development of soybean plants. J Exp Bot 1998, 49:2013-2018. The stem injection method has been optimised for soybean plants as an example of a non-cereal species. It was possible to administer concentrated sugar solutions into soybean plants continuously for almost their entire life cycle which was shown to suppress photosynthesis while positively affect-ing growth.
10. Chiou T-J, Bush DR: Sucrose is a signal molecule in assimilate • partitioning. Proc Natl Acad Sci USA 1998, 95:4784-4788. The authors demonstrate that the feeding of sucrose, but not of hexoses, decreased the activity of a proton–sucrose symporter in plasma membrane vesicles from leaves, which correlated with a repression of the correspond-ing gene expression. Inhibitor studies suggest that this novel sucrose-spe-cific response pathway does not involve hexokinase. These results are in contrast to a study by Harms et al.[74] that demonstrated that the mRNA for a potato sucrose transporter is not regulated by sucrose.
11. Rook F, Gerrits N, Kortstee A, van Kampen M, Borrias M, Weisbeek P, • Smeekens S: Sucrose-specific signalling represses translation of
the Arabidopsis ATB2 bZIP transcription factor gene. Plant J
1998, 15:253-263.
The expression pattern of bZIP suggests a role in the control of processes associated with the transport of metabolites. The expression of a GUS-reporter gene construct was specifically repressed by sucrose, but not by hexose sugars, indicating regulation by a sucrose specific pathway. 12. Dijkwel PP, Huijser C, Weisbeek PJ, Chua NH, Smeekens SCM: • Sucrose control of phytochrome A signaling in Arabidopsis. Plant
Cell 1997,9:583-595.
A plastocyanin promoter-luciferase reporter gene fusion was used to identi-fy mutants showing reduced repression of luminescence by sucrose. The specific repression of phytochrome responses of seedlings suggested the interaction between a sucrose specific sugar signal transduction pathway and light regulation.
13. Geiger M, Stitt M, Geigenberger P: Metabolism in slices from growing potato tubers responds differently to addition of sucrose and glucose. Planta 1998, 206:234-244.
14. Weber H, Heim U, Golombek S, Borisjuk L, Manteuffel R, Wobus U: • Expression of a yeast-derived invertase in developing cotyledons
of Vicia narbonensisalters the carbohydrate state and affects storage functions.Plant J 1998, 16:163-172.
To change the sugar status during seed development, a yeast-derived inver-tase was expressed in Vicia narbonensisunder the control of a LeguminB4 promoter. There was a positive correlation of sucrose content to the levels of mRNA for sucrose-synthase and a most pronounced correlation to ADP-glucose pyrophosphorylase as well as to starch content. The authors sug-gest a sucrose-mediated induction of storage-associated differentiation. 15. Weber H, Borisjuk L, Wobus U: Sugar import and metabolism
during seed development.Trends Plant Sci 1997, 2:169-174. 16. Jang J-C, León P, Zhou L, Sheen J: Hexokinase as a sugar sensor • in higher plants. Plant Cell 1997, 9:5-19.
The authors present evidence for a role of hexokinase in sugar signaling by modulating the in planta level of hexokinase activity. Arabidopsis plants which overexpress hexokinase showed increased sugar sensitivity whereas anti-sense suppression of hexokinase resulted in a decreased sugar sensitivity 17. Jang JC, Sheen J: Sugar sensing in higher plants.Trends Plant Sci
1997, 6:208-214.
19. Smeekens S, Rook F: Sugar sensing and sugar-mediated signal transduction in plants.Plant Physiol 1997, 115:7-13.
20. Umemura T-A, Perata P, Futsuhara Y, Yamaguchi J: Sugar sensing and a-amylase gene repression in rice embryos. Planta1998, 204:420-428.
21. Herbers K, Meuwly P, Frommer WB, Métraux J-P, Sonnewald U: Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway.
Plant Cell 1996, 8:793-803.
22. Cangelose GA, Ankenbauer RG, Nester EW: Sugars induce the
Agrobacteriumvirulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc Natl Acad Sci USA 1990, 87:6708-6712.
23. Özan S, Dover J, Rosenwald AG, Wölfl S, Johnston M: Two glucose transporters in Saccharomyces cerevisiaeare glucose sensors that generate a signal for induction of gene expression. Proc Natl Acad Sci USA1996, 93:12428-12432.
24. Zhou L, Jan J-C, Jones TL, Sheen J: Glucose and ethylene signal • transduction crosstalk revealed by an Arabidopsis
glucose-insensitive mutant.Proc Natl Acad Sci USA 1998, 95:10294-10299. In the isolated Arabidopsis gin1 mutant, glucose repression of different steps of seedling development is impaired. The phenotype of the mutant can be copied by ethylene precursor treatment of wild-type plants or by consti-tutive ethylene biosynthesis or signaling mutants. In contrast, an ethylene insensitive mutant was shown to exhibit glucose hypersensitivity. This study demonstrates a convergence between the glucose and the ethylene signal transduction pathways.
25. Németh K, Salchert K, Putnoky P, Bhalerao R, Koncz-Kálmán Z, • Stankovic-Stangeland B, Bakó L, Mathur J, Ökrész L, Stabel S et al.:
Pleiotropic control of glucose and hormone responses by PRL1, a nuclear WD protein, in Arabidopsis.Genes Dev 1998,
12:3059-3073.
A T-DNA insertional mutation conferring hypersensitivity to glucose and sucrose has been identified. This mutation results in a pleiotropic phenotype including an increased sensitivity of plants to growth hormones. The corre-sponding gene encodes a nuclear WD protein.
26. Das SB, Bowley SR, McKersie BD: A high leaf-starch mutant in alfalfa has altered invertase activity. Crop Sci 1998,38:722-728. 27. Stitt M, Sonnewald U: Regulation of metabolism in transgenic
plants. Annu Rev Plant Physiol Plant Mol Biol 1995, 46:341-368. 28. Caddick MX, Greenland AJ, Jepson I, Krause KP, Qu N, Ridell KV, • Salter MG, Schuch W, Sonnewald U, Tomsett AB: An ethanol
inducible gene switch for plants used to manipulate carbon metabolism.Nat Biotechnol1998, 16:177-180.
A chemically inducible plant gene expression system, with negligible back-ground activities has been engineered. The potential of this system was demonstrated by inducible manipulation of carbon metabolism in transgenic plants.
29. Hirt H: Multiple roles of MAP kinases in plant signal transduction.
Trends Plant Sci 1997, 2:11-15.
30. Barker LDP, Templeton MD, Ferguson IB:A 67-kDa plasma • membrane-bound Ca2+-stimulated protein kinase active in sink
tissue of higher plants. Planta1998, 205:197-204.
A novel protein kinase was identified in microsomal membrane fractions of apple and a homologous protein kinase from maize with similar properties was demonstrated to be specifically active in sink tissues.
31. Iwata Y, Kuriyama M, Nakakita M, Kojima H, Ohto M-A, Nakamura K: Characterization of a calcium-dependent protein kinase of tobacco leaves that is associated with the plasma membrane and is inducible by sucrose. Plant Cell Physiol 1998, 39:1176-1183. 32. Man AL, Purcell PC, Hannappel U, Halford NG: Potato SNF1-related
protein kinase: molecular cloning, expression analysis and peptide kinase activity measurements. Plant Mol Biol 1997, 34:31-43.
33. Annen F, Stockhaus J: Characterization of a Sorghum bicolorgene family encoding putative protein kinases with a high similarity to the yeast SNF1 protein kinase. Plant Mol Biol 1998, 36:529-539. 34. Purcell PC, Smith AM, Halford NG: Antisense expression of a • sucrose non-fermenting-1-related protein kinase sequence in
potato results in decreased expression of sucrose synthase in tubers and loss of sucrose-inducibility of sucrose synthase transcripts in leaves. Plant J 1998, 14:195-202.
SNF1 regulates the expression of many genes encoding enzymes of carbo-hydrate metabolism in yeast. Antisense repression of a homologous potato kinase results in specific effects on sucrose synthase expression and sugar
regulation, whereas the activities of other sink specific enzymes was not affected.
35. Halford NG, Hardie DG: SNF1-related protein kinases: global regulators of carbon metabolism in plants? Plant Mol Biol 1998, 37:735-748.
36. Salchert K, Bhalerao R, Koncz-Kálmán Z, Koncz C: Control of cell elongation and stress responses by steroid hormones and carbon catabolic repression in plants. Phil Trans R Soc Lond B 1998, 353:1517-1520.
37. Urwin NAR, Jenkins GI: A sucrose repression element in the
Phaseolus vulgaris rbcS2gene promoter resembles elements responsible for sugar stimulation of plant and mammalian genes.
Plant Mol Biol 1997, 35:929-942.
38. Hwang Y-S, Karrer EE, Thomas BR, Chen L, Rodriguez RL: Three cis -elements required for rice a-amylase Amy3Dexpression during sugar starvation. Plant Mol Biol 1998, 36:331-341.
39. Toyofuku K, Umemura T-A, Yamaguchi J: Promoter elements • required for a sugar-repression of the TAmy3Dgene for a
-amylase in rice.FEBS Lett 1998, 428:275-280.
The authors show by in situ hybridisation the tissue specific sugar repression of rice α-amylase gene RAmy3D in scutellar epithelium cells of callus-form-ing rice embryos. A transient expression and site-directed mutageneis was used to identify the cis-acting elements required for sugar repression. 40. Morita A, Umemura T-A, Kuroyanagi M, Futsuhara Y, Perata P, Yamaguchi
J: Functional dissection of a sugar-repressed a-amylase gene
(RAmy1A)promoter in rice embryos. FEBS Lett1998, 423:81-85. 41. Davidson RL: Effect of soil nutrients and moisture on root/shoot
ratio in Lolium perenne L. and Trifolium repens. Annals Bot 1969, 33:571-577.
42. Berman ME, Dejong TM: Water stress and crop load effects on fruit fresh and dry weights in peach (Prunus persica). Tree Physiol
1996, 16:859-864.
43. Van den Boogaard R, Alewijnse D, Veneklaas EJ, Lambers H: Growth and water-use efficiency of 10 Triticum aestivumcultivars at different water availability in relation to allocation of biomass.
Plant Cell Env 1997, 20:200-210.
44. Sowder CM, Tarpley L, Vietor DM, Miller FR: Leaf photoassimilation and partitioning in stress-tolerant Sorghum.Crop Sci1997, 37:833-838.
45. Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M: Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 1997, 201:502-518. 46. Escobar-Gutiérrez AJ, Zipperlin B, Carbonne F, Moing A, Gaudillère JP: Photosynthesis, carbon partitioning and metabolite content during drought stress in peach seedlings. Aust J Plant Physiol
1998, 25:197-205.
47. Dorion S, Lalonde S, Saini HS: Induction of male sterility in wheat by meiotic-stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiol 1996, 111:137-145.
48. Pelleschi S, Rocher J-P, Prioul J-L: Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves.Plant Cell Env 1997, 20:493-503.
49. Schellenbaum L, Müller J, Boller A, Wiemken A, Schüepp H: Effects of drought on non-mycorrhizal and mycorrhizal maize: changes in the pools of non-structural carbohydrates, in the activities of invertase and trehalase, and in the pools of amino acids and imino acids. New Phytol 1998, 138:59-66.
50. Pelleschi S, Guy S, Kim J-Y, Pointe C, Mahé A, Barthes L, Leonardi A, • Prioul J-L: Ivr2, a candidate gene for a QTL of vacuolar invertase
activity in maize leaves. Gene specific expression under water stress.Plant Mol Biol1999, in press.
The authors demonstrate that water shortage results in a early and large stim-ulation of vacuolar invertase activity which was tightly correlated to the steady-state level of only one specific invertase isoenzyme. This analysis was linked to a quantitative trait loci (QTL) analysis, which demonstrated that not all of the identified QTLs were located near the corresponding locus. This indicates that other genes are also likely to be involved in the invertase stress response. 51. Gilbert GA, Wilson C, Madore MA: Root-zone salinity alters • raffinose oligosaccharide metabolism and transport in coleus.
Plant Physiol 1997, 115:1267-1276.
novel carbohydrates. The authors provide the novel and interesting sugges-tion that alterasugges-tion in the type of carbohydrate may act as a signalling system for the activation of acclimation responses in the sink tissue.
52. Gucci R, Moing A, Gravano E, Gaudillère JP: Partitioning of photosynthetic carbohydrates in leaves of salt-stressed olive plants.Aust J Plant Physiol 1998, 25:571-579.
53. Lucas WJ, Wolf S: Connections between virus movement, macromolecular signalling and assimilate allocation. Curr Opin Plant Biol 1999, 2:192-197.
54. Gerant D, Podor M, Grieu P, Afif D, Cornu S, Morabito D, Banvoy J, Robin C, Dizengremel P: Carbon metabolism enzyme activities and carbon partitioning in Pinus halepensismill. exposed to mild drought and ozone. J Plant Physiol 1996, 148:142-147. 55. Hibberd JM, Whitbread R, Farrar JF: Effect of 700 µmol mol-1CO
2
and infection with powdery mildew on the growth and carbon partitioning of barley. New Phytol1996, 134:309-315. 56. Miller JE, Heagle AS, Pursley WA: Influence of ozone stress on • soybean response to carbon dioxide enrichment: II. Biomass and
Development. Crop Sci 1998, 38:122-128.
The authors analyse the effect of the expected increase of the levels of both ozone and CO2 in the environment on biomass and development. It has been
shown that elevated CO2ameliorated ozone effects on main stem biomass,
root biomass, and leaf area.
57. Lichtenthaler HK, Miehé JA: Technical focus: fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci 1997, 2:285-323. 58. Rosema A, Snel JFH, Zahn H, Buurmeijer WF, Van Hove LWA: The
relation between laser-induced chlorophyll fluorescence and photosynthesis. Remote Sens Environ 1998, 65:143-154. 59. Skoog F, Miller CO: Chemical regulation of growth and organ
formation in plant tissue cultures in vitro. In: Molecular Aspects and Cellular Aspects of Development.Edited by Bell E. New York: Chapman and Hall; 1965:481-494.
60. Perata P, Matsukura C, Vernieri P, Yamaguchi J: Sugar repression of • a gibberellin-dependent signaling pathway in barley embryos.
Plant Cell1997, 9:2197-2208.
The results demonstrate that sugar and hormonal signalling specifically interact in the regulation of gibberellin acid-induced gene expression in barley grains in a highly tissue specific manner. Whereas the induction of α-amylase by gib-berellic acid in the aleurone layer is unaffected by the presence of the sugar, carbohydrate repression is effective in the scutellar epithelium of the embryo. 61. Bustos MM, Iyer M, Gagliardi S: Induction of a b-phaseolin
promoter by exogenous abscisic acid in tobacco: developmental regulation and modulation by external sucrose and Ca2+ions.
Plant Mol Biol 1998, 37:265-274.
62. Sadka A, DeWald DB, May GD, Park WD, Mullet JE: Phosphate modulates transcription of soybean VspBand other sugar-inducible genes. Plant Cell 1994, 6:737-749.
63. Nielsen TH, Krapp A, Röper-Schwarz U, Stitt M: The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP glucose pyrophosphorylase is modified by phosphate and nitrogen.Plant Cell Env 1998, 21:443-454.
64. Jang J-C, Sheen J: Sugar sensing in higher plants. Plant Cell 1994, 6:1665-1679.
65. Tymowska-Lalanne Z, Kreis M: The plant invertases: physiology, biochemistry and molecular biology. Adv Bot Res 1998, 28:71-117. 66. Tang G-Q, Lüscher M, Sturm A: Antisense repression and vacuolar • and cell wall invertase in transgenic carrot alters early plant
development and sucrose partitioning. Plant Cell 1999, 11:1-14. Antisense repression of both invertase isoenzymes resulted in phenotypic alterations that appeared very early in development. This study further sup-ports an important role for both extracellular and vacuolar invertases in plant growth and development. The data suggest that extracellular invertase plays an important role in early development, whereas both isoforms seem to have important functions in sucrose partitioning.
67. Roitsch T, Bittner M, Godt DE: Induction of apoplastic invertase of
Chenopodium rubrum by D-glucose and a glucose analog and tissue-specific expression suggest a role in sink-source regulation. Plant Physiol 1995, 108:285-294.
68. Ehness R, Roitsch T: Co-ordinated induction of mRNAs for • extracellular invertase and a glucose transporter in Chenopodium
rubrumby cytokinins. Plant J 1997, 11:539-548.
This study shows that extracellular invertase and hexose transporters are not only functionally linked but also coordinately regulated. It has been shown that upregulation of the two enzymes by cytokinin results in increased glu-cose and sucrose uptake (via hexose monomers). This regulatory mecha-nism was suggested to be one of the molecular mechamecha-nisms required for the stimulation of growth and cell division and the retardation of senescence. 69. Sturm A, Chrispeels MJ: cDNA cloning of carrot extracellular b
-fructosidase and its expression in response to wounding and bacterial infection. Plant Cell 1990, 2:1107-1119.
70. Linden JC, Ehneß R, Roitsch T: Ethylene regulation of apoplastic invertase expression in autotrophic cells of Chenopodium rubrum.
Plant Growth Regul 1996, 19:219-222.
71. Tymowska-Lalanne Z, Kreis M: Expression of the Arabidopsis thalianainvertase gene family. Planta 1998, 207:259-265. 72. Prioul JL, Quarrie S, Causse M, de Vienne D: Dissecting complex
physiological functions through the use of molecular quantitative genetics.J Exp Bot1997:1151-1163.
73. Herbers K, Sonnewald U: Molecular determinants of sink strength.
Curr Opin Plant Biol 1998, 1:207-216.