Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Temperature & Density for Castings
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
FIGURE 5.1 (a) Temperature as a function of time for the solidification of pure metals. Note that freezing takes place at a constant temperature. (b) Density as a function of time.
T
emperature
Time Cooling of liquid
Cooling of solid
B A
Liquid
Liquid + solid
Solid Freezing begins
Freezing ends Freezing
temperature
Specific density
Time Shrinkage of liquid Shrinkage of solid
Solidification shrinkage
Two-Phased Alloys
FIGURE 5.2 (a) Schematic illustration of grains, grain boundaries, and particles dispersed throughout the structure of a two-phase system, such as lead-copper alloy. The grains represent lead in solid solution of copper, and the particles are lead as a second phase. (b) Schematic illustration of a two-phase system, consisting of two sets of grains: dark and light. Dark and light grains have their own compositions and properties.
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Phase Diagram for Nickel-Copper
FIGURE 5.3 Phase diagram for nickel-copper alloy system obtained by a low rate of solidification. Note that pure nickel and pure copper each have one freezing or melting temperature. The top circle on the right depicts the nucleation of crystals; the second circle shows the formation of dendrites; and the bottom circle shows the solidified alloy with grain boundaries.
Solid
(42% Cu-58% Ni) Liquid
(50% Cu-50% Ni) First solid
(36% Cu-64% Ni)
Liquid
(58% Cu-42% Ni)
0 36 42 50 58 67 100
Solid solution
Alloy composition
Liquid solution
Fraction liquid
0 1
Solidus Liquidus
L + S
Solid solution (50% Cu-50% Ni)
1981
CS CO CL 1082 1980
2280 2350 2395 2651 1455
1249 1288 1313
T
emperature (°F)
°C
Composition (% by weight)
Copper (Cu)
Irn-Iron Carbide Phase Diagram
FIGURE 5.4 (a) The iron-iron carbide phase diagram. (b) Detailed view of the microstructures above and below the eutectoid temperature of 727°C (1341°F). Because of the importance of steel as an engineering material, this diagram is one of the most important phase diagrams.
400 500 600 700 800 900 1000 1100
0 0.5 1.0 1.5 2.0 2.5
1000 1500 2000 727°C °F T emperature (°C)
Carbon (% by weight)
! + Fe3C
"
" + Fe3C
Fe3C
Ferrite
! ! ! !
!
"+ !
T
emperature (°C)
Carbon (% by weight) 1000 1200 1400 800 600 400 1600
0 1 2 3 4 5 6 6.67
1000 1500 2500
2000
Cementite (Fe3C)
Liquid 727°C 1495°C 1538°C 1394°C 912°C °F 0.77% 0.022% 4.30% 2.11% 1148°C
" (ferrite)
" + cementite
! + cementite
! + liquid
# (Ferrite)
Detail view
(a) (b)
! (austenite)
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Texture in Castings
FIGURE 5.5 Schematic illustration of three cast structures of metals solidified in a square mold: (a) pure metals, with preferred texture at the cool mold wall. Note in the middle of the figure that only favorable oriented grains grow away from the mold surface; (b) solid-solution alloys; and (c) structure obtained by heterogeneous nucleation of grains.
(a) Chill zone
Equiaxed structure Equiaxed zone
(b) (c)
Alloy Solidification & Temperature
FIGURE 5.6 Schematic illustration of alloy solidification and temperature distribution in the solidifying metal. Note the formation of dendrites in the semi-solid (mushy) zone.
L + S
TS TL
Liquid
Solid
Solid Solid
Mushy zone
Dendrites Mold
wall
Liquid
Liquid
T
emperature
Alloying element (%)
S
L Liquidu
s S
olidu s
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Solidification Patterns for Gray Cast Iron
FIGURE 5.7 Schematic illustration of three basic types of cast structures: (a) columnar dendritic; (b) equiaxed dendritic; and (c) equiaxed nondendritic. Source: After D. Apelian.
(a)
(b) 0.05–0.10% C
Steel
0.25–0.30% C Steel
Minutes after pouring Minutes after pouring
0.55–0.60% C Steel
8 11 40 60 90 102
5 2 15 2 16 2
Sand mold
Chill mold
Sand mold
Chill mold
Sand mold
Cast Structures
FIGURE 5.9 Schematic illustration of cast structures in (a) plane front, single phase, and (b) plane front, two phase. Source: After D. Apelian.
(a) (b) (c)
Solid Solid
Solid Liquid Liquid Liquid
Mold wall
(a)
Solid Liquid Mold
wall Liquid
(b)
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Mold Features
FIGURE 5.10 Schematic illustration of a typical sand mold showing various features. Open riser
Vent
Pouring basin (cup)
Drag Cope
Sand Sprue
Sand Flask
Parting line Mold
cavity Well
Gate
Core (sand)
Blind riser
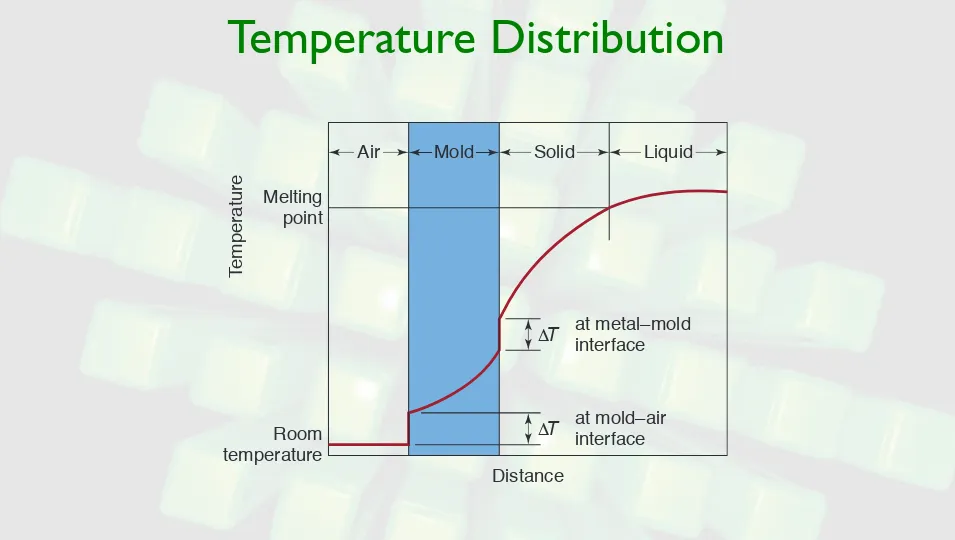
Temperature Distribution
FIGURE 5.11 Temperature distribution at the mold wall and liquid-metal interface during solidification of metals in casting. Room
temperature
Distance
at mold–air interface
at metal–mold interface
Melting point
T
emperature
Air Solid Liquid
!T
!T
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
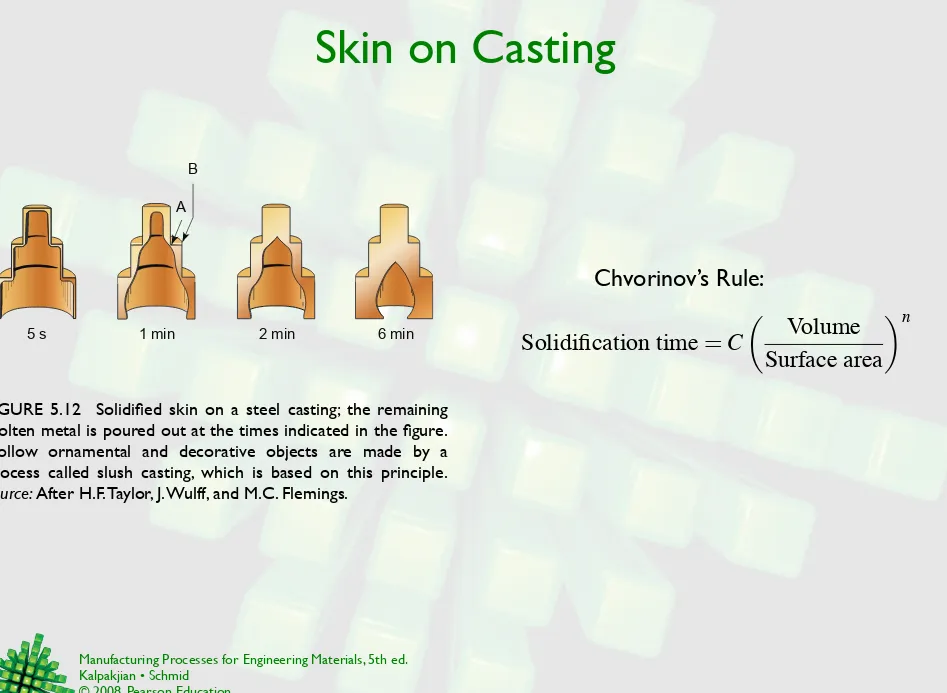
Skin on Casting
FIGURE 5.12 Solidified skin on a steel casting; the remaining molten metal is poured out at the times indicated in the figure. Hollow ornamental and decorative objects are made by a process called slush casting, which is based on this principle. Source: After H.F. Taylor, J. Wulff, and M.C. Flemings.
5 s 1 min 2 min 6 min
A B
Chvorinov’s Rule:
Solidification time
=
C
!
Volume
Surface area
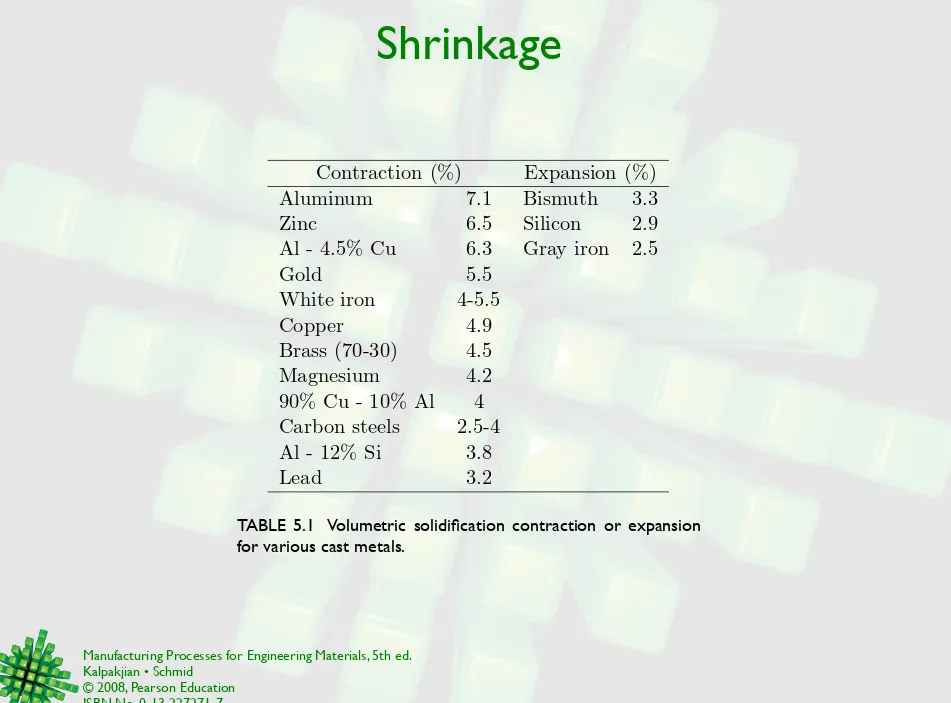
Shrinkage
Contraction (%)
Expansion (%)
Aluminum
7.1
Bismuth
3.3
Zinc
6.5
Silicon
2.9
Al - 4.5% Cu
6.3
Gray iron
2.5
Gold
5.5
White iron
4-5.5
Copper
4.9
Brass (70-30)
4.5
Magnesium
4.2
90% Cu - 10% Al
4
Carbon steels
2.5-4
Al - 12% Si
3.8
Lead
3.2
Man
ufacturing Pr
ocesses f
or Engineering Materials,
5th ed. Kalpakjian • Schmid © 2008, P earson Education ISBN No . 0-13-227271-7
Cast Material
Pr
oper
ties
FIGURE 5.13 Mechanical pr oper ties for various gr oups of cast allo ys. Compar e with various tables of pr oper ties in Cha pter 3. Source: Courtesy of Steel F
ounders' Society of
America. Steel Nodular iron Gray iron Malleable iron Aluminum based Copper based Magnesium based Nickel based Zinc based
Ultimate tensile strength (psi x 103)
300 280 260 240 220 200 180 160 140 120 100 80 60 40 20 0 2000 1800 2000 1800 1600 1400 1200 1000 800 600 400 200 MPa (a) 300 280 260 240 220 200 180 160 140 120 100 80 60 40 20 0 1600 1400 1200 1000 800 600 400 200 Steel Nodular iron Gray iron Malleable iron Aluminum based Copper based Magnesium based Nickel based Zinc based
Yield strength (psi x 103)
MPa (b) Nodular iron Gray iron Malleable iron Aluminum based Copper based Magnesium based Nickel based Zinc based Titanium metal Titanium alloys Cast steel
0 5 10 15 20 25 30
0 50 100 150 200
GPa
Modulus of elasticity (psi x 106)
0 2 4 6 8 10 12
W rought Cast Steel Nodular iron Gray iron Malleable iron Aluminum based Magnesium based Titanium metal Titanium alloy Tensile strength/density ratio (in x 105)
(d) (c) Nodular iron Gray iron Aluminum based Copper based Magnesium based Nickel based Zinc based Steel Malleable iron 800 700 600 500 400 300 200 100 0
Brinell harbness (HB)
(e) Malleable iron 70 60 50 40 30 20 10 0 90 80 70 60 50 40 30 20 10 0 Steel Nodular iron Gray iron J
Impact energy (ft-lb, Charpy V-notch)
(f ) 70 60 50 40 30 20 10 0 Nodular iron Malleable iron Copper based Nickel based Steel Gray iron Copper based Nickel based Reduction of area (%)
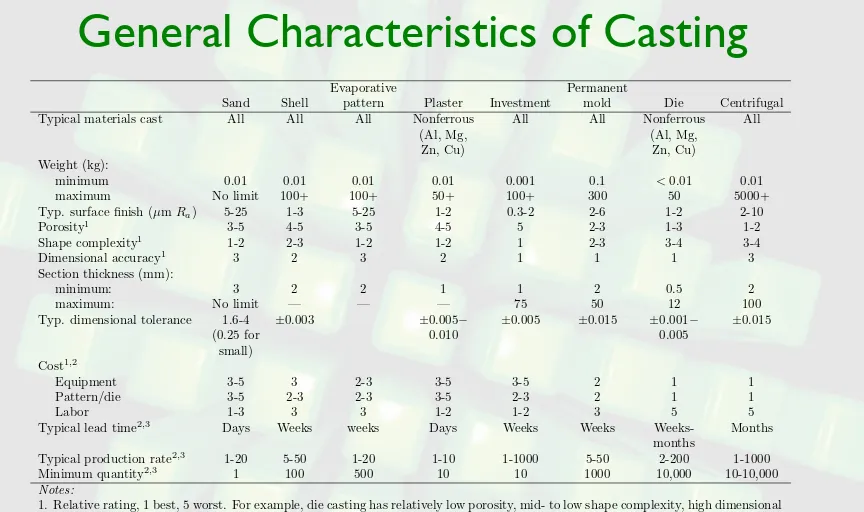
General Characteristics of Casting
TABLE 5.2 General characteristics of casting processes.
Evaporative Permanent
Sand Shell pattern Plaster Investment mold Die Centrifugal
Typical materials cast All All All Nonferrous All All Nonferrous All
(Al, Mg, (Al, Mg,
Zn, Cu) Zn, Cu)
Weight (kg):
minimum 0.01 0.01 0.01 0.01 0.001 0.1 <0.01 0.01
maximum No limit 100+ 100+ 50+ 100+ 300 50 5000+
Typ. surface finish (µm Ra) 5-25 1-3 5-25 1-2 0.3-2 2-6 1-2 2-10
Porosity1
3-5 4-5 3-5 4-5 5 2-3 1-3 1-2
Shape complexity1
1-2 2-3 1-2 1-2 1 2-3 3-4 3-4
Dimensional accuracy1
3 2 3 2 1 1 1 3
Section thickness (mm):
minimum: 3 2 2 1 1 2 0.5 2
maximum: No limit — — — 75 50 12 100
Typ. dimensional tolerance 1.6-4 ±0.003 ±0.005− ±0.005 ±0.015 ±0.001− ±0.015
(0.25 for 0.010 0.005
small)
Cost1,2
Equipment 3-5 3 2-3 3-5 3-5 2 1 1
Pattern/die 3-5 2-3 2-3 3-5 2-3 2 1 1
Labor 1-3 3 3 1-2 1-2 3 5 5
Typical lead time2,3
Days Weeks weeks Days Weeks Weeks Weeks- Months
months
Typical production rate2,3
1-20 5-50 1-20 1-10 1-1000 5-50 2-200 1-1000
Minimum quantity2,3
1 100 500 10 10 1000 10,000 10-10,000
Notes:
1. Relative rating, 1 best, 5 worst. For example, die casting has relatively low porosity, mid- to low shape complexity, high dimensional accuracy, high equipment and die costs and low labor costs. These ratings are only general; significant variations can occur depending on the manufacturing methods used.
2. Data taken from Schey, J.A., Introduction to Manufacturing Processes, 3rd ed, 2000.
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
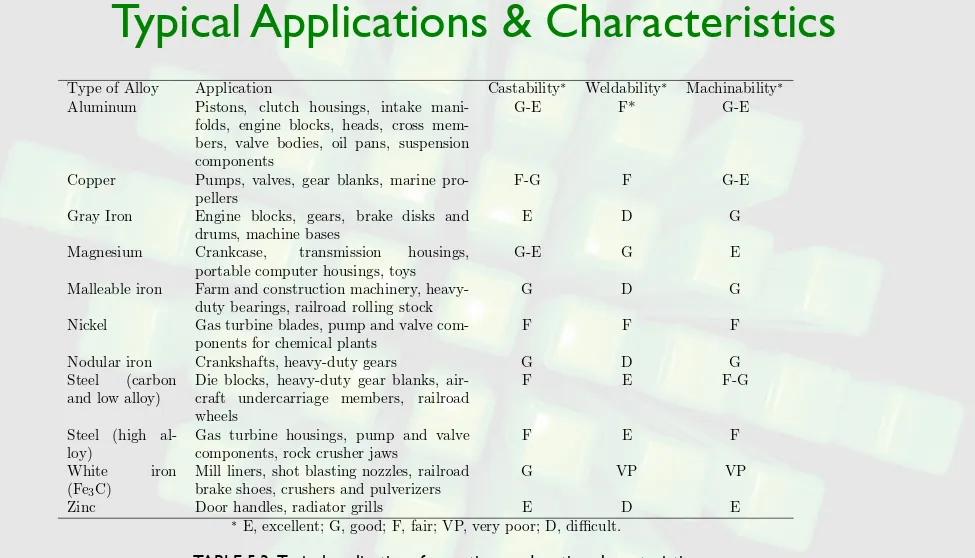
Typical Applications & Characteristics
TABLE 5.3 Typical applications for castings and casting characteristics.
Type of Alloy Application Castability∗ Weldability∗ Machinability∗
Aluminum Pistons, clutch housings, intake
mani-folds, engine blocks, heads, cross mem-bers, valve bodies, oil pans, suspension components
G-E F* G-E
Copper Pumps, valves, gear blanks, marine
pro-pellers
F-G F G-E
Gray Iron Engine blocks, gears, brake disks and
drums, machine bases
E D G
Magnesium Crankcase, transmission housings,
portable computer housings, toys
G-E G E
Malleable iron Farm and construction machinery,
heavy-duty bearings, railroad rolling stock
G D G
Nickel Gas turbine blades, pump and valve
com-ponents for chemical plants
F F F
Nodular iron Crankshafts, heavy-duty gears G D G
Steel (carbon
and low alloy)
Die blocks, heavy-duty gear blanks, air-craft undercarriage members, railroad wheels
F E F-G
Steel (high al-loy)
Gas turbine housings, pump and valve components, rock crusher jaws
F E F
White iron
(Fe3C)
Mill liners, shot blasting nozzles, railroad brake shoes, crushers and pulverizers
G VP VP
Zinc Door handles, radiator grills E D E
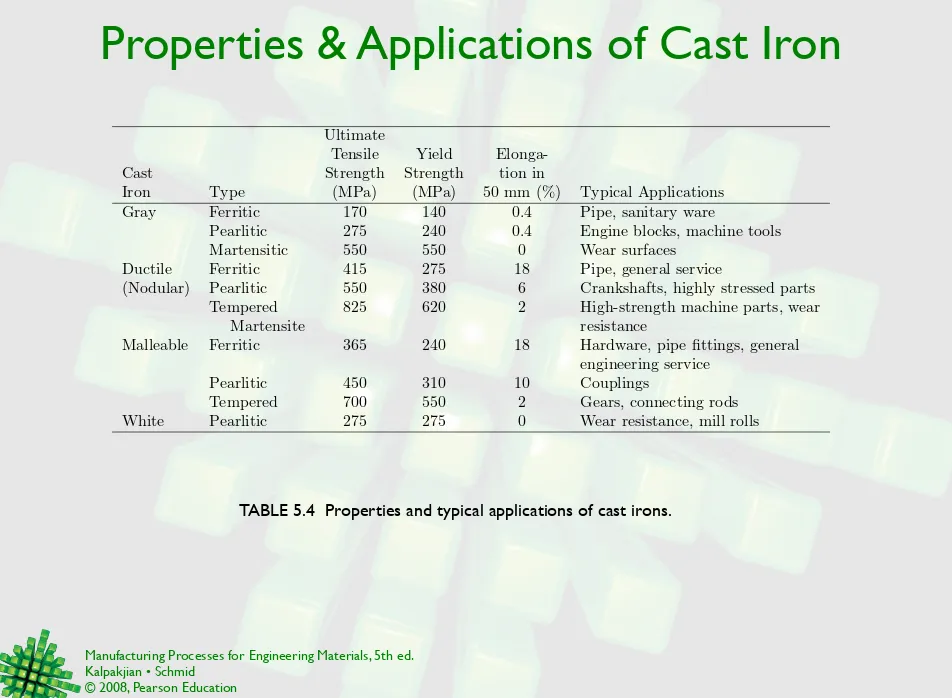
Properties & Applications of Cast Iron
TABLE 5.4 Properties and typical applications of cast irons.
Ultimate
Tensile Yield
Elonga-Cast Strength Strength tion in
Iron Type (MPa) (MPa) 50 mm (%) Typical Applications
Gray Ferritic 170 140 0.4 Pipe, sanitary ware
Pearlitic 275 240 0.4 Engine blocks, machine tools
Martensitic 550 550 0 Wear surfaces
Ductile Ferritic 415 275 18 Pipe, general service
(Nodular) Pearlitic 550 380 6 Crankshafts, highly stressed parts
Tempered 825 620 2 High-strength machine parts, wear
Martensite resistance
Malleable Ferritic 365 240 18 Hardware, pipe fittings, general
engineering service
Pearlitic 450 310 10 Couplings
Tempered 700 550 2 Gears, connecting rods
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Nonferrous Alloys
TABLE 5.5 Typical properties of nonferrous casting alloys.
Casting UTS Yield Strength Elongation Hardness Alloy Condition Method∗ (MPa) (MPa) in 50 mm (%) (HB) Aluminum
357 T6 S 345 296 2.0 90
380 F D 331 165 3.0 80
390 F D 279 241 1.0 120
Magnesium
AZ63A T4 S, P 275 95 12 —
AZ91A F D 230 150 3 —
QE22A T6 S 275 205 4 —
Copper
Brass C83600 — S 255 177 30 60
Bronze C86500 — S 490 193 30 98
Bronze C93700 — P 240 124 20 60
Zinc
No. 3 — D 283 — 10 82
No. 5 — D 331 — 7 91
ZA27 — P 425 365 1 115
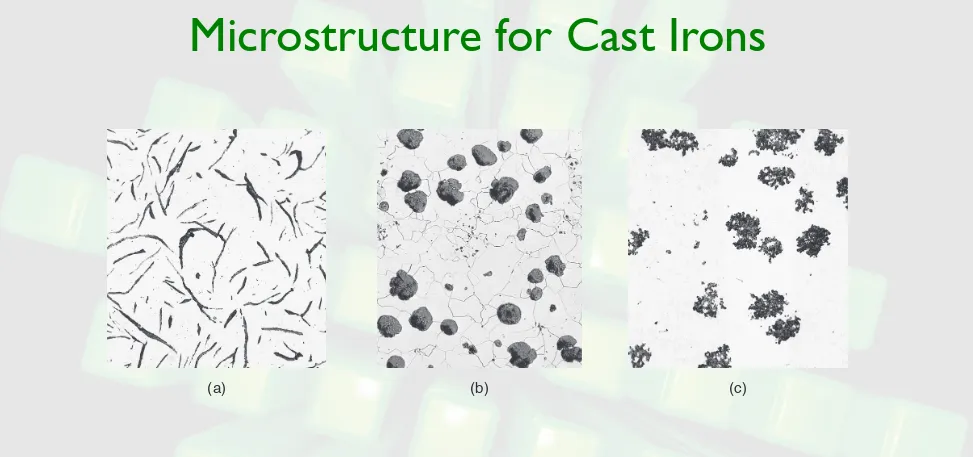
Microstructure for Cast Irons
FIGURE 5.14 Microstructure for cast irons. (a) ferritic gray iron with graphite flakes; (b) ferritic nodular iron, (ductile iron) with graphite in nodular form; and (c) ferritic malleable iron. This cast iron solidified as white cast iron, with the carbon present as cementite (Fe3C), and was heat treated to graphitize the carbon.
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
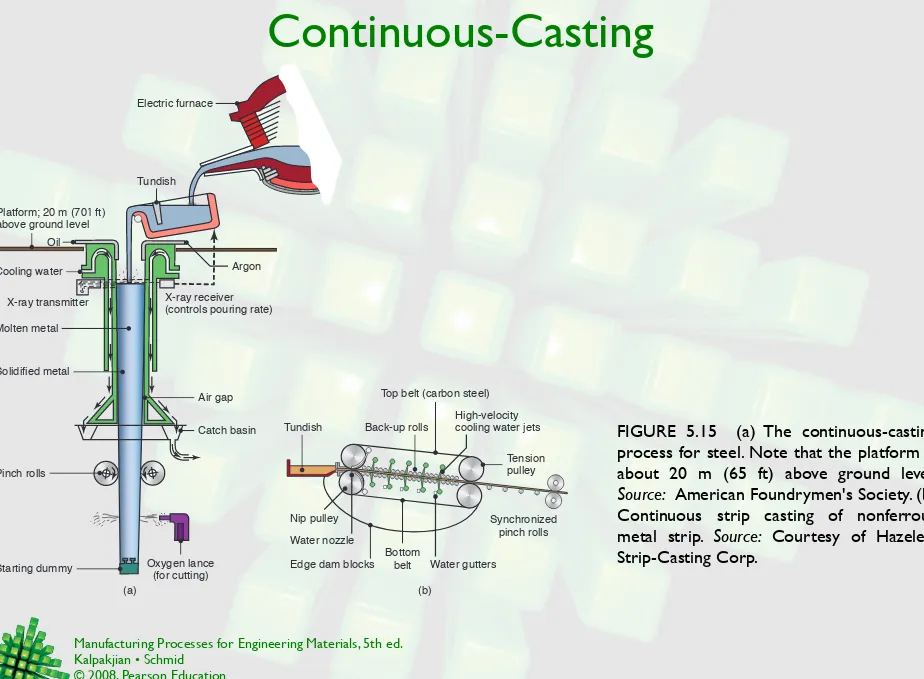
Continuous-Casting
FIGURE 5.15 (a) The continuous-casting process for steel. Note that the platform is about 20 m (65 ft) above ground level. Source: American Foundrymen's Society. (b) Continuous strip casting of nonferrous metal strip. Source: Courtesy of Hazelett Strip-Casting Corp.
Electric furnace
Tundish
Argon
X-ray receiver
(controls pouring rate) X-ray transmitter
Molten metal
Solidified metal Oil Cooling water
Platform; 20 m (701 ft) above ground level
Air gap
Catch basin
Pinch rolls
Oxygen lance (for cutting) Starting dummy
Tundish
Top belt (carbon steel) High-velocity cooling water jets Back-up rolls
Bottom
belt Water gutters
Nip pulley Synchronized pinch rolls
Tension pulley
Edge dam blocks Water nozzle
Sand
Casting
FIGURE 5.16 Schematic illustration of the sequence of operations in sand casting. (a) A mechanical drawing of the part, used to create patterns. (b-c) Patterns mounted on plates equipped with pins for alignment. Note the presence of core prints designed to hold the core in place. (d-e) Core boxes produce core halves, which are pasted together. The cores will be used to produce the hollow area of the part shown in (a). (f) The cope half of the mold is assembled by securing the cope pattern plate to the flask with aligning pins, and attaching inserts to form the sprue and risers. (g) The flask is rammed with sand and the plate and inserts are removed. (h) The drag half is produced in a similar manner. (j) The core is set in place within the drag cavity. (k) The mold is closed by placing the cope on top of the drag and securing the assembly with pins. (l) After the metal solidifies, the casting is removed from the mold. (m) The sprue and risers are cut off and recycled, and the casting is cleaned, inspected, and heat treated (when necessary). Source: Courtesy of Steel Founders' Society of America.
Cope ready for sand
Cope after ramming with sand and removing pattern,
sprue, and risers
Drag ready for sand
Drag after removing pattern Core halves
pasted together
(e) (f) (g) (h) (i)
Sprue Risers Flask
Drag with core set in place
(j)
Cope and drag assembled and ready for pouring
(k) Cope
Drag Closing pins
Casting as removed from mold; heat treated
(l)
Casting ready for shipment
(m) (a)
Mechanical drawing of part Cope pattern plate Drag pattern plate Core boxes (d)
(b) (c)
Core prints Gate
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
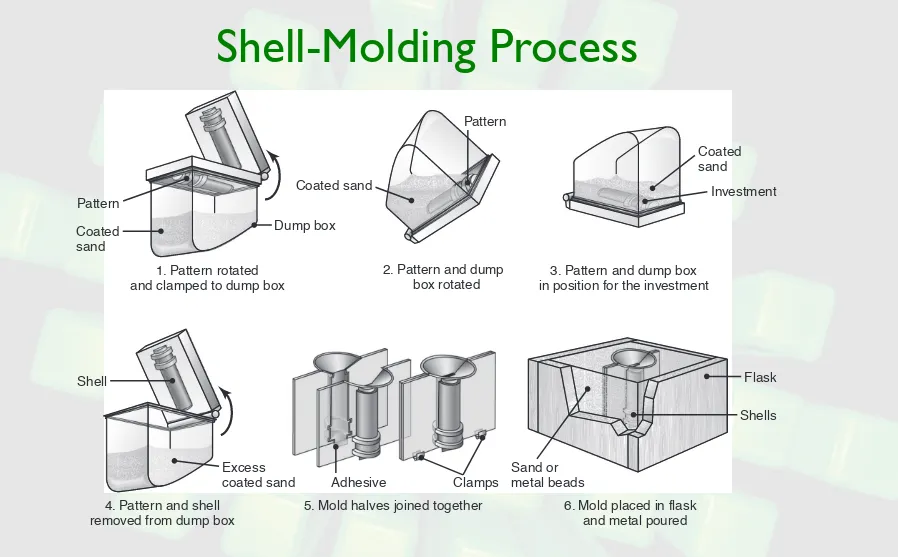
Shell-Molding Process
FIGURE 5.17 Schematic illustration of the shell-molding process, also called the dump-box technique.
Pattern
Coated sand
Dump box
1. Pattern rotated and clamped to dump box
Shell
Excess coated sand
4. Pattern and shell removed from dump box
Coated sand
3. Pattern and dump box in position for the investment
Investment Pattern
Coated sand
2. Pattern and dump box rotated
Adhesive Clamps
5. Mold halves joined together
Flask
Sand or metal beads
Shells
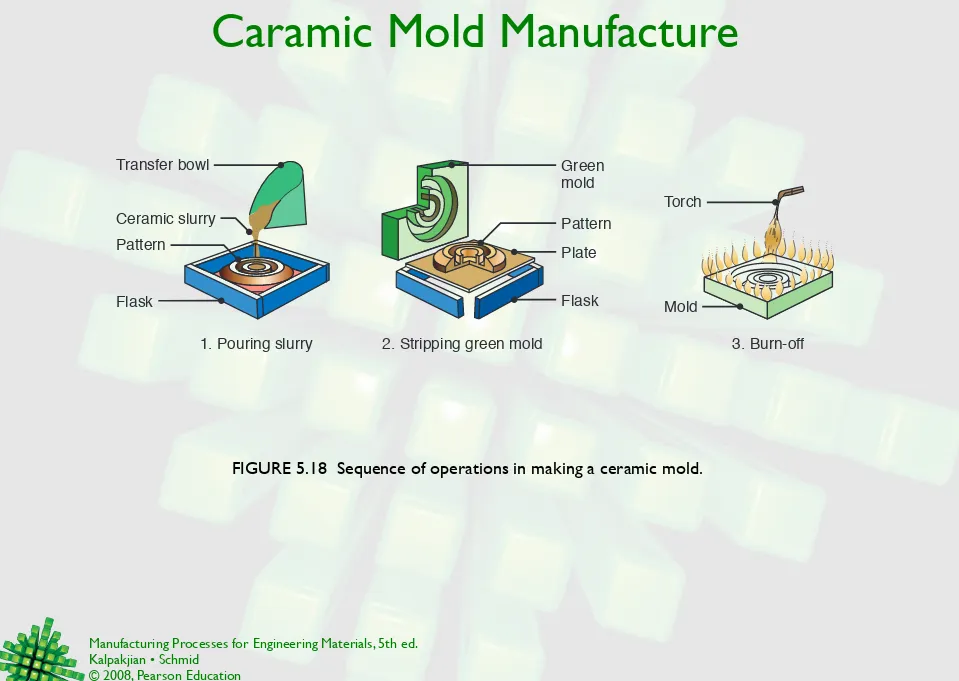
Caramic Mold Manufacture
FIGURE 5.18 Sequence of operations in making a ceramic mold.
3. Burn-off 2. Stripping green mold
1. Pouring slurry
Flask Green mold
Pattern
Plate Ceramic slurry
Pattern
Transfer bowl
Flask
Torch
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Vacuum-Casting Process
FIGURE 5.19 Schematic illustration of the vacuum-casting process. Note that the mold has a bottom gate. (a) before and (b) after immersion of the mold into the molten metal. Source: After R. Blackburn.
Mold
(a) (b)
Induction furnace
Vacuum
Casting
Evaporative Pattern Casting
FIGURE 5.20 Schematic illustration of the expendable-pattern casting process, also known as lost-foam or evaporative-pattern casting.
1. Pattern molding
4. Compacted in sand 5. Casting
6. Shakeout 2. Cluster assembly
3. Coating
Cluster
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Investment Casting
FIGURE 5.21 Schematic illustration of investment casting (lost wax process). Castings by this method can be made with very fine detail and from a variety of metals. Source: Steel Founders' Society of America.
9. Shakeout 8. Pouring
7. Pattern meltout 6. Completed mold
Casting
10. Pattern Molten
metal Autoclaved
Molten wax or plastic
Heat Heat
1. Injection wax or plastic pattern
4. Slurry coating 2. Ejecting
pattern
5. Stucco coating 3. Pattern
assembly (tree) Wax
Rotor Microstructure
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Pressure & Hot-Chamber Die Casting
FIGURE 5.23 The pressure casting process, utilizing graphite molds for the production of steel railroad wheels. Source: Griffin Wheel Division of Amsted Industries Incorporated.
Airtight chamber
Ladle Refractory tube
Molten metal Air pressure Railroad wheel Graphite mold
FIGURE 5.24 Schematic illustration of the hot-chamber die-casting process.
Gooseneck Nozzle
Die cavity
Hydraulic shot cylinder
Plunger rod
Plunger
Molten metal
Pot Ejector die
Cover die
Cold-Chamber Die Casting
FIGURE 5.25 Schematic illustration of the cold-chamber die-casting process. These machines are large compared to the size of the casting, because high forces are required to keep the two halves of the die closed under pressure.
Shot cylinder Metal
sleeve Cover
disc Closing
cylinder
Ejector box
Ejector platen (Moves)
Ejector die half
Hydraulic cylinder
Shot sleeve Ejector box
Ladle
Stationary die half
Plunger rod Stationary platen Cavity
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Properties of Die-Casting Alloys
Ultimate
Elonga-Tensile Yield tion Strength Strength in 50 mm
Alloy (MPa) (MPa) (%) Applications
Aluminum 380 320 160 2.5 Appliances, automotive
(3.5 Cu-8.5 Si) components, electrical motor
frames and housings, engine blocks.
Aluminum 13 300 150 2.5 Complex shapes with thin
(12 Si) walls, parts requiring strength at
elevated temperatures
Brass 858 (60 Cu) 380 200 15 Plumbing fixtures, lock hard-ware, bushings, ornamental cast-ings
Magnesium 230 160 3 Power tools, automotive
AZ91B (9 Al - 0.7 Zn) parts, sporting goods
Zinc No. 3 (4 Al) 280 — 10 Automotive parts, office equip-ment, household utensils, build-ing hardware, toys
Zinc No. 5 (4 Al - 1 Cu) 320 — 7 Appliances, automotive parts, building hardware, business equipment
Source: The North American Die Casting Association
Centrifugal Casting
FIGURE 5.26 Schematic illustration of the centrifugal casting process. Pipes, cylinder liners, and similarly shaped hollow parts can be cast by this process.
Free roller Drive roller
Mold
(a) (b)
Drive shaft
Spout
Rollers
Ladle Molten metal
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Semicentrifugal Casting
FIGURE 5.27 (a) Schematic illustration of the semicentrifugal casting process. Wheels with spokes can be cast by this process. (b) Schematic illustration of casting by centrifuging. The molds are placed at the periphery of the machine, and the molten metal is forced into the molds by centrifugal forces.
(a) (b)
Mold
Molten metal
Casting Flasks
Holding fixture
Drag
Revolving table
Casting Pouring basin
and gate
Squeeze-Casting
FIGURE 5.28 Sequence of operations in the squeeze-casting process. This process combines the advantages of casting and forging.
1. Melt metal 2. Pour molten metal into die
3. Close die and apply pressure
4. Eject squeeze casting, charge melt stock,
repeat cycle Die
Ejector pin
Finished casting
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Turbine Blade Casting
FIGURE 5.29 Methods of casting turbine blades: (a) directional solidification; (b) method to produce a single-crystal blade; and (c) a single-crystal blade with the constriction portion still attached. Source: (a) and (b) After B.H. Kear, (c) Courtesy of ASM International.
(c)
(a) (b)
Radiant heat
Columnar
crystals Constriction
Chill plate Columnar
crystals Heat baffles
Radiant heat
Crystal Growing
FIGURE 5.30 Two methods of crystal growing: (a) crystal pulling (Czochralski process) and (b) floating-zone method. Crystal growing is especially important in the semiconductor industry. (c) A single-crystal silicon ingot produced by the Czochralski process. Source: Courtesy of Intel Corp.
(c)
(a) (b)
~1 rev/s
10
µ
m/s
Liquid Seed
20
µ
m/s
Induction coil
Single crystal
Polycrystalline
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Melt-Spinning Process
FIGURE 5.31 (a) Schematic illustration of the melt-spinning process to produce thin strips of amorphous metal. (b) Photograph of nickel-alloy production through melt-spinning. Source: Courtesy of Siemens AG.
(b) (a)
Crucible Induction coil
Melt
Strip Gas
Austenite-Pearlite Transformation
FIGURE 5.32 (a) Austenite to pearlite transformation of iron-carbon alloys as a function of time and temperature. (b) Isothermal transformation diagram obtained from (a) for a transformation temperature of 675°C (1247°F). (c) Microstructures obtained for a eutectoid iron-carbon alloy as a function of cooling rate. Source: Courtest of ASM International.
(a) 25 75 50 0 100 75 25 50 100 0 Austenite (%) Pearlite (%) Time (s)
600°C 650° 675°
1 10 102 103
50
0 100
Percent of austenite
transformed to pearlite
T emperature ( ° C) Austenite (%) ° F 50 100 0 600 500 700 800 1000 1200 1400
50% Completion curve Pearlite Completion curve (~100% pearlite) Eutectoid temperature Austenite (unstable) Begin curve (~0% pearlite) Transformation temperature 675°C
Transformation begins
1 10 103 104 105
Transformation ends Austenite (stable) 102 Time (s) T emperature ( °C) 200
100 200
400 600 800 1000 1200 1400 300 400 500 35°C/s 140 °C/s 600 700 800 Eutectoid temperature
M (start) Critical cooling rate °F Martensite Martensite
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Phase Diagram for Aluminum-Copper
FIGURE 5.33 (a) Phase diagram for the aluminum-copper alloy system. (b) Various microstructures obtained during the age-hardening process.
X
T
emperature
X—solid solution
XA—quenched, solid solution retained
AB—age-hardened, precipitation starts
(submicroscopic)
AC—over-aging, precipitate
agglomerates
100 95 90 Aluminum (Al)
0 5 10 Copper (Cu)
70 400 900 1100 Liquid
+ liquid
20 200 500 600 700
(b) (a)
1300
Composition (% by weight)
T
emperature (°C)
°F
A B C
Time
Outline of Heat Treating
TABLE 5.7 Outline of heat treatment processes for surface hardening.
Element
Metals added to General Typical
Process hardened surface Procedure characteristics applications Carburizing Low-carbon
steel (0.2% C), alloy steels (0.08-0.2% C)
C Heat steel at 870-950◦
(1600-1750◦F) in an
at-mosphere of carboaceous gases (gas carburizing) or carbon-containing solids (pack carburizing). Then quench.
A hard, high-carbon surface is produced. Hardness 55-65 HRC. Case depth <
0.5-1.5 mm (<0.020 to 0.060 in.).
Some distortion of part dur-ing heat treatment.
Gears, cams, shafts, bearings, piston pins, sprockets, clutch plates Carbonitriding Low-carbon steel
C and N Heat steel at 700-800◦C
(1300-1600◦F) in an
atmo-sphere of carbonaceous gas and ammonia. Then quench in oil.
Surface hardness 55-62 HRC. Case depth 0.07-0.5 mm (0.003-0.020 in.). Less distor-tion than in carburizing.
Bolts, nuts, gears.
Cyaniding Low-carbon steel (0.2% C), alloy steels (0.08-0.2% C)
C and N Heat steel at 760-845◦C
(1400-1550◦F) in a molten
bath of solutions of cyanide (e.g., 30% sodium cyanide) and other salts.
Surface hardness up to 65 HRC. Case depth 0.025-0.25 mm (0.001-0.010 in.). Some distortion.
Bolts, nuts, screws, small gears.
Nitriding Steels (1% Al, 1.5% Cr, 0.3% Mo), alloy steels (Cr, Mo), stain-less steels, high-speed steels
N Heat steel at 500-600◦C
(925-1100◦F) in an atmosphere of
ammonia gas or mixtures of molten cyanide salts. No fur-ther treatment.
Surface hardness up to 1100 HV. Case depth 0.1-0.6 mm (0.005-0.030 in.) and 0.02-0.07 mm (0.001-0.003 in.) for high speed steel.
Geards, shafts, sprockets, valves, cutters, boring bars
Boronizing Steels B Part is heated using boron-containing gas or solid in con-tact with part.
Extremely hard and wear-resistance surface. Case depth 0.025-0.075 mm (0.001-0.003 in.).
Tool and die steels.
Flame hardening
Medium-carbon steels, cast irons
None Surface is heated with an oxyacetylene torch, then quenched with water spray or other quenching methods.
Surface hardness 50-60 HRC. Case depth 0.7-6 mm (0.030-0.25 in.). Little distortion.
Axles, crankshafts, piston rods, lathe beds, and centers.
Induction hardening
Same as above None Metal part is placed in cop-per induction coils and is heated by high frequency cur-rent, then quenched
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Heat Treatment Temperature Ranges
FIGURE 5.34 Temperature ranges for heat treating plain-carbon steels, as indicated on the iron-iron carbide phase diagram.
0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6
T
emperature (
°C
)
600 700 800 900 1000
1200 1400 1600 1800
Normalizing
Full
annealing
Acm
Spheroidizing 738°C
A3
A1
Composition (% C)
Casting Processes Comparison
TABLE 5.8 Casting Processes, and their Advantages and Limitations.
Process Advantages Limitations
Sand Almost any metal is cast; no limit to size, shape or weight; low tooling cost.
Some finishing required; somewhat coarse finish; wide tolerances.
Shell mold Good dimensional accuracy and sur-face finish; high production rate.
Part size limited; expensive patterns and equipment required.
Expendable pattern Most metals cast with no limit to size; complex shapes
Patterns have low strength and can be costly for low quantities.
Plaster mold Intricate shapes; good dimensional accuracy and finish; low porosity.
Limited to nonferrous metals; limited size and volume of production; mold making time relatively long.
Ceramic mold Intricate shapes; close tolerance parts; good surface finish.
Limited size.
Investment Intricate shapes; excellent surface fin-ish and accuracy; almost any metal cast.
Part size limited; expensive patterns, molds, and labor.
Permanent mold Good surface finish and dimensional accuracy; low porosity; high produc-tion rate.
High mold cost; limited shape and in-tricacy; not suitable for high-melting-point metals.
Die Excellent dimensional accuracy and surface finish; high production rate.
Die cost is high; part size limited; usu-ally limited to nonferrous metals; long lead time.
Centrifugal Large cylindrical parts with good quality; high production rate.
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Chills
FIGURE 5.35 Various types of (a) internal and (b) external chills (dark areas at corners), used in castings to eliminate porosity caused by shrinkage. Chills are placed in regions where there is a larger volume of metal, as shown in (c).
(a)
(b)
(c)
Porosity Chill
Casting
Boss Chill Sand
Casting Chill
Hydrogen Solubility in Aluminum
FIGURE 5.36 Solubility of hydrogen in aluminum. Note the sharp decrease in solubility as the molten metal begins to solidify.
Hydrogen solubility
Fusion
Solid
Liquid
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Elimination of Porosity in Castings
FIGURE 5.37 (a) Suggested design modifications to avoid defects in castings. Note that sharp corners are avoided to reduce stress concentrations; (b, c, d) examples of designs showing the importance of maintaining uniform cross-sections in castings to avoid hot spots and shrinkage cavities.
(a) Poor
Good
(b) (c) (d)
Shrinkage cavity
Poor Poor
Design Modifications
FIGURE 5.38 Suggested design modifications to avoid defects in castings. Source: Courtesy of The North American Die Casting Association.
Use radii or fillets to avoid corners and provide uniform cross-section.
Wall sections should be uniform.
Sloping bosses can be designed for straight die parting to simplify die design.
Ribs and/or fillets improve bosses.
Side cores can be eliminated with this hole design. Deep cavities should be on one side of the casting where possible. Poor Good
Poor Good
Poor Good
Poor Good Poor Good Poor Good
Core in cover half
Manufacturing Processes for Engineering Materials, 5th ed. Kalpakjian • Schmid
© 2008, Pearson Education ISBN No. 0-13-227271-7
Economics of Casting
FIGURE 5.39 Economic comparison of making a part by two different casting processes. Note that because of the high cost of equipment, die casting is economical mainly for large production runs. Source: The North American Die Casting Association.
Cost per piece (relative)
8
7
6
5
4
3
2
1
0
100 101 102 103 104 105 106
Number of pieces
Die cast
Sand cast
Lost-Foam Casting of Engine Blocks
FIGURE 5.40 (a) An engine block for a 60-hp 3-cylinder marine engine, produced by the lost-foam casting process; (b) a robot pouring molten aluminum into a flask containing a polystyrene pattern. In the pressurized lost-foam process, the flask is then pressurized to 150 psi (1000 kPa). Source: Courtesy of Mercury Marine