Purification and partial characterisation of geranylgeranyl
diphosphate synthase, from
Taxus baccata
cell cultures
An enzyme that regulates taxane biosynthesis
Gregory Laskaris *, Robert van der Heijden, Rob Verpoorte
Di6ision of Pharmacognosy,Leiden/Amsterdam Centre for Drug Research,Leiden Uni6ersity,P.O.Box9502,2300RA Leiden,The Netherlands
Received 8 September 1999; received in revised form 2 November 1999; accepted 6 November 1999
Abstract
Geranylgeranyl diphosphate (GGPP) synthase, an enzyme that regulates taxane biosynthesis, was purified to homogeneity from cell cultures ofTaxus baccata. The molecular weight of the native protein was estimated to be 7692 kDa, resulting from the association of two apparently identical subunits having a molecular weight of 38 kDa. Farnesyl diphosphate (Km 2.46mM) in
combination with isopentenyl diphosphate (Km1.5mM) was the most effective substrate. Dimethyl allyl diphosphate was a poorer
substrate (Km12.7mM). Mn2+ ion at 4 mM in combination with Mg2+ of 2 mM gave the greatest stimulation of activity. The
pI of the enzyme was lower than 4 and the pH optimum was between 6.9 and 7.2. The enzyme activity was found in the 20 000×g
(centrifugal force) pellet and a non-ionic detergent was used for its extraction. The inclusion of detergent was not necessary during subsequent chromatographic steps. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Taxus baccata; GGPP synthase; Purification
www.elsevier.com/locate/plantsci
1. Introduction
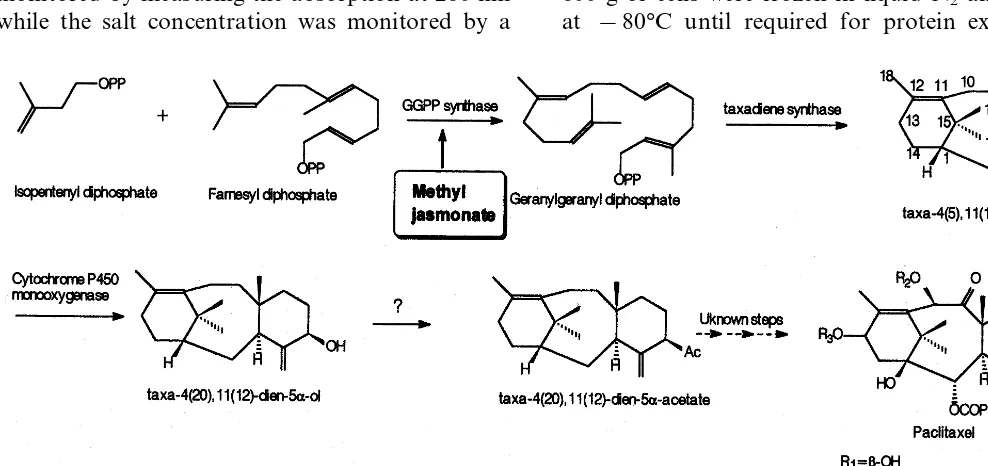
Geranylgeranyl diphosphate (GGPP) synthase (E.C. 2.5.1.29) provides the C-20 building block for various classes of compounds, i.e. diterpenes, carotenoids and chlorophylls in plants.
Paclitaxel is a diterpene originating from vari-ous Taxus species and is one of the most impor-tant anticancer agents. The efficacy of paclitaxel has urged scientists to gain insight in the pathway flux towards taxane formation in order to use this knowledge to increase taxane production by means of metabolic engineering. The three first steps of paclitaxel biosynthesis are known:
cyclisa-tion of geranylgeranyl diphosphate to taxa-4(5),11(12)-diene [1], hydroxylation to taxa-4(20),11(12)-dien-5a-ol [2] and the conversion of this alcohol to the corresponding acetate ester [3] (Fig. 1). Taxadiene synthase, the enzyme catalysing the cyclisation of GGPP, has been purified [4] and the corresponding cDNA cloned [5]. Although the low level of taxadiene synthase activity in Taxus bre6ifolia stem disks suggests a crucial role for the regulation of the pathway, this could not be confirmed by usingT. canadensiscell cultures [6]. An insight into the molecular mecha-nisms governing taxane biosynthesis is important in order to ensure an increased production of paclitaxel and to achieve biotransformations of various other taxoids (i.e. taxines) to pharmaceuti-cally important taxanes. Thus far, GGPP synthase is the sole enzyme having shown to control the rate of taxane skeleton formation [7,8] and conse-quently further characterisation seemed necessary. In this paper the purification and some properties Abbre6iations: NAA, naphthalene acetic acid; IPP, isopentenyln
pOp(isopentenyl diphosphate); FPP, farnesyln pOp(farnesyl diphos-phate); GGPP, geranylgeranyl n pOp (geranylgeranyl diphosphate). * Corresponding author. Present address: Laboratory of Pharma-cognosy, Department of Pharmacy, Aristotle University of Thessa-loniki, 540 06 ThessaThessa-loniki, Greece. Tel.: +30-31-997617, 997619; fax: +30-31-997645.
E-mail address:[email protected] (G. Laskaris)
of this key enzyme from Taxus baccata cell cul-tures are presented.
2. Methods
2.1. Materials
[1-14C] Isopentenyl diphosphate (IPP) with a
specific activity of 1.96 Gbq mmol−1 was
pur-chased from Amersham Pharmacia Biotech. Non-labelled IPP, farnesyl diphosphate (FPP) and dimethyl allyl diphosphate (DMAPP) were synthe-sised according to Davisson et al. [9]. Leupeptin hemisulfate was from ICN and PVPP from Sigma. PD-10 columns, Phenyl Sepharose 6 Fast Flow, Mono Q 10/10, Mono P 5/20 ready-made columns were purchased from Amersham Pharmacia Bio-tech, while TSK-GEL G3000 SW (0.0075×0.6 m) was from Tosohaas. A Waters HPLC system con-sisting of a 616 pump, a 486 tuneable UV detector, a 600 S system controller and a Rheodyne 7215 injection valve was used for the gel filtration (room temperature). All other purification steps were performed at 4°C with a FPLC system (Phar-macia) consisting of a LCC-500 Liquid Chro-matography Controller, two P-500 pumps and a MV-8 Motor Valve. Protein in the effluent was monitored by measuring the absorption at 280 nm while the salt concentration was monitored by a
conductivity meter. Gel electrophoresis was per-formed on a PhastSystem (Amersham Pharmacia Biotech) using microgels and protein bands were visualised by silver staining [10]. Ultrafiltration concentrator, YK-30 (30 kDa cut-off point) filters and the Microcon-30 concentrators were from Amicon.
All the chemicals were of the highest purity commercially available.
2.2. Plant material, extraction, enzyme assay
The explants were derived from the T. baccata 621 tree from Pinetum Blijdestein in Hilversum, Holland, in June 1995. Cell suspension line T. baccata C (TBC) was established in a B5M11 medium (B5 salts supplemented with 100 mg/l meso-inositol, 10 mg/l thiamine dihydrochloride, 1 mg/l pyridoxine hydrochloride, 1.86 mg/l NAA and 20 g/l sucrose) from leaves-derived callus that grew on the same medium. Cultures were main-tained under continuous light of 1000 Lux (Philips TL 40W/33 RS) on a gyratory shaker at 110 rpm and 25°C in 2 l flasks containing 500 ml of medium and they were harvested 2 days after inocculation, at the time when enzyme activity was the highest [7]. Cells were filtered over a sintered glass under vacuum and then washed with water. 800 g of cells were frozen in liquid N2 and stored
at −80°C until required for protein extraction.
The frozen cells were ground in a liquid N2chilled
Waring blender for 2×30 s at maximum speed and a fine powder was obtained. Immediately 0.15 g of polyvinylpolypyrrolidone (PVPP) and 1 ml of extraction buffer per gram of fresh weight, were added. The extraction buffer contained 50 mM Tris – HCl pH 7.6, 1% Brij-35, 2 mM DTT, 10mM leupeptin, 4 mM MnCl2, 2 mM MgCl2 and 20%
glycerol. The homogenate was mixed in a 30°C waterbath until the temperature reached 4°C and was then passed through Miracloth and cen-trifuged at 20 000×g for 60 min and the superna-tant was used for the subsequent purification steps.
The assay is based on the acid lability of the allylic diphosphates [11] and is described in detail in [7,8]. GGPP synthase activity was measured using the following incubation mixture: 50 ml of desalted protein extract, 50 mM Tris – HCl pH 7.2, 10 mM leupeptin, 4 mM MnCl2, 2 mM MgCl2,
20% glycerol and 25 mM KF in a total volume of 200 ml. Before addition of the substrates the mix-ture was preincubated for 10 min in the presence of 10 mM iodoacetamide in order to inhibit the activity of IPP isomerase. The reaction was started by addition of [1-14C]-IPP (4.86
mM final concen-tration, 55 mCi/mmol) and FPP (43.5 mM final concentration). After incubation for 30 min at 30°C the enzyme reaction was stopped by addition of 500 ml EtOH:HCl (1:1). The hydrolysis of the allylic diphosphates was allowed to proceed for 20 min and then 2 ml of toluene were added in order to extract the allylic alcohols. 1 ml of the toluene layer was removed and mixed with 10 ml of Opti-Fluor (Packard) and the radioactivity incorpora-tion was determined by liquid scintillation counting (Tri-Carb 4530, Packard). Blanks were performed with addition solely of [1-14C]-IPP,
protein extract and omitting acidic hydrolysis as well as incubation with [1-14C]-IPP and no protein
extract, showed negligible incorporation of radioactivity.
Protein concentration was determined by using the DC Protein Assay kit from Bio-Rad.
2.3. Purification of GGPP synthase
Ammonium sulphate was added in the 20 000×
g supernatant at 4°C and the activity precipitated between 40 – 60% saturation. The precipitate was resuspended in a buffer consisting of 20 mM Tris –
HCl (pH 7.4), 2 mM DTT, 15% glycerol, 4 mM MnCl2, 2 mM MgCl2 (buffer A), supplemented
with 1 M (NH4)2SO4 and it was loaded onto a
Phenyl Sepharose 6 Fast Flow column equili-brated in the same buffer. The proteins were eluted with a 6 h linear gradient ranging from 1 M to 0 M NH4SO4 of buffer A at a flow rate of 2
ml/min. Fractions of 12.5 ml were collected. Ac-tive fractions were pooled and concentrated by ultrafiltration using an Amicon YK-30 filter. This concentrate was desalted over PD-10 columns.
The concentrated active extract was repeatedly chromatographed over a Mono Q strong anion exchange column equilibrated with buffer A and the proteins were eluted at a rate of 0.4 ml/min with the following NaCl gradient: 0 – 0.1 M linear gradient over 12.5 min, isocratic at 0.1 M NaCl until 25 min and then linear to 0.25 M until 75 min. Fractions of 1 ml were collected.
Active fractions were pooled and concentrated with Microcon filters and they were repeatedly chromatographed at a flow rate of 0.5 ml/min on a Tosohaas G-3000 SW column equilibrated with 20 mM Hepes (pH 7.1), 2 mM DTT, 15% glycerol, 4 mM MnCl2 and 2 mM MgCl2 (buffer B). The
fraction size was 0.5 ml.
Active fractions were pooled, concentrated with Microcon filters and applied on a Mono P weak anion exchange column. Proteins were eluted at a rate of 0.4 ml/min with a linear gradient of 0 to 0.5 M NaCl in buffer B, in 100 min. 0.3 ml fractions were collected. The Mono P step resulted in a homogeneous GGPP synthase.
2.4. Molecular mass determination
Table 1
Purification of GGPP synthase fromTaxus baccata cell cultures
Total protein Activity Specific activity (nkat/g of protein) Yield (%)
Purification Purification (-fold)
TSK-G3000 6.9 18 158 698 6.5
0.01
Mono-P 2.3 230 000 8846 2.1
2.5. Electrophoresis
Native and SDS PAGE was performed with PhastSystem in PhastGel homogeneous medium 12.5%. Detection of proteins was with silver staining.
2.6. Enzyme kinetics
Kinetic data were fitted to various models using the EZ-FIT curve-fitting computer programme. Initial rates were measured in duplicate using the above described GGPP synthase assay at 10 differ-ent concdiffer-entrations, under conditions where the rate of product formation was linear.
The Km for FPP was calculated in the range of
0.5 to 45 mM. The IPP concentration was held constant at 25mM. The Km for IPP was measured
between 0.05 an 25 mM of IPP, while FPP’s con-centration was held constant at 45 mM.
3. Results and discussion
In order to extract GGPP synthase activity from T.baccatacells a non-ionic detergent (Brij-35) was used [7]. The inclusion of the detergent was not necessary in the subsequent chromatographic steps.
Concerning the enzyme assay, the inclusion of iodoacetamide, an inhibitor of IPP isomerase-was deemed necessary because the activity of IPP iso-merase masked the GGPP synthase activity, when using acid hydrolysis. The Mono Q purification step resulted in the separation of the two enzymes and iodoacetamide was excluded from the assay buffer thereafter. Similar problems have been re-solved in the literature by use of hydroxylapatite chromatography [13].
The recoveries of GGPP synthase activity dur-ing the purification steps are summarised in Table 1. The initial cell extract volume was 800 ml and the ammonium sulphate precipitation step was attempted in order to concentrate the protein. The 40 – 60% precipitate was dissolved in 50 ml of buffer A and then applied on a 0.03×0.4 m hydrophobic interaction chromatography column by means of a Superloop (Pharmacia) of 50 ml. The active fractions 91 – 115 (Fig. 2A) were pooled (:300 ml) and concentrated by ultrafiltrating through an Amicon YK-30 filter and then they were desalted through PD-10 columns (final vol-ume of :30 ml). Fractions of 2 ml were succes-sively chromatographed on a Mono Q column as described in Section 2.1. Active fractions (Fig. 2B) were collected, pooled and concentrated by ultrafi-ltration and by using Microcon-30 filters (final volume :3 ml). This volume was injected in fractions of 50 ml on top of a gel filtration TSK-GEL G3000 SW column (Fig. 2C). This step was carried out in room temperature and maybe this accounts for the significant loss of activity. The active fractions were pooled and inserted on top of a Mono P column without prior concentration. This step resulted in the isolation of pure GGPP synthase (Fig. 2D and Fig. 3): fractions 27 and 28 were pure while fractions 25 and 26 were pooled and after desalting they were rechromatographed on the Mono P column under the same conditions as before in order to collect additional amount of pure enzyme.
The estimation of the native molecular weight was performed on a calibrated TSK-GEL G3000 SW gel filtration column. The enzyme eluted with a Kav ratio corresponding to a molecular weight of
poly-Fig. 2. Chromatographic purification of GGPP synthase. (A) Elution profile from Phenyl Sepharose 6 Fast Flow column. Elution was carried out with a linear gradient of 1 M to 0 M (NH4)2SO4in 20 mM Tris – HCl (pH 7.4), 2 mM DTT, 15% glycerol, 4 mM
MnCl2, 2 mM MgCl2(buffer A). (B) Elution profile from Mono Q column with 0 – 0.1 M linear gradient over 12.5 min, stable
at 0.1 M NaCl until 25 min and then linear to 0.25 M until 75 min, in buffer A. (C) Elution profile from Tosohaas G-3000 SW column equilibrated with 20 mM Hepes (pH 7.1), 2 mM DTT, 15% glycerol, 4 mM MnCl2 and 2 mM MgCl2(buffer B). (D)
Elution profile from Mono P column. The column was eluted with a linear gradient of 0 to 0.5 M NaCl in buffer B.
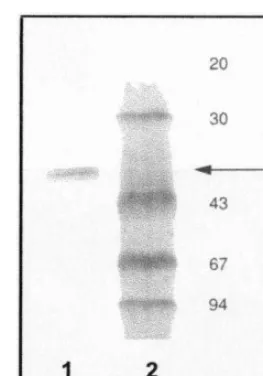
acrylamide microgels, and revealed a band of ap-proximately 38 kDa (Fig. 4). From these data we conclude that native GGPP synthase fromT. bac-cata is a homodimeric enzyme.
The pH-activity profile showed an optimum be-tween pH 6.9 and 7.2 (data not shown). The enzyme like all prenyltransferases showed a pro-nounced metal dependency on Mn2+ and Mg2+. When used individually Mn2+ was the most effec-tive cofactor while Zn2+ was less effective in enhancing the enzyme activity (Fig. 5). The best combination of divalent cations was Mn2+ at 4 mM together with Mg2+ at 2 mM.
GGPP synthase was totally devoid of IPP iso-merase activity and catalysed the synthesis of GGPP using IPP (Km=1.5 mM) as homoallylic
donor and FPP (Km 2.46 mM) or less efficiently
DMAPP (Km12.7mM) as allylic donors. The pI of
the enzyme was lower than 4. This was deduced
Fig. 4. SDS PAGE of Mono P purified GGPP synthase. Lane 1. Mono P-purified GGPP synthase. Lane 2. Low molecular weight markers (kDa) values are indicated on the right-hand side of the figure. Gel electrophoresis was performed with the PhastSystem (Pharmacia): a 12.5% homogeneous PhastGel with SDS bufferstrips was used. Protein visualisation was obtained by silver staining.
Although not conclusively shown, the enzyme seems to be membrane associated. Brij-35 or Tri-ton-X-100 do not increase the enzyme’s activity, so the use of the detergent in the extraction buffer in order to extract activity seems to reflect a genuine behaviour of this enzyme. The inclusion of the detergent was not necessary in the subsequent chromatographic steps. This behaviour is typical of peripheral (membrane associated) enzymes. In the case of Taxus this phenomenon seems to be restricted only in T. baccata since enzyme activity was easily extracted without inclusion of detergent in the closely related species T. canadensis. A similar case has been reported inGinkgo bilobacell cultures [12] but since no further purification steps were performed in that case, no speculation about the nature of this enzyme can be done.
GGPP synthase is considered to be located in the stroma of plastids [14] and this is reflected in its increased activity at higher pH [11,13]. This is not the case in T. baccatawhere the pH optimum is encountered in the neutral pH area. Further-more the very low pI value (lower than 4) is a unique characteristic of GGPP synthase of T. baccata. These findings, together with the use of detergent, suggest an enzyme localisation different than the plastidial stroma.
Divalent metal ions are typically required as cofactors in prenyltransferase reactions: GGPP synthase from Capsicum annuum [14],Sinapis alba [15], Ricinus communis [13], rat liver [16], and bovine brain [17] show a preference towards Mg2+ while Mn2+ was more effective than Mg2+ in the case of pumpkin [18], carrot root [19], pig liver [20] and T. baccata. Concerning substrate specificity the T. baccataenzyme shows a marked preference towards IPP and FPP while the Kmfor DMAPP is
higher but not so high to be considered practically unusable, as it is the case with Ricinus, or so low to be considered as the favourite substrate, like Capsicum (Km 0.95 mM). The enzyme’s preference
for FPP over DMAPP was confirmed by using the Vmax/Km ratio which is a measure of the catalytic
efficiency. Thus the Vmax/Km for IPP is 0.00245
s−1, for FPP 0.001 s−1 and for DMAPP 0.00016
s−1. We conclude that GGPP synthase uses FPP 6
times more efficiently than DMAPP. This together with the low pH optimum plead for an extraplas-tid localisation of the enzyme, considering that free FPP seems to be absent from this organelle and that the stroma pH is alkaline. A related question concerns the presence of different
isoen-Fig. 5. Metal ion dependency of GGPP synthase activity. Enzyme activity values are shown as percentage of the highest activity.
during purification attempts by chromatofocusing on a Mono P column, when the enzyme failed to elute normally with gradient between 4 and 9. It was eluted after the end of the run, only when 1 M NaCl was included in the eluent.
zymes of GGPP synthase inT. baccata. No isoen-zymes were observed during the purification pro-cess and this could reflect the developmental stage of the cells. The cells that were used for the purification were 2 days old and since at the second day of the culture the taxane biosynthesis is vigorous [7,8] the isolated GGPP synthase should be the one that is responsible for taxane biosynthesis. In this respect any other isoenzymes that could occur later in the cell’s life stage can be considered auxiliary or even competitive and it would be interesting to have a measure of the concentration of other cell’s substances that com-pete with taxanes for the use of GGPP (i.e. carotenoids). We note here that as many as six GGPP synthase genes have been isolated from Arabidopsis thaliana (GGPS1 – 6) [25 – 29] and one of these is of mitochondrial origin [29]. These findings call for a more extensive study of the localisation of the enzyme in T. baccata and it is
hoped that the purification which is presented in this paper will shed some light towards this direction.
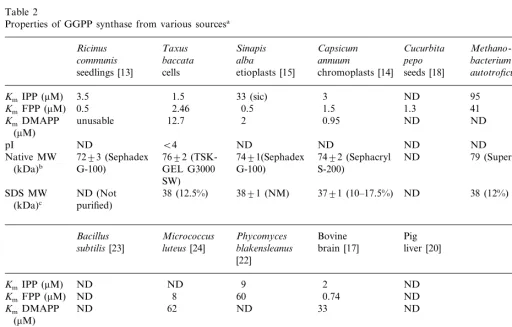
The molecular weight and the homodimeric na-ture of the enzyme is in full accordance with all GGPP synthases isolated from plant sources like Sinapis alba etioplasts, Capsicum chromoplasts andRicinus communis seedlings as well as from the Methanobacterium thermoautotrophicum [21]. The enzyme isolated from Phycomyces blakesleanus [22] is a homodimer of 60 kDa. Native GGPP synthase from animal sources has higher molecu-lar weight but it seems to be comprised of monomers of similar weight (i.e. GGPP synthase from bovine brain seems to be a tetramer of a 37.5 kDa unit). Km values, pI and molecular weight of
GGPP synthases from various sources are pre-sented in Table 2.
As previously mentioned GGPP synthase seems to regulate the carbon flux towards taxane
forma-Table 2
Properties of GGPP synthase from various sourcesa
Ricinus Taxus Sinapis Capsicum Cucurbita Methano
-annuum baccata
communis alba pepo bacterium thermo
-seeds [18] autotroficum [21] etioplasts [15] chromoplasts [14]
seedlings [13] cells
(kDa)b GEL G3000 G-100)
SW)
luteus[24] liver [20]
subtilis [23]
85 (gel filtration) 60 (Sephadex G-Native MW 70(Sephadex
G-100) 100) hadex G-200)
(kDa)b
ND (partially
SDS MW ND (partially 30 (10%) 37.5 (NM) ND (partially purified) purified)
(kDa)c purified)
aND: not determined, NM: not mentioned.
bThe method used for the determination of the MW of the native form of the enzyme is in parentheses.
tion in T. baccata. In this regard the isolation of the native form of the enzyme will lead to the development of antibodies for localisation studies and via the determination of its amino acid se-quence to the cloning of the gene in order to shed light to the regulation of its expression in molecu-lar level.
Acknowledgements
Gregory Laskaris is grateful to the Committee of the European Union for financial support through an individual fellowship (BIO4-CT975015) of the Biotechnology program.
References
[1] A.E. Koepp, M. Hezari, J. Zajicek, B. Stofer Vogel, R.E. Lafever, N.G. Lewis, R. Croteau, Cyclization of ger-anylgeranyl diphosphate to taxa-4(5),11(12)-diene is the committed step of taxol biosynthesis in Pacific yew, J. Biol. Chem. 270 (1995) 8686 – 8690.
[2] J. Hefner, S.M. Rubenstein, R.E.B. Ketchum, D.M. Gibson, R.M. Williams, R. Croteau, Cytochrome P450-catalyzed hydroxylation of taxa-4(5),11(12)-diene to taxa-4(20),11(12)-dien-5a-ol: the first oxygenation step in
taxol biosynthesis, Chem. Biol. 3 (1996) 479 – 489. [3] M. Hezari, R. Croteau, Taxol biosynthesis: an update,
Planta Med. 63 (1997) 291 – 295.
[4] M. Hezari, N.G. Lewis, R. Croteau, Purification and characterization of taxa-4(5),11(12)-diene synthase from Pacific yew (Taxus bre6ifolia) that catalyzes the first
committed step of taxol biosynthesis, Arch. Biochem. Biophys. 322 (1995) 437 – 444.
[5] M.R. Wildung, R. Croteau, A cDNA clone for taxadiene synthase, the diterpenecyclase that catalyzes the commit-ted step of taxol biosynthesis, J. Biol. Chem. 271 (1996) 9201 – 9204.
[6] M. Hezari, R.E.B. Ketchum, D.M. Gibson, R. Croteau, Taxol production and taxadiene synthase activity in
Taxus canadensis cell suspension cultures, Arch. Biochem. Biophys. 337 (1997) 185 – 190.
[7] G. Laskaris, C.F. de Jong, M. Jaziri, R. van der Heijden, G. Theodoridis, R. Verpoorte, Geranylgeranyl diphos-phate synthase activity and taxane production inTaxus baccata cells, Phytochemistry 50 (1999) 939 – 946. [8] G. Laskaris, M. Bounkhay, G. Theodoridis, R. van der
Heijden, R. Verpoorte, M. Jaziri, Induction of ger-anylgeranyl diphosphate synthase activity and taxane accumulation in Taxus baccata cell cultures after elicita-tion by methyl jasmonate, Plant Sci. 147 (1999) 1 – 8. [9] V.J. Davisson, A.B. Woodside, C.D. Poulter, Synthesis
of allylic and homoallylic isoprenoid pyrophosphates, Methods Enzymol. 110 (1985) 130 – 144.
[10] L.G. Davies, M.D. Dibner, J.F. Battey, Silver staining of gels for proteins or RNA, In: Basic Methods in Molecu-lar Biology, 1986, Elsevier, New York, pp. 315 – 317. [11] D.R. Threlfall, I.M. Whitehead, In: Molecular plant
pathology, a practical approach, vol 2, 1992, IRL Press, pp. 91 – 92.
[12] D.J. Carrier, J. Archambault, R. van der Heijden, R. Verpoorte, Formation of terpenoid products in Ginkgo biloba L. cultivated cells, Plant Cell Rep. 15 (1996) 88 – 94.
[13] M.W. Dudley, T.R. Green, C.H. West, Biosynthesis of the macrocyclic diterpene casbene in castor bean (Ricinus communis L.) seedlings, Plant Physiol. 81 (1986) 343 – 348.
[14] O. Dogbo, B. Camara, Purification of IPP isomerase and GGPP synthase fromCapsicumchromoplasts by affinity chromatography, Biochim. Biophys. Acta 920 (1987) 140 – 148.
[15] A. Laferriere, P. Beyer, Purification of geranylgeranyl diphosphate synthase from Sinapis alba etioplasts, Biochim. Biophys. Acta 1077 (1991) 167 – 172.
[16] H. Sagami, S. Matsuoka, K. Ogura, Formation of Z,E,E-GGPP by rat liver microsomes, J. Biol. Chem. 266 (1991) 3458 – 3463.
[17] H. Sagami, Y. Morita, K. Ogura, Purification and prop-erties of GGPP synthase from bovine brain, J. Biol. Chem. 269 (1994) 20561 – 20566.
[18] K. Ogura, T. Nishino, T. Shinka, S. Seto, Prenyltrans-ferases of Pumpkin fruit, Methods Enzymol. 110, (1985) Ch. 19.
[19] D.L. Nandi, J.W. Porter, The enzymatic synthesis of GGPP by enzymes of carrot root and pig liver, Arch. Biochem. Biophys. 105 (1964) 7 – 19.
[20] H. Sagami, K. Ishii, K. Ogura, GGPP synthetase of pig liver, Methods Enzymol. 110 (1985) Ch. 21.
[21] A. Chen, C.D. Poultier, Purification and characterisation of FPP/GGPP synthase, a thermostable bifunctional en-zyme from Methanobacterium thermoautotrophicum, J. Biol. Chem. 268 (1993) 11002 – 11007.
[22] F.L. Brinkhaus, H.C. Rilling, Purification of GGPP syn-thase from Phycomyces blakesleanus, Arch. Biochem. Biophys. 266 (1988) 607 – 612.
[23] I. Takahashi, K. Ogura, Prenyltransferases of Bacillus subtilis: undecaprenyl pyrophosphate synthetase and ger-anylgeranylpyrophosphate synthetase, J. Biochem. 92 (1982) 1527 – 1537.
[24] H. Sagami, K. Ogura, Geranyl pyrophosphate syn-thetase-geranylgeranyl pyrophosphate synthetase from
Micrococcus luteus, Methods Enzymol. 110 (1985) 188 – 192.
[25] P.A. Scolnik, G.E. Bartley, Two more members of an
Arabidopsis geranylgeranylpyrophosphate synthase gene family, Plant Physiol. 110 (1996) 1435 – 1440.
[26] P.A. Scolnik, G.E. Bartley, Nucleotide sequence of an
Arabidopsis cDNA for geranylgeranyl pyrophosphate synthase, Plant Physiol. 104 (1994) 1469 – 1470.
[27] P.A. Scolnik, G.E. Bartley, Nucleotide sequence of a putative geranylgeranyl pyrophosphate synthase from
[28] X.F. Zhu, K. Suzuki, K. Okada, K. Tanaka, T. Naka-gawa, M. Kawamukai, K. Matsuda, Cloning and func-tional espression of a novel geranylgeranyl pyrophos-phate synthase from Arabidopsis thaliana in Escherichia coli, Plant Cell Physiol. 38 (1997) 357 – 361.
[29] X.F. Zhu, K. Suzuki, T. Saito, K. Okada, K. Tanaka, T. Nakagawa, H. Matsuda, M. Kawamukai, Geranylger-anyl pyrophosphate synthase encoded by the newly iso-lated gene GGPS6 fromArabidopsis thalianais localized in mitochondria, Plant Mol. Biol. 35 (1997) 331 – 341.