Synthesis and properties of water-resistant
poly(glucaramides)
Geoffrey A.R. Nobes *, William J. Orts, Gregory M. Glenn
United States Department of Agriculture,Agricultural Research Ser6ice,Western Regional Research Center, 800 Buchanan Street,Albany,CA 94710, USA
Received 8 August 1999; accepted 26 March 2000
Abstract
Replacing nonrenewable polymers with renewable ones is desirable for packaging applications. For this purpose, a number of poly(glucaramides) were synthesized and tested for applications requiring water resistance. The polymers were prepared from the polycondensation of esterified glucaric acid and a diamine, the former being obtained from the oxidation of glucose, thus providing a renewable source for the monomer. Poly(glucaramides) were prepared with
up to a 74% yield. The polymers were characterized by differential scanning calorimetry (DSC),1H and13C nuclear
magnetic resonance (NMR) spectroscopy, X-ray diffraction and gel permeation chromatography (GPC). The poly(glucaramides) were tested for their usefulness by incorporating them into pressed fiber-reinforced composite panels. Two poly(glucaramides) were chosen for further testing. These imparted a detectable degree of water resistance to the panels. Published by Elsevier Science B.V.
Keywords:Fiber-reinforced panels; Mechanical properties; Poly(glucaramides); Water resistance
www.elsevier.com/locate/indcrop
1. Introduction
Commodity polymers derived from nonrenew-able resources such as fossil fuels are extremely widespread in modern life, being used in numer-ous plastics applications. However, despite their inherent usefulness, they present a major environ-mental problem since typical disposal involves deposition into the largely anaerobic conditions of landfill. The consequence is that plastic products
may languish in the environment for many years. Because commodity plastics are derived from petroleum feedstocks, alternative renewable re-sources derived from plant material are being developed.
Starch is an abundant biopolymer, which repre-sents one type of renewable resource from which plastics can be produced (Galliard and Bowler, 1987; Koch and Ro¨per, 1988; Zobel, 1988; Mad-dever and Chapman, 1989; Ro¨per and Koch, 1990; Doane, 1992; Kim and Pometto, 1994; Glenn and Hsu, 1997; Ellis et al., 1998). Starch can also be foamed to make products with low density and good insulating properties (Glenn and * Corresponding author. Tel.: +1-510-5595628; fax: +
1-510-5595936.
E-mail address:[email protected] (G.A.R. Nobes)
Irving, 1995; Glenn et al., 1996), but they do not have good water resistance. Therefore, a water-re-sistant coating must be applied to the starch-based product in order to impart water resistivity to it. The purpose of the present study was to incorporate a water-insoluble polymer derived from renewable resources in the formulation of starch-based plastic products in order to avoid the need for a coating.
Poly(glucaramides) or hydroxylated nylons are one class of water-insoluble polymer derived from renewable resources. These polymers are struc-turally analogous to nylons in that they are com-posed of a diacid component and a diamine component. With poly(glucaramides), the diacid component is glucaric acid, a six-carbon diacid with hydroxyl groups at positions 2, 3, 4 and 5, hence the term hydroxylated nylon. Glucaric acid is a product of the oxidation of glucose. The chemistry of poly(glucaramides) was developed by Kiely and coworkers beginning in the late 1980s (Kiely and Lin, 1989; Kiely and Chen, 1994; Kiely et al., 1994; Chen and Kiely, 1996). The synthesis of poly(glucaramides) involves a reaction se-quence based on unprotected esterified D-glucaric acid by simple condensation reactions. This sys-tem is efficient and allows for a great deal of versatility in polymer composition because of the large variety of diamines that can be used.
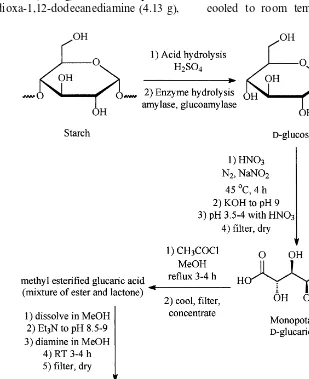
The first step in the production of poly(glu-caramides) is the hydrolysis of starch by either chemical (i.e. acid) or enzymatic methods (or a combined approach) to glucose (see Fig. 1). In the second step, the glucose is oxidized to form glu-caric acid. Following a direct esterification of glucaric acid, it can be reacted with a multifunc-tional amine, usually a diamine, to form the poly(glucaramide) (see Fig. 1). The polymeriza-tion reacpolymeriza-tion occurs at room temperature and no specialized reaction conditions, such as an inert atmosphere, are needed. The polymer is isolated by filtration.
In this study, semicrystalline poly(glu-caramides) were prepared and were processed with various agricultural fibers to form composite panels.
2. Materials and methods
2.1. Synthesis of D-glucaric acid
D-glucaric acid was prepared according to a method modified from those of Mehltretter (1963) and Kiely et al. (1997). D-glucose (25.0 g; Sigma, St. Louis, MO) and 26.4 ml of concentrated HNO3 (Trace Metal Grade; 70% w/w; Fisher,
Pittsburgh, PA) were placed in a 500 ml round bottom flask. Bubbling N2 gas was delivered to
the mixture by way of a 10 ml pipette. The temperature of the stirred mixture was raised to 45°C. After the glucose dissolved, the flask was transferred to an ice bath and a few crystals of NaNO2 (Mallinckrodt, Paris, KY) were added to
initiate the oxidation. The temperature of the mixture immediately began to increase and a large volume of brown gas was produced. The mixture temperature was controlled with N2 bubbling so
that it did not exceed 60°C. After the temperature decreased to approximately 40°C, the flask was transferred to a 45°C water bath for approxi-mately 4 h and the N2 bubbling was continued.
After the production of brown gas subsided, an additional 8.9 ml of concentrated HNO3 was
added and the flask was heated as before, at 45°C with N2, for an additional 4 h. At this point, the
oxidation was deemed complete. The mixture was transferred to a 400 ml beaker in an ice bath and the pH was adjusted to 9.0 with 8 M KOH (Baker, Phillipsburg, NJ). Following cooling to room temperature, the solution was diluted with distilled water and passed through a column (4× 40 cm) containing an ion retardation resin (Bio-Rad AG®
11 A8; Bio – Rad, Hercules, CA). Fractions of approximately 200 ml were collected from the column. The fractions of the product were then combined together and concentrated by rotary evaporation to produce a viscous syrup. The syrup was transferred to a 400 ml beaker in an ice bath and the pH was adjusted to between 3.5 and 4.0 with concentrated HNO3 or until the
2.2. Synthesis of poly(glucaramides)
A series of ten diamine compounds were used to synthesize the poly(glucaramides) in this study. In all cases, the diamine was added to the es-terified glucaric acid as a 1.14 M methanol solu-tion. The diamines (Aldrich) used are listed with the masses used of each in parentheses: 1,4-di-aminobutane (1.78 g), 1,6-diaminohexane (2.35 g), 1,8-diaminooctane (2.91 g), 1,10-diaminodecane (3.48 g), 1,12-diaminododecane (4.05 g), 2-methyl-1,5-pentanediamine (2.35 g), 1,3-diaminopentane (2.06 g), 4,9-dioxa-1,12-dodecanediamine (4.13 g),
4,7,10-trioxa-1,13-tridecanediamine (4.45 g), and m-xylylenediamine (2.75 g).
Poly(glucaramides) were all synthesized by a direct esterification method adapted from Kiely et al. (1994). Acetyl chloride (3.0 ml; Aldrich, Mil-waukee, WI) was added dropwise with stirring to 25 ml of methanol (HPLC grade; Fisher) in a 250 ml round bottom flask cooled in an ice bath. Then, 5 g of monopotassium glucaric acid (20.1 mmol) was added to the mixture. The round bottom flask was transferred to an oil bath and the mixture was refluxed for 3 h. The mixture was cooled to room temperature and filtered to
Table 1
Properties of synthesized poly(glucaramides)
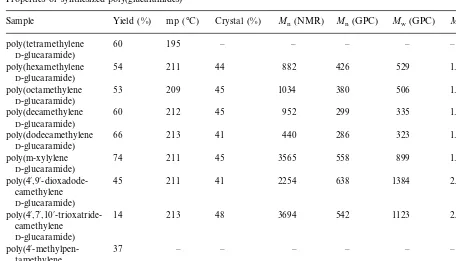
Sample Yield (%) mp (°C) Crystal (%) Mn(NMR) Mn(GPC) Mw (GPC) Mw/Mn(GPC)
poly(tetramethylene 60 195 – – – – –
D-glucaramide)
54 211 44
poly(hexamethylene 882 426 529 1.2
D-glucaramide)
poly(octamethylene 53 209 45 1034 380 506 1.3
D-glucaramide)
60 212 45
poly(decamethylene 952 299 335 1.1
D-glucaramide)
66 213 41
poly(dodecamethylene 440 286 323 1.1
D-glucaramide)
74 211 45
poly(m-xylylene 3565 558 899 1.6
D-glucaramide)
2.2
45 211 41
poly(4%,9%-dioxadode- 2254 638 1384
camethylene
D-glucaramide)
2.1
14 213
poly(4%,7%,10%-trioxatride- 48 3694 542 1123
camethylene
D-glucaramide)
37 – – – – –
poly(4%-methylpen- –
tamethylene
D-glucaramide)
37 – – – – – –
poly(1%-ethyltrimethylene
D-glucaramide)
move KCl. The filtrate was concentrated by ro-tary evaporation at 65°C to a viscous syrup. The syrup was then dissolved in 20 ml of methanol. Triethylamine (Aldrich) was added until the pH was between 8.5 and 9.0. A methanol solution of the diamine (20.2 mmol) was added and the entire mixture stirred at room temperature for 3 – 4 h. The polymer typically began to precipitate within 10 – 20 min. After 3 – 4 h, the reaction mixture was filtered to isolate the polymer. The polymer was washed three times with 10 ml methanol and three times with 10 ml acetone. The poly(glucaramide) was dried in vacuo at 65°C for 12 h. Melting points (determined by Differential Scanning Calorimetry (DSC)), molecular weights (deter-mined by Nuclear Magnetic Resonance (NMR) spectroscopy and Gel Permeation Chromatogra-phy(GPC)), and crystallinity (determined from the X-ray powder patterns) of the polymers are listed in Table 1.
The structures of the poly(glucaramides) were confirmed with NMR spectroscopy. Individual
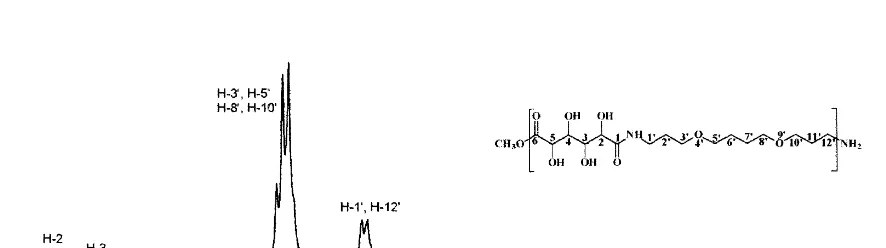
results for the polymers are: Poly(hexamethylene D-glucaramide):1H NMR (DMSO)d3.98 (d, 1H,
H-2), 3.88 (broad s, 1H, H-3), 3.69 (dd, 1H, H-4), 3.93 (dd, 1H, H-5), 3.07 (broad d, 4H, H-1% and H-6%), 1.40 (broad s, 4H, H-2% and H-5%), 1.25 (broad s, 4H, H-3% and H-4%). Poly(octamethylene D-glucaramide):1H NMR (DMSO)d3.98 (d, 1H,
H-2), 3.87 (t, 1H, H-3), 3.68 (dd, 1H, H-4), 3.93 (dd, 1H, H-5), 3.07 (broad d, 4H, H-1%and H-8%),
1.40 (broad s, 4H, H-2% and H-7%), 1.24 (s, 8H,
H-3%, H-4%, H-5% and H-6%). Poly(decamethylene
D-glucaramide):1H NMR (DMSO)d3.97 (d, 1H,
H-2), 3.86 (broad s, 1H, H-3), 3.68 (dd, 1H, H-4), 3.92 (broad d, 1H, H- 5), 3.07 (m, 4H, H-1% and
H-10%), 1.40 (broad s, 4H, H-2%and H-9%), 1.24 (s,
12H, H-3%, H-4%, H-5%, H-6%, H-7%and H-8%). Poly(-dodecamethylene D-glucaramide): 1H NMR
D-glu-caramide): 1H NMR (DMSO) d 4.12 (d, 1H,
H-2), 3.92 (s, 1H, H-3), 3.80 (dd, 1H, H-4), 3.99 (d, 1H, H-5), 7.30 (s, 1H, Ar-H), 7.21 (d, 2H, Ar-H), 7.16 (s, 1H, Ar-H), 4.30 (dd, 4H, CH2-Ar).
Poly(4%,9%-dioxadodecamethylene D-glucaramide):
1H NMR (DMSO) d 3.98 (d, 1H, H-2), 3.87 (t,
1H, H-3), 3.69 (dd, 1H, H-4), 3.92 (d, 1H, H-5), 3.36 (d, 8H, H-3%, H-5%, H-8% and H-10%), 3.14 (d,
4H, H-1%and H-12%), 1.65 (t, 4H, H-2%and H-11%),
1.52 (s, 4H, H-6% and H-7%). Poly(4%,7%,10%
-trioxa-tridecamethylene D-glucaramide): 1
H NMR (DMSO) d 3.99 (d, 1H, H-2), 3.88 (t, 1H, H-3), 3.70 (dd, 1H, H-4), 3.92 (d, 1H, H-5), 3.51 (dd, 8H, H-5%, H-6%, H-8% and H-9%), 3.41 (t, 4H, H-3%
and H-11%), 3.14 (d, 4H, H-1% and H-13%), 1.65 (t, 4H, H-2% and H-12%).
2.3. General analytical methods
Nuclear magnetic resonance (NMR) spectra of the poly(glucaramides) were recorded at 298 K from samples in dimethyl sulfoxide (DMSO) with tetramethylsilane (TMS) as an internal standard on a Bruker ARX400 (Billerica, MA) spectrome-ter operating at 400.14 MHZ for 1
H and 100.62
MHZ for 13C. Single pulse experiments were run
for both nuclei. A 30° pulse at a 2.3 s repetition rate was used for the carbon spectra, and a 90° pulse at a 7 – 8 s repetition rate was used for the proton spectra. The structures of the polymer products determined by NMR spectroscopy were in agreement with previously published references (Kiely et al., 1994; Chen and Kiely, 1996). NMR was also used to estimate the molecular weight of the polymers obtained. Representative proton spectra are shown for poly(hexamethylene D-glu-caramide) and poly(4%,9%-dioxadodecamethylene
D-glucaramide) in Fig. 3 and Fig. 4, respectively. Differential scanning calorimetry (DSC) profi-les of the polymers were measured using a TA Instruments DSC 2910 modulated DSC (New Castle, DE). In modulated DSC a sinusoidal os-cillation is overlaid on the linear heating ramp to yield a heating profile in which the average sample temperature increases with time, but not in a linear fashion. The effects of a more complex heating profile are as if multiple experiments were determined simultaneously on the same sample; one at a linear heating rate and one at an
Fig. 3.1H NMR Spectrum of poly(hexamethylene
D-glucaramide)
Fig. 4.1H NMR Spectrum of poly(4%,9%-dioxadodecamethyleneD-glucaramide)
taneous heating rate. The DSC profiles were run from room temperature to 300°C at a heating rate of 5°C/min. All DSC scans were featureless from room temperature to the melting points near 210°C.
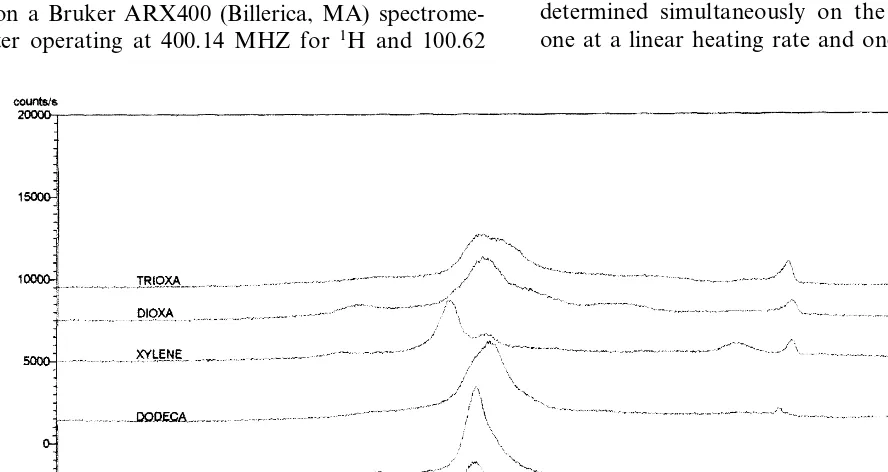
X-ray powder patterns (Fig. 2) were measured on a Philips X’Pert diffractometer (Mahwah, NJ). Sample scans were run in continuous mode from 4.0 to 34.0° at a scan speed of 0.02°/s. A fixed 15° primary mask was used along with a fixed 0.5° divergence slit. Crystallinity was determined by an integration method where the percent crystallinity was taken as the area of the diffractogram due to the crystalline content divided by the total area under the diffractogram (both crystalline and amorphous content).
Molecular weights of the synthesized polymers were determined using NMR and gel permeation chromatography (GPC). For NMR analysis the method of Shit and Maiti (1986) was used wherein the molecular weight is determined based on the integration of the1H NMR spectrum. The
integration for the end group protons of the poly-mer is compared to the integration for a known set of protons in the backbone of the polymer in order to calculate the average degree of polymer-ization, and thusMn. For GPC analysis, aliquots
2.4. Formation of pressed test panels
Fiber reinforced test panels were fabricated us-ing poly(glucaramides) as a water-insoluble binder with poly(vinyl alcohol), which is a water soluble polymer chosen to highlight the water resistance of poly(glucaramides) and cellulose fiber. The panels (125×32×2 mm) were prepared by press-ing the components in a mold on a laboratory Carver Press (Model C; Carver Inc., Wabash, IN). Panels contained 0 – 66% poly(vinyl alcohol) (Airvol 203S; Air Products, Allentown, PA), 0 – 66% poly(glucaramide) and 33% a-cellulose (Sigma), which had previously been ground in a Wiley mill (c20 mesh). The various mixtures were dry-mixed by passing them through a Wiley mill (c10 mesh) prior to introduction into the mold. The panels were pressed at 210°C and 34474 kPa (5000 psi) for 1 min. The mold was removed from the press and allowed to cool for 2 min before removing the panel. All of the poly(glucaramide) fabricated panels were tested for quality including visual appearance, presence of voids and uniformity. The best candidates were selected for further testing. These were the poly-(octamethylene D-glucaramide) and poly(de-camethyleneD-glucaramide) panels. Six panels of each of the composition listed in Table 2 were
prepared and tested for mechanical properties and water absorption.
2.5. Mechanical testing of pressed test panels
Panels were tested mechanically in an Instron universal testing machine (Model 4500; Canton, MA) using Series IX Automated Materials Test-ing System version 7.50. The panels were tested using a standard plastics tensile test derived from ASTM method D638M-91a (Annual Book of ASTM Standards, 1992). The crosshead speed was 25.0 mm/min with a 1.0 kN load. The tensile strength and Young’s modulus were calculated by the computer software. The tensile strength was calculated as the maximum tensile stress reached by the sample during the tensile test. Young’s modulus was taken as the initial slope of the stress – strain curve.
2.6. Water absorption test
The water absorption test to determine the water resistance of the pressed panels was based on ASTM D 570-95 (Annual Book of ASTM Standards, 1996) entitled Standard Test Method for Water Absorption of Plastics. The specimens were cut to size (36×32 mm) using a utility knife. Each specimen was weighed to the nearest 0.0001 g and its dimensions (width, length and thickness) were measured. The specimens were then condi-tioned for 16 h at 50°C. They were weighed (conditioned weight) and immersed in distilled water for 24 h at room temperature (2392°C). After 24 h, the specimens were removed from the water, the surface water wiped off with a dry cloth and weighed (wet weight). The physical state of specimens after immersion was also noted. After weighing, the specimens were reconditioned by drying for 24 h at 50°C, and reweighed (recon-ditioned weight). This test provides two useful values. The first is the percentage increase in weight during immersion:
Increase in weight,
%=[(wet weight−conditioned weight) /conditioned weight]×100
The second is the percent soluble matter lost during immersion:
Table 2
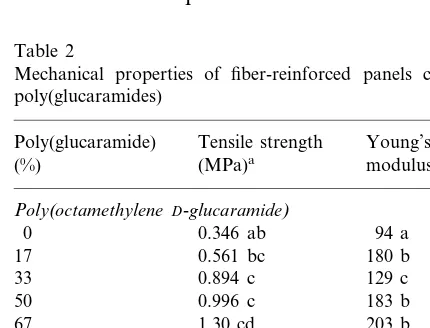
Mechanical properties of fiber-reinforced panels containing poly(glucaramides)
Tensile strength
Poly(glucaramide) Young’s
(%) (MPa)a modulus (MPa)
Poly(octamethyleneD-glucaramide)
94 a
Poly(decamethyleneD-glucaramide)
0 0.346 a 94 a
aWithin columns, means followed by the same letter do not
Soluble matter lost, %=[(conditioned weight− reconditioned weight)/conditioned weight]×100
In addition to panels containing no poly(glu-caramide), two additional controls were used in this test. One was a pressed panel containing only the poly(vinyl alcohol) (Airvol 203S) used in the pressed test panels. The other sample was a stan-dard fiber-reinforced test panel containing 24% soft wood fiber, 24% hard wood fiber, 19%a -cel-lulose and 33% filler (other agricultural fibers). This panel is typical of agricultural fiber-based composite panels that need to be coated with water-resistant materials.
3. Results and discussion
3.1. Synthesis of poly(glucaramides)
The results of the polymerization are summa-rized in Table 1. The yields were generally in the range of 45 to 75%. The one exception was the trioxa polymer, poly(4%,7%,10%
-trioxatride-camethylene D-glucaramide) where the yield was only 14%. The melting points of the resultant polymers were typically between 209 and 213°C. The only exception was poly(tetramethylene D-glucaramide) at 195°C in which the short chain length of the diamine component contributes to a lower melting point. X-ray powder patterns (Fig. 2) showed that the synthesized poly(glucaramides) were semicrystalline polymers with between 40 and 50% crystallinity.
The synthesis of the poly(glucaramides) has been found to be simple, efficient and versatile. The initial step in the path to poly(glucaramides) is the production ofD-glucaric acid obtained from D-glucose. This reaction occurs readily by oxida-tion of D-glucose with concentrated nitric acid. This step limits the overall efficiency of the entire production of poly(glucaramides) from glucose to polymer. The reason for this is the temperature increase during the oxidation. If the temperature increases beyond 60°C, the glucose decomposes rather than being oxidized. The use of N2
bub-bling (Kiely et al., 1997) in the system prevents the drastic increase in temperature. In addition,
we found that placing the reaction flask in an ice bath during the initial stage of the oxidation further reduced the extent of glucose degradation. After stabilization of the mixture, the reaction was returned to the 45°C water bath for the duration of the oxidation. D-glucaric acid was obtained in 55% yield from the reaction. Interest-ingly, when the oxidation was scaled to 50 and 300 g of glucose, the yield increased to 60 and 79% respectively. Thus, it seems from this study that larger batches are feasible, an important fac-tor in the commercialization of this technology.
The polymerization reaction is likewise facile and proceeded without complication. The poly-merization also demonstrates the versatility of the synthesis. Virtually any diamine could be used to produce a poly(glucaramide) depending on the desired properties of the final polymer. In the present study, the nonlinear diamines, 2-methyl-1,5-pentanediamine and 1,3-diaminopentane, did not yield solid polymers. While these products may be useful for some applications, they did not suit the needs of the present project, which was based on mixing solid polymers and pressing them into panels. As a result, these polymers were not fully characterized. Also, compounds with multi-ple primary amino groups could be used in the polymerization, assuming proper stoichiometric control of both monomers. For example, the use of a triamine should enable a network polymer structure to be formed.
tri-ethylamine in the reaction ensured that the poly-merization environment was sufficiently alkaline to continue lactonization throughout the polymer-ization. Other studies (Kiely and Chen, 1994; Chen and Kiely, 1996) were specifically directed toward the synthesis of lactones of esterified glu-caric acid in order to increase polymerization efficiency. Our goal was to keep the system as simple as possible with for potential commercial applications. From the results of the polymeriza-tion, it is evident that the triethylamine was suffi-cient to promote the formation of glucaric acid lactones to produce adequate poly(glucaramides) with desirable properties.
One of the objectives of this project was to investigate the commercial applicability of poly(glucaramide) production from starch as a renewable source for the glucaric acid monomer. The conversion of starch to glucose is readily accomplished (Lloyd and Nelson, 1992). How-ever, to commercialize poly(glucaramides), large quantities of the polymers must be economically produced. To date, poly(glucaramides) have only been produced on a relatively small scale. In previous studies (Kiely and Chen, 1994; Kiely et al., 1994; Chen and Kiely, 1996) poly(glu-caramides) were made in batches of less than 10 g. In this study, poly(glucaramides) were produced in 100 g batches. The commercial demand for nylon resin (excluding nylon fiber) is currently over half a billion kg per year with approximately 5% annual growth predicted. Nylon 6,6 resin cur-rently sells for between $1.10 and $1.30/kg. The cost of glucose is about $0.26/kg which indicates that poly(glucaramides) could compete economi-cally with nylon. Another factor to consider is that the properties of the poly(glucaramides) must to be optimized to fit into specific applications. As with other polymers from renewable resources, their physical properties must match or exceed those of polymers already in use in order to be accepted commercially. With the versatility avail-able in the chemistry of the production of poly(glucaramides), these polymers could be pro-duced in quantities competitive with commercial nylon resins although it is likely that they will be used for different end-use applications.
3.2. Mechanical testing of fiber-reinforced panels
Mechanical testing was performed in order to assess whether there was any increase in the strength of the pressed composite panels due to the incorporation of the poly(glucaramides). Table 2 shows the effect on tensile strength and Young’s modulus of an increase of poly(glu-caramide). Panels with poly(octamethylene D-glu-caramide) showed an increase in tensile strength proportional to an increase in poly(glucaramide) content. A binomial regression analysis of the data gave an R2
value of 0.982. For panels con-taining poly(decamethylene D-glucaramide), ten-sile strength reached a maximum at a concentration of 33 – 37% (determined by bino-mial regression analysis;R2
=0.774) and a further increase in poly(glucaramide) corresponded to a decrease in tensile strength. Similar behavior was seen for Young’s modulus. Panels containing poly(octamethyleneD-glucaramide) showed an in-crease in Young’s modulus proportional to an increase in poly(glucaramide) content (R2=
0.618). For panels containing poly(decamethylene D-glucaramide), it again appeared that there was a maximum in Young’s modulus at between 33 and 37% of the poly(glucaramide) (R2
=0.456). It is not clear from the experiment why there was a difference in the behavior of the poly(oc-tamethylene D-glucaramide) versus poly(deca-methylene D-glucaramide).
The inclusion of poly(glucaramides) in the pan-els did not correspond to an improvement in the strength of the test panels. Thus, the primary advantage of poly(glucaramides) in a pressed panel formulation is to impart water resistance.
3.3. Water resistance of fiber-reinforced panels
Table 3
Water resistance of fiber-reinforced panels containing poly(glucaramides)
Soluble matter lost (%)
Poly(glucaramide) (%) Increase in weight (%)a State of panels after immersion
Poly(octamethyleneD-glucaramide)
nd
nd disintegrated
0
46 a
17 228 a slight deformation
33 ab
183 ab retained shape
33
50 172 ab 24 ac retained shape
6 d retained shape
130 c 67
Poly(decamethyleneD-glucaramide)
nd
nd disintegrated
0
17 184 a 45 a very slight deformation
34 b
171 a retained shape
33
170 a
50 17 c retained shape
173 a
67 1 d retained shape
aWithin columns, means followed by the same letter do not differ significantly at a 95% confidence interval.
nearly 560% after immersion and lost 3.4% of its soluble matter. Samples containing only poly(vinyl alcohol) and a-cellulose but no poly(glucaramide) also completely disintegrated during the immersion.
For panels containing poly(octamethylene D-glucaramide), the increase in weight after immer-sion and the amount of soluble matter lost during immersion both decreased with increasing amounts of poly(octamethylene D-glucaramide) (Table 3). Linear regression analysis of the data for weight increase gave an R2 value of 0.955
while for soluble matter lost R2 was 0.980. For
panels containing poly(decamethylene D-glu-caramide), there was no significant change in weight increase after immersion (Table 3; R2=
0.474). However, the amount of soluble matter lost decreased significantly so that there was virtu-ally no loss at the highest concentration of poly(decamethylene D-glucaramide) (R2=0.995).
It was also observed that panels containing the poly(glucaramides) retained their shape during immersion in contrast to the control samples which disintegrated during immersion.
These results show that the water resistance of fiber-reinforced pressed panels can be greatly im-proved by including poly(glucaramides) in the formulation. In addition to improved water resis-tance, inclusion of the poly(glucaramides) main-tained the structural integrity of the panels during
immersion in water. As the amount of poly(glu-caramide) in the panel increased, both the in-crease in weight and the amount of soluble matter lost decreased substantially.
4. Conclusions
Poly(glucaramides) can be readily made from glucaric acid in good yield. Glucaric acid, in turn, can be readily derived from glucose, thus provid-ing a renewable source to these useful polymers. The synthetic method allows for a great deal of flexibility in the design of poly(glucaramides) in that a variety of primary diamines (or higher polyamine) can be used in the polymerization. Thus, poly(glucaramides) with tailored properties can be produced. The poly(glucaramides) in this study were chosen for their water insolubility. While molecular weights were not high, which is not exceptional given that the polymerization is a polycondensation reaction, they were adequate.
class of materials derived from renewable resources.
Acknowledgements
The authors thank Don Stern, Gregory Gray and Rosalind Wong for the X-ray powder pat-terns, GPC and NMR analysis respectively. The financial support of the Washington Wheat Com-mission is also gratefully acknowledged.
References
Annual Book of ASTM Standards, Vol. 8.01 1992. Annual Book of ASTM Standards, Vol. 8.01 1996.
Chen, L., Kiely, D.E., 1996. Synthesis of stereoregularhead,
tail hydroxylated nylons derived from D-glucose. J. Org.
Chem. 61, 5847 – 5851.
Doane, W.M., 1992. New uses for starch. In: New Crops, New Uses, New Markets, 1992 Yearbook of Agriculture. US Department of Agriculture, Washington, DC, pp. 147 – 153.
Ellis, R.P., Cochrane, M.P., Dale, M.F.B., et al., 1998. Starch production and industrial use. J. Sci. Food Agric. 77, 289 – 311.
Galliard, T., Bowler, P., 1987. Morphology and composition of starch. In: Galliard, T. (Ed.), Starch: Properties and Potential. In: Critical Reports on Applied Chemistry, vol. 13. John Wiley and Sons, Chichester, UK, pp. 55 – 77. Glenn, G.M., Irving, D.W., 1995. Starch-based microcellular
foams. Cereal Chem. 72, 155 – 161.
Glenn, G.M., Miller, R.E., Irving, D.W., 1996. Microcellular starch-based foams. In: Fuller, G., McKeon, T.A., Bills, D.D. III (Eds.), Agricultural Materials as Renewable Re-sources: Nonfood and Industrial Applications. In: ACS Symposium Series, vol. 647. American Chemical Society, Washington, DC, pp. 88 – 106.
Glenn, G.M., Hsu, J., 1997. Compression-formed starch-based plastic. Industr. Crops Prod. 7, 37 – 44.
Hoagland, P.D., 1981. The formation of intermediate lactones during aminolysis of diethyl galactarate. Carbohydr. Res. 98, 203 – 208.
Hoagland, P.D., Pessen, H., McDonald, G.G., 1987. The formation of intermediate lactones during aminolysis of diethyl xylarate. J. Carbohydr. Chem. 6, 495 – 499. Kiely, D.E., Lin, T.-H., 1989. Polyhydroxypolyamides and
process for making same. U.S. Patent 4,833,230, May 23, 1989
Kiely, D.E., Chen, L., 1994. Glucaric acid monoamides and their use to prepare poly(glucaramides). U.S. Patent 5,329,044, July 12, 1994
Kiely, D.E., Chen, L., Lin, T.-H., 1994. Hydroxylated nylons based on unprotected esterifiedD-glucaric acid by simple condensation reactions. J. Am. Chem. Soc. 116, 571 – 578. Kiely, D.E., Carter, A., Shrout, D.P., 1997. Oxidation process.
U.S. Patent 5,599,977, February 4, 1997
Kim, M., Pometto, A.L. III, 1994. Food packaging potential of some novel degradable starch-polyethylene plastics. J. Food Protection 57, 1007 – 1012.
Koch, H., Ro¨per, H., 1988. New industrial products from starch. Starch 40 (4), 121 – 131.
Lloyd, N.E., Nelson, W.J., 1992. Glucose- and fructose-con-taining sweeteners from starch. In: Alexander, R.J., Zobel, H.F. (Eds.), Developments in Carbohydrate Chemistry. American Association of Cereal Chemists, St. Paul, MN, pp. 611 – 660.
Maddever, W.J., Chapman, G.M., 1989. Modified starch-based biodegradable plastics. Plastics Engineering July, 31 – 34.
Mehltretter, C.L., 1963. D-glucaric acid. In: Whistler, R.L., Wolfrom, M.L. (Eds.), Methods in Carbohydrate Chem-istry, vol. II. Academic Press, New York, pp. 46 – 48. Ro¨per, H., Koch, H., 1990. The role of starch in
biodegrad-able thermoplastic materials. Starch 42 (4), 123 – 130. Shit, S.C., Maiti, S., 1986. Application of NMR spectroscopy
in molecular weight determination of polymers. Eur. Polym. J. 22, 1001 – 1008.
Zobel, H.F., 1988. Molecules to granules: a comprehensive starch review. Starch 40 (2), 44 – 50.