Purification and properties of growth stage-dependent antiviral
proteins from the leaves of
Celosia cristata
A. Balasubrahmanyam
a, V.K. Baranwal
b, M.L. Lodha
a, A. Varma
b, H.C. Kapoor
a,*
aDi6ision of Biochemistry,Indian Agricultural Research Institute,Pusa,New Delhi110012,India bDi6ision of Plant Pathology,Indian Agricultural Research Institute,Pusa,New Delhi110012,India
Received 16 June 1999; received in revised form 21 September 1999; accepted 21 September 1999
Abstract
Two antiviral glycoproteins, active against mechanical transmission of two tobamoviruses, tobacco mosaic virus and sunnhemp rosette virus, and citrus ring spot virus (ungrouped), were purified from the dried leaves ofCelosia cristata. These proteins, called CCP-25 and CCP-27, haveMr25 and 27 kDa, respectively. Their concentration was found to vary between the pre-flowering and
post-flowering stages of C. cristata. Either of these proteins, obtained at different growth stages, inhibited by \90% lesion formation at a concentration of 20 – 30mg ml−1. They were resistant to proteases in the native state, but were readily digested
when denatured. Both of them imparted actinomycin D sensitive resistance by inhibiting local lesions onNicotiana tabacumcv. Samsun NN by tobacco mosaic virus. Their application, individually, also resulted in high resistance in systemic hosts to sunnhemp rosette virus, and citrus ring spot virus, respectively. © 2000 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Celosia cristata; Antiviral; Basic glycoproteins; Stage-dependent; Protein purification
www.elsevier.com/locate/plantsci
1. Introduction
A large number of higher plants possess potent inhibitors of plant viruses and a few have been purified and characterized [1,2]. They either inacti-vate virus in vitro [3], or interfere with mechanical transmission, e.g. those from spinach [4] and
Mirabilis jalapa [5], and sometimes may induce systemic resistance [6] when applied at least some time prior to virus challenge. These antiviral prin-ciples are generally basic proteins, with or without a sugar moiety, with sizes ranging from 24 to 32 kDa and act against a range of plant viruses [1,7,8]. The leaf extract ofCelosia cristatahas been
found to prevent lesion production by sunnhemp rosette virus (SRV), tobacco mosaic virus (TMV), and potato virus X (PVX) in several hosts [9]. Studies on partially purified inhibitor from this leaf extract indicated that it might interfere with virus establishment in the host [10]. The purifica-tion of the virus inhibitor to apparent homogene-ity is described here. Further evidence is presented that there could be two related glycoproteins (whose levels might vary according to the growth stage) in the leaf extract and both of which can impart a very high level of resistance to TMV and SRV on their respective local lesion hosts. They also provoke high resistance to SRV and citrus ring spot virus (CRSV) on their respective sys-temic hosts namely, Crotalaria juncea (sunnhemp) and Phaseolus 6ulgaris (French bean). These
proteins are referred as CCP-25 and CCP-27, based on their molecular weights.
* Corresponding author. Tel.:+91-11-578-4038; fax:+ 91-11-576-6420.
E-mail address:hck –[email protected] (H.C. Kapoor)
2. Materials and methods
Preparations of virus inoculum and inhibitor (antiviral principle), testing the activity, raising of test plants and inoculation procedures were car-ried out as described before [9]. The different virus-host (local lesion host) combinations used in the current study are Nicotiana glutinosa, N.
tabacum cv. Samsun NN against TMV, and
Cyamopsis tetragonoloba against SRV, depending on availability of test plants and seasons. The percentage inhibition was calculated by the for-mula (C−T)/C×100, where C is the number of lesions in control and T is the number on treated leaves.
2.1. Preparation of purified 6irus inoculum
Purified preparations of TMV and SRV were obtained using polyethylene glycol (PEG) and dif-ferential centrifugation as described by Noordam [11]. CRSV was purified by extraction with seven volumes of phosphate buffer (0.05 M, pH 7.8) containing 0.005 M DIECA, 0.01 M EDTA and 0.02 M sodium sulphite from virus infected French bean tissue followed by treatment with chloroform (10%, v/v). The suspension was centrifuged at 10 000×g for 10 min and the supernatant was subjected to two cycles of high speed (40 000×g, 2.5 h) and low speed (10 000×g, 5 min) centrifu-gation. The supernatant obtained after final low speed centrifugation was used as purified virus preparation.
2.2. Optimum pH for extraction of maximum anti6iral acti6ity
Leaf extract of C. cristata was prepared using different buffers of varying pH and applied on to the well-developed leaves of the test plants. The extraction buffers used were: (i) acetate buffer (20 mM, pH 5.2); (ii) phosphate buffers (20 mM) with pH (a) 6.0 and (b) 7.0; and (iii) Tris – HCl buffer (20 mM, pH 8.6).
2.3. Purification of 6irus inhibitor
2.3.1. Extraction
The leaves of C. cristata were harvested sepa-rately at three different stages of plant growth, i.e. pre-flowering, flowering and post-flowering (20 – 25
days after flowering). They were washed with tap water and dried at room temperature or at 40 – 45°C. This material (40 g) was homogenized with 7 volumes of extraction buffer (0.1 M sodium acetate buffer, pH 5.2, containing 12 mM b -mer-captoethanol) and 20 mg of polyvinyl polypyrol-lidone in a waring blender. The slurry was filtered through muslin cloth and centrifuged at 12 000×g
for 15 min. The clear supernatant was collected for further purification.
2.3.2. Ammonium sulphate precipitation
The precipitation of protein by ammonium sul-phate was initially carried out at 25, 60, 80 and 100% saturation. The protein obtained at each step was centrifuged (12 000×g, 10 min) and su-pernatant was used for further precipitation pro-cess. The pellet obtained at each step was suspended in buffer A (20 mM sodium phosphate, pH 6.2, 10 mM NaCl) and dialyzed against the same buffer. The clear sample was tested for the antiviral activity on the test hosts. Since activity was found in two protein fractions precipitates between 25 and 60%, and between 60 and 80% ammonium sulphate saturation, all of the protein precipitation between 25 and 80% saturation was used for further purification.
2.3.3. Ion exchange chromatography
2.3.3.1. DEAE-cellulose chromatography. Treat-ment of DEAE-cellulose was carried out as de-scribed [10]. The active protein (18.8 mg) obtained after ammonium sulphate precipitation was loaded on a DEAE-cellulose column (15×1.5 cm) and washed with buffer A. Fractions of 10 ml were collected from the column until no protein was found in the eluant. The bound protein was eluted with 0.5 M NaCl in the same buffer. Both frac-tions were tested for their antiviral activity. The active unadsorbed fractions were pooled and sub-jected to cation exchange chromatography.
discontinuous gradient of NaCl (0.1 and 0.2 M) prepared in the same buffer. About four to five bed volumes of buffer were used at each step and fractions (3.0 ml) were collected on an LKB frac-tion collector system at a flow rate of 30 ml h−1.
Protein elution was simultaneously monitored at 280 nm and signals were traced on to a printer. Fractions falling within the protein peak were pooled and tested for antiviral activity.
2.3.4. Gel filtration chromatography
The pooled protein sample (1.9 mg) was either lyophilized at −20°C or concentrated in a dialy-sis bag on a sucrose pad at 4°C. Later it was chromatographed on a Sephadex G-75 (Pharma-cia Fine Chemicals, Uppsala, Sweden) column (61×1.5 cm) equilibrated with buffer C (20 mM sodium acetate, pH 5.2 containing 0.01% sodium azide). The protein was eluted at a constant flow rate of 40 ml h−1 and fractions (3.1 ml) were
monitored at 280 nm. Fractions falling within the protein peak were pooled and tested for antiviral activity.
2.4. Protein and carbohydrate estimation
Protein estimation was done by the Bradford method [12] using bovine serum albumin (BSA) as a standard. The presence of carbohydrate was tested by the Molisch test [13] and the estimation of total neutral sugars was done by the phenol-sulfuric acid method [14].
2.5. Protein characterization
SDS-PAGE was performed in a 12% separating gel according to Laemmli [15]. The proteins were also analyzed in a 10 – 20% linear gradient gel. The gradient was prepared using an LKB gradi-ent maker. The proteins were visualised with Coomassie blue or by silver staining [16]. Glyco-proteins were detected on polyacrylamide gels us-ing the periodic acid-Schiff reagent (PAS) [17]. Ovalbumin was used as a positive control on the gel, while BSA served as the negative control. The molecular weights of the purified proteins were determined under native conditions by cali-brated gel-permeation chromatography using MW GF-70 kit (contains aprotinin, 6.5 kDa; cy-tochrome c, 12.4 kDa; carbonic anhydrase, 29 kDa and bovine serum albumin, 66 kDa) (Sigma)
in a Sephadex G-75 column. The Rf of molecular
marker and purified proteins were plotted against the molecular weight on a semi-log graph paper. The molecular weights of the purified proteins were also determined under denatured conditions in a 12% polyacrylamide gel and a 10 – 20% linear gradient gel by SDS-PAGE using MW SDS-70 kit.
2.6. Stability to protease digestion
The purified proteins, obtained by the above mentioned purification procedure, were incubated at 37°C for 12 h with trypsin, chymotrypsin,
Staphylococcus aureus protease XVII-B or papain. The incubation mixture contained purified protein and protease in a ratio of 25:1 (w/w) in 50 mM Tris – HCl buffer, pH 6.8. The mixture was then applied onto test plants to determine its antiviral activity. Both proteins, after incubation, were also monitored by SDS-PAGE. The antivi-ral proteins were also denatured in Cleveland buffer [18] and digested with each of the above proteases for 15 or 30 min and subjected to SDS-PAGE.
2.7. Effect on systemic hosts
The purified protein was tested for its ability to impart resistance in different systemic host-virus combinations. About 60 mg of purified protein in 1 ml was mixed with each of the purified viruses (TMV, SRV and CRSV), and the mixture was applied on carborundum dusted leaves of three test plants each of tobacco cv NP33, sunnhemp and French bean, respectively. The symptoms were observed after 25-35 days of virus infection in case of TMV and SRV, and after 6 days in case of CRSV. Back-inoculation or electron mi-croscopy or both were carried out to detect the virus in the treated systemic host plant.
3. Results
Preliminary studies had shown that an extract of dry leaf material (dried at 40 – 45°C) of C.
Table 1
Purification of antiviral protein fromCelosia cristataa
Test sample concentration (mg ml−1)
Fraction Total protein (mg) Percent of inhibitionb
0.024
Crude extract 24.52 75.0
25–80% ammonium sulphate 18.80 0.117 95.0
0.164 95.0
DEAE-cellulose unadsorbed 9.30 CM-Sepharose purified
0.142
Peak I 4.49 13.5
0.125
1.98 97.5
Peak II
Peak III 2.50 0.175 −5.9
0.060
1.55 98.0
Sephadex G-75 (single peak)
aData refers to 40 g ofC.cristata dried leaf material.
bThree to four well-developed leaves of four test plants of Nicotiana tabacumcv. Samsun NN, each for all sets, were treated
with 2 ml of test sample. After 1 h the leaves were washed with distilled water and blotted dry. The leaves were dusted with carborundum and inoculated with tobacco mosaic virus.
3.1. Optimum pH for extraction
Initial studies showed that the inhibitor ex-tracted with 20 mM sodium acetate buffer, pH 5.2, exhibited maximum activity (95.6% local lesion inhibition) compared to that of other extracts made with different buffers of varying pH. Fur-ther, the few lesions produced in this case were very small and not very well developed compared to those in other treatments (results not shown). Therefore, this buffer, with higher molarity (0.1 M) and supplemented with 12 mM b -mercap-toethanol was used for extracting the antiviral activity for subsequent purification process.
3.2. Purification of inhibitor
Upon stepwise ammonium sulphate precipita-tion, most of the antiviral activity was recovered in the fraction precipitating between 25 and 80% ammonium sulphate. This pellet was redissolved in buffer A, dialyzed against the same buffer and subjected to DEAE-cellulose chromatography. The antiviral activity that came through in the void volume was further fractionated by CM-sep-harose cation exchange chromatography, using a discontinuous linear NaCl gradient (0.1 and 0.2 M) to elute bound proteins. The elution at 0.1 M NaCl resulted in one protein peak, peak I, whereas 0.2 M NaCl gave rise to two peaks, peaks II and III. Negligible amounts of protein were eluted at higher salt concentrations (results not shown). Of these, the peak II fractions exhibited maximum activity (Table 1). The relevant pooled fractions were subjected to size exclusion chromatography
on Sephadex G-75. This resulted in a single protein peak, which was subsequently found to possess the antiviral activity.
3.3. Characterization of C. cristata anti6iral protein
3.3.1. SDS-PAGE
Electrophoresis of the Sephadex G-75 chro-matography purified inhibitor under denaturing
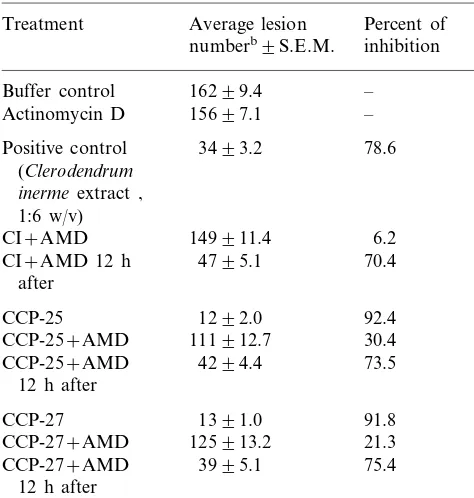
Fig. 1. SDS-PAGE (12%) of antiviral proteins of Celosia cristata at different stages of purification. Lanes (a) and (h) carry molecular weight (kDa) markers, and lane (b) crude extract (30mg), lane (c) 25 – 80% ammonium sulphate precipi-tated (80 mg), lane (d) DEAE-cellulose unadsorbed (30 mg), lane (e) CM-sepharose purified (30mg), lane (f) 2 and (g) 3.5
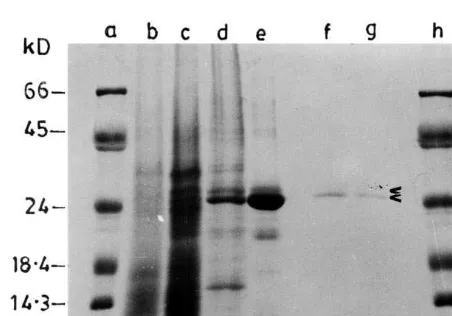
Fig. 2. SDS-PAGE of antiviral proteins purified at different growth stages of Celosia cristata. Lanes (b) and (c) show 8 and 16 mg of antiviral protein, respectively, purified at pre-flowering stage, lanes (e) and (f) 7 and 15 mg of protein purified at flowering stage, lane (a) 8 mg protein purified at post-flowering stage, and lane (d) molecular weight markers as in Fig. 1. Protein in each sample was concentrated by TCA precipitation as described in the legend for Fig. 1.
ing), they were present in equal concentration when they were purified from the leaves harvested at flowering stage. The leaves harvested at post-flowering stage (20 – 25 days after post-flowering, DAF) showed a prominent higher molecular weight polypeptide band (Fig. 2). Both of these proteins independently exhibited antiviral activity against TMV on N. tabacum Samsun NN.
3.3.2. Molecular weight of anti6iral proteins
Molecular weights (Mr) of nativeCelosia
antivi-ral proteins were determined by calibrated gel permeation chromatography. A Sephadex G-75 column was calibrated by using the Sigma MW-GF-70 marker kit. Proteins purified from different lots showing a single polypeptide on SDS-PAGE were passed through the calibrated Sephadex G-75 column separately. One of them showed a lar weight of 33 kDa and other showed a molecu-lar weight of 37 kDa. However, on SDS-PAGE the Mr of the higher mobility species was 33 kDa,
while for the second polypeptide, it was slightly different with a Mr of 35 kDa (Fig. 2). Since the
estimation of Mr of glycoproteins by gel fitration
or non-gradient SDS-PAGE may give erroneous results [19,20], electrophoresis was also carried out in a 10 – 20% linear gradient gel to confirm the above data. The results produced Mr of 25 and 27
kDa, respectively, for the aforesaid polypeptides (Fig. 3). Therefore, these proteins will be referred as CCP-25 and CCP-27, respectively (CCP stands for C. cristata protein).
3.3.3. Presence of carbohydrates
Both the 25 and 27 kDa Celosia antiviral proteins stained positively for glycoproteins on polyacrylamide gels when PAS reagent was used (not shown). Ovalbumin, which served as a posi-tive glycoprotein control, also stained pink, while BSA, which is not a glycoprotein, did not stain. Total neutral sugar contents, as determined by the phenol-sulfuric acid method were 26 and 28%, respectively (not shown).
3.3.4. Effect of dilution, temperature and exogenous proteases
CCP-25 and CCP-27 obtained after Sephadex G-75 chromatography were used for these studies. Various concentrations of the two proteins, rang-ing from 10 – 150 mg ml−1 as determined by the
Bradford method [11], were applied on tobacco cv. Samsun NN plants to test for the resistance
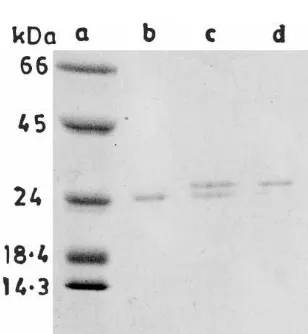
re-Fig. 3. Linear gradient (10 – 20%) SDS-PAGE of antiviral proteins purified at different growth stages ofCelosia cristata. Lane (a) molecular weight markers as in Fig. 1; lane (b) 4mg of CCP-25 purified at pre-flowering stage; lane (c) CCP-25 and CCP-27 (8mg) purified at flowering stage; and lane (d) 4
mg of CCP-27 purified at post-flowering stage. Protein in each sample was concentrated by TCA precipitation as described in the legend for Fig. 1.
(pre-flower-Fig. 4. SDS-PAGE analysis of CCP-25 treated with different proteases. Approximately 15mg of CCP-25, after dissociation with Cleveland buffer [17], were incubated with different proteases (0.6 mg) for 15 or 30 min. After digestion, the samples were made to 10%b-mercaptoethanol and 2% SDS, heated at 100°C for 2 min and electrophoresed on 15% polyacrylamide gel. Lane (a) untreated CCP-25; lanes (b) and (c) trypsin treated; lanes (d) and (e) chymotrypsin treated; lanes (f) and (g) Staphylococcus aureus protease XVII-B treated; and lanes (h) and (i) papain treated CCP-25. The first lane for each enzyme represents the treatment for 15 min and the second one represents the same for 30 min.
lose the ability to impart resistance (not shown). CCP-25 and CCP-27 were tested for their sensi-tivity to proteases by incubation with trypsin, chymotrypsin, S. aureusprotease XVII-B and pa-pain. Application of protease-treated CCP-25 and CCP-27 on tobacco cv. Samsun NN, did not result in any increase in lesion number. However, the proteases caused some reduction in TMV lesion number themselves, when applied alone on test plants followed by challenge inoculation with TMV. SDS-PAGE of native antiviral proteins in-cubated with the proteases for 24 h revealed that their degradation was minimal, but after denatura-tion with Cleveland buffer [18] they were digested to a large extent within 30 min of incubation (treatment of CCP-25 with different proteases is shown in Fig. 4). In contrast, BSA was completely digested when incubated either in native or dena-tured form with each of these proteases (not shown).
3.4. Effect of purified proteins on systemic hosts
Both CCP-25 and CCP-27 were independently tested for their ability to induce resistance on systemic host plants. However, the results are pre-sented only for CCP-25 in Table 2. In the case of tobacco-TMV system, symptoms appeared on both control (26 days post-inoculation, pi) and treated (35 days pi) plants, albeit symptoms were delayed on treated plants. Subsequent back inocu-lation on local lesion host (N. tabacumcv. Samsun sponse. CCP-25 inhibited lesion formation by
more than 90% at a concentration as low as 20mg ml−1, whereas CCP-27 exhibited the same
re-sponse at a concentration of 30 mg ml−1 (results
not shown). Both CCP-25 and CCP-27 displayed tolerance to temperature and remained entirely unaffected even after 10 min of incubation at 90°C. However, at around 95°C, they began to
Table 2
Effect of antiviral protein CCP-25 on different virus–systemic host combinationsa
Symptom Treatment
Systemic host–virus Lesion number upon back Virus particles observed by EM
inoculationb9S.E.M.
expression
N.tabacumcv NP33, Control Systemic mosaic 285912.3 Very high TMV
15699.1 High
Treated Mosaic
8797.2 High
Crotalaria juncea, SRV Control Systemic mosaic
Few 791.0
Nil Treated
Very high Control
Phaseolus6ulgaris, Systemic mosaic –
CRSV
– Nil
Treated Nil
aThe purified protein CCP-25 (60mg ml−1) was mixed with the isolated virus in each case and applied on carborundum dusted
lower leaves of respective systemic host plants. After 25–35 days of virus infection in case of tobacco mosaic virus (TMV) and sunnhemp rosette virus (SRV), and after 6 days in case of citrus ring spot virus (CRSV), top leaves were harvested and analyzed for the presence of virus by back inoculation on respective local lesion hosts or by electron microscopy or both.
Table 3
Effect of actinomycin D on antiviral propertya
Treatment Average lesion Percent of numberb9S.E.M. inhibition
16299.4 Positive control 3493.2
(Clerodendrum
separately applied on leaves of tobacco cv. Samsun NN. In one set, actinomycin D (AMD, 20 mg ml−1) was applied
immediately after the treatment, while in another set AMD treatment was given after 12 h of CCP application. Challenge inoculations were made as usual. Control sets were treated with buffer alone or with the AMD solution alone. Dry leaf extract of Clerodendrum inerme (CI) prepared with 20 mM sodium acetate, pH 5.2 (1:6, w/v) was used as a positive control [6].
bMean number of lesions obtained from 12 leaves of four
test plants.
3.5. Effect of actinomycin D on anti6iral property
Both CCP-25 and CCP-27 imparted resistance to tobacco cv. Samsun NN against TMV. This resistance could be largely inhibited when actino-mycin D (AMD, 20mg ml−1) was applied
immedi-ately after treating the plant with either CCP-25 or CCP-27. When AMD was applied 12 h after CCP treatment, it failed to inhibit the resistance re-sponse (Table 3).
4. Discussion
Two proteins were purified from C. cristata
leaves and appeared homogeneous on polyacry-lamide gel electrophoresis and on gel filtration. Both of them individually exhibited more than 90% inhibition of local lesions on test host plants. Their respective sizes appeared to be 33 and 35 kDa in continuous 12% polyacrylamide gel, and 33 and 37 kDa on gel filtration. However, elec-trophoresis in 10 – 20% polyacrylamide linear gra-dient gel gave sizes of 25 and 27 kDa. Therefore, these proteins have been designated as CCP-25 and CCP-27, respectively. The difference is not surprising, since glycoproteins containing more than 10% carbohydrates, often behave anoma-lously upon gel filtration [19], and polyacrylamide gel electrophoresis [20]. In the case of gel filtration, the error is the result of greater hydration of carbohydrates in solution compared with the polypeptide chain; for SDS-PAGE, it is because of decreased binding of SDS by the glycoprotein as compared with the standard proteins. However, with increasing polyacrylamide gel cross-linking, of the two factors (charge and molecular sieving) involved in electrophoresis in SDS gels, molecular sieving predominates and the anomalously high apparent molecular weights of glycoproteins de-crease, approaching values close to their real molecular weights in an asymptotic manner [20]. It was, therefore, concluded that in the present case, the values of 25 and 27 kDa, respectively, ob-tained on 10 – 20% linear gradient gel are probably more nearly correct.
Baranwal and Verma [10] in their preliminary studies reported the partial purification of a virus inhibitor with glycoprotein characteristics from C.
cristata. Without demonstrating the homogeneity of the inhibitor, they suggested that its Mr might
range between 21 and 22 kDa. Since the determi-NN) and electron microscopy confirmed the
nation of molecular weights of glycoproteins by gel filtration as well as non-homogeneity of proteins may give erroneous results, this value may not represent the correct figure. The present findings, however, clearly show that the inhibitor might consist of two apparently related proteins, either of which can induce the resistance response. The C. cristata antiviral proteins seem to be very similar to the antiviral proteins already known from other plants, e.g.Dianthus caryophyl
-lus [21],Phytolacca americana [22], M. jalapa [23],
Bougain6illea spectabilis[24], Clerodendrum inerme
[6] and Clerodendrum aculeatum [25]. They have similar molecular weights, are glycoproteinaceous in nature, bind to CM-Sepharose, and have high activity in slightly acidic medium.
C. cristata, an ornamental plant, grows from July to January. The leaves were harvested at pre-flowering, flowering and post-flowering stages of plant growth and the purification of antiviral proteins was carried out at each stage. This was carried out for three consecutive seasons. The results consistently showed the predomination of 25 at the pre-flowering stage, while the CCP-27 concentration steadily increased during flower-ing, and at the post-flowering stage it was the predominant protein. Repeated efforts to separate the two proteins found at the flowering stage by ion exchange chromatography, gel filtration by Sephadex G-75 or Superose did not succeed. It is likely that these two proteins are isoforms and their appearance is not an artifact of the purifica-tion procedure. Such forms are reported in the case ofP. americana[22],D.caryophyllus[21], and
C. inerme [6]. In the case of pokeweed, the major form is PAP (pokeweed antiviral protein) of 29 kDa and the other form (PAP II) of 30 kDa has been observed in plants grown under summer conditions [22]. PAP II concentration was found to be highly variable from year to year. PAP II was eluted behind PAP at a higher salt concentra-tion, and contained 3 N acetylglucosamine residues. However, there are no reports suggesting that they might be produced at the same time. In
D. caryophyllus [21], the two isolated proteins Dianthin 30 and Dianthin 32 eluted at different salt concentrations, but it was suggested that Di-anthin 30 might be derived from DiDi-anthin 32 or from another protein during chromatographic purification. Similarly, inC.inerme[6] the proteins CIP-29 and CIP-34 were eluted at different salt
concentrations. However, the exact nature of CIP-34 is not clear because the protein exhibited anMr
of 100 kDa under native conditions. It also showed two other minor bands, 33 and 35 kDa, as well as the 34 kDa species. The role of the other two proteins and regulation of their production remain unclear. Therefore, the current findings may be the first indicating the variation in level of two antiviral proteins based on the developmental stage of plant. It is not clear whether there is more than one gene encoding the antiviral proteins with regulation in a stage-dependent manner.
The inhibition of antiviral activity by immediate application of AMD following the protein treat-ment on N. tabacum cv Samsun NN suggests that these proteins might be acting as inducers by altering the susceptibility of the host plant. How-ever, the antiviral property is not reversed if AMD is applied 12 h after the protein treatment. This property is quite similar to that exhibited by C.
inerme and C. aculeatum antiviral proteins, where host-mediated formation/accumulation of a new virus inhibitory protein in the treated test plants was reported [25]. The only difference is that the
C. cristata antiviral proteins do not show the systemic induction phenomenon in the test hosts (local lesion hosts), tobacco and guar. It may be interesting to study the differences in these two systems. However, this observation of reversal of inhibition following AMD application is in con-trast with the preliminary findings of Baranwal and Verma [10], where the inhibitory activity of partially purified inhibitor was not reversed fol-lowing simultaneous application of AMD to the leaf surface of N. glutinosa. It is not clear whether this is because of the lower purity of the protein in the latter case or the test host used.
Antiviral proteins have been generally found effective only in local lesion hosts. Very few of them have also been reported to be effective in systemic hosts, e.g. PAP [26]. The studies with C.
for antiviral action, but rather may help generate a signal that renders the plant resistant to virus infection [27].
Acknowledgements
The authors are grateful to Mr Sushil Kumar Sharma and Mr Dayanand for technical assis-tance, maintenance of test plants and virus cul-tures. Financial support provided by ICAR Center of Advanced Studies in Biochemistry is gratefully acknowledged.
References
[1] H.N. Verma, Varsha, V.K. Baranwal, Endogenous virus inhibitors from plants, their physical and biological properties, In: M. Chessin, D. Deborde, A. Zipf (Eds.), Antiviral Proteins in Higher Plants, CRC Press, Boca Raton, FL, 1995, pp. 1 – 21.
[2] H.N. Verma, Varsha, V.K. Baranwal, Agricultural role of endogenous antiviral substances of plant origin, In: M. Chessin, D. Deborde, A. Zipf (Eds.), Antiviral Proteins in Higher Plants, CRC Press, Boca Raton, FL, 1995, pp. 23 – 37.
[3] K. Kumar, J. Sasaki, H. Sejima, Y. Takeuchi, Y. Hayashi, Interactions between TMV, pokeweed antiviral protein, and tobacco cell wall, Phytopathology 80 (1990) 636 – 641.
[4] J.E. Kuntz, J.C. Walker, Virus inhibition by extracts of spinach, Phytopathology 37 (1947) 561 – 579.
[5] N. Habuka, K. Akiyama, M. Miyano, T. Matsumota, M. Noma, Expression and secretion ofMirabilisantiviral protein in Escherichia coli and its inhibition of in vitro eukaryotic and prokaryotic protein synthesis, J. Biol. Chem. 265 (1990) 10988 – 10992.
[6] V. Prasad, S. Srivatsava, Varsha, H.N. Verma, Two basic proteins isolated from Clerodendrum inerme
Gaertn. are inducers of systemic antiviral resistance in susceptible plants, Plant Sci. 110 (1995) 73 – 82.
[7] S. Kubo, T. Ikeda, S. Imaizumi, Y. Takanami, Y. Mikami, A potent plant virus inhibitor found in
Mirabilis jalapa L., Ann. Phytopathol. Soc. Jpn. 56 (1990) 481 – 482.
[8] J.K. Lodge, W.K. Kaniewski, N.E. Tumer, Broad-spec-trum virus resistance in transgenic plants expressing pokeweed antiviral protein, Proc. Natl. Acad. Sci. USA 90 (1993) 7089 – 7093.
[9] V.K. Baranwal, H.N. Verma, Localized resistance against virus infection by leaf extract ofCelosia cristata, Plant Pathol. 41 (1992) 633 – 638.
[10] V.K. Baranwal, H.N. Verma, Characteristics of a virus inhibitor from the leaf extract of Celosia cristata, Plant Pathol. 46 (1997) 523 – 529.
[11] D. Noordam, Identification of plant viruses. Methods and Experiments. Oxford and IBH, London, 1973. [12] M.M. Bradford, A rapid and sensitive method for the
quantitation of microgram quantities of protein utilizing the principle of protein dye binding, Anal. Biochem. 72 (1976) 248 – 254.
[13] A.I. Vogel, A Textbook of Practical Chemistry Including Qualitative Organic Analysis, 3rd ed., English Language Book Society and Longman Group Limited, London, 1971.
[14] G. Ashwell, The phenol-sulfuric acid reaction for carbo-hydrates, Methods Enzymol. 8 (1966) 93 – 95.
[15] U.K. Laemmli, Cleavage of structural proteins during assembly of the head of bacteriophage T4, Nature 227 (1970) 680 – 685.
[16] H. Blum, H. Beler, H.J. Gross, Silver staining of plant proteins, RNA and DNA in polyacrylamide gels, Elec-trophoresis 8 (1987) 33 – 39.
[17] J.M. Matthieu, R.H. Quarels, Quantitative scanning of glycoproteins on polyacrylamide gels stained with peri-odic acid-Schiff reagent (PAS), Anal. Biochem. 55 (1973) 313 – 316.
[18] D.W. Cleveland, S.G. Fischer, M.W. Kirschner, U.K. Laemmli, Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophore-sis, J. Biol. Chem. 252 (1977) 1102 – 1106.
[19] P. Andrews, Estimation of molecular size and molecular weights of biological compounds by gel filtration, Meth-ods Biochem. Anal. 18 (1970) 1 – 53.
[20] J.P. Segrest, R.L. Jackson, Molecular weight determina-tion of glycoproteins by polyacrylamide gel electrophore-sis in sodium dodecyl sulphate, Methods Enzymol. 28 (1972) 54 – 63.
[21] F. Stirpe, D.G. Williams, L.G. Onyon, R.F. Legg, Di-anthins, ribosome-damaging proteins with anti-viral properties from Dianthus caryophyllus L. (carnation), Biochem. J. 195 (1981) 399 – 405.
[22] J.D. Irvin, Antiviral proteins from Phytolacca, in: M. Chessin, D. Deborde, A. Zipf (Eds.), Antiviral Proteins in Higher Plants, CRC Press, Boca Raton, FL, 1995, pp. 65 – 91.
[23] T. Ikeda, Y. Takamani, S. Imaizumi, T. Matumoto, Y. Mikami, S. Kubo, Formation of anti-plant viral protein by Mirabilis jalapa, Plant Cell Rep. 6 (1987) 216 – 219. [24] R. Balasaraswathi, S. Sadasivam, M. Ward, J.M.
Walker, Antiviral protein fromBougain6illea spectabilis
roots; purification and characterisation, Phytochemistry 47 (1998) 1561 – 1565.
[25] H.N. Verma, S. Srivastava, Varsha, D. Kumar, Induc-tion of systemic resistance in plants against viruses by a basic protein fromC.aculeatum leaves, Phytopathology 86 (1996) 485 – 492.
[26] Z.C. Chen, R.F. White, J.F. Antoniw, Q. Lin, Effect of pokeweed antiviral protein (PAP) on the infection of plant viruses, Plant Pathol. 40 (1991) 612 – 620.
[27] S. Smirnov, V. Shulaev, N. Tumer, Expression of poke-weed antiviral protein in transgenic plants induces virus resistance in grafted wild type plants independently of salicylic acid accumulation and pathogenesis-related protein synthesis, Plant Physiol. 114 (1997) 1113 – 1121.
![Fig. 4. SDS-PAGE analysis of CCP-25 treated with differentproteases. Approximately 15 �g of CCP-25, after dissociationwith Cleveland buffer [17], were incubated with differentproteases (0.6 �g) for 15 or 30 min](https://thumb-ap.123doks.com/thumbv2/123dok/1034547.928855/6.612.36.541.511.655/analysis-treated-differentproteases-approximately-dissociationwith-cleveland-incubated-differentproteases.webp)