The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
76
Dilute Ionic Liquids Pretreatment of Palm Empty Bunch and
Its Impact to Produce Bioethanol
Lucy Arianie,1* Utin Dewi Pebriyana,1 Yudiansyah,2 Nora Idiawati1 and Deana Wahyuningrum2
1
Department of Chemistry – Faculty of Mathematics and Natural Science, Universitas Tanjungpura, Jl. A.Yani 73 Pontianak 78124. 2Department of Chemistry – Faculty of Mathematics and Natural Sciences,
Institut Teknologi Bandung, Jl. Ganeca 10 Bandung 40132.
*
Corresponding author: Email : lucy205@yahoo.com
Received 6 November 2013; Revision 11 December 2013; Accepted 7 May 2014; Published online for edition May-August 2014
ABSTRACT
Ethanol production through ionic liquids pretreatment of palm empty bunch (PEB) was carried out. This research aims to investigate impact of ionic liquids synthetic i.e 1-butyl-3-methyl imidazoliumbromide or [BMIM]bromide toward cellulose’s palm empty bunch and convert its cellulose into bioethanol. Ionic liquid was synthesized through reflux and microwave assisted synthesis methods. Research investigation showed that microwave assisted synthesis produce [BMIM]bromide 90% faster than reflux method. The characterization of synthesized product using FTIR, 1H-NMR, 13C-NMR and LC-MS showed that these reactions have been carried out successfully. Scanning electron microscope figure out changes morphological surface of palm empty bunch caused by ionic liquid pretreatment. Crystallinity index of PEB milled and cellulose of PEFB after [BMIM]bromide dissolution were identified using comparison of PEB FTIR spectrum. Cellulose without dilute [BMIM]bromide have higher LOI number than cellulose after [BMIM]bromide dissolution. It indicated that a large part of cellulose after dissolution has been changed into amorf. Hydrolysis residue of palm empty bunch hydrolyzed by sulfuric acids 5%, 100 0C for 5 hours and produce 685 ppm of reducing sugar. Simultaneous Saccharification and Fermentation using Trichoderma viride and Saccharomyce cerevisiae for 5 days produce 0,69% of bioethanol.
.Key word: palm empty bundh, [BMIM]bromide, ethanol.
INTRODUCTION
Lignocellulose pretreatment is primary step to break off chain between cellulose, hemicellulose and lignin. Pretreatment is due to split chain of cellulose, hemicelluloses and lignin, decreasing crystallinity index of cellulose and enhance material porosity [1]. Palm empty bunch (PEB) is one of abundance sources natural biomass that has not been optimally used yet [1]. Lignocellulose fibril in PEB is difficult to degraded and to be extracted into cellulose because lignocellulose fibril have high crystallinity index.
Dilute ionic liquid pretreatment is up to date pretreatment. Ionic liquid is salt consists of large portion organic cation and small anorganic anion. Ionic liquid is interesting solvent that has chemistry and thermal stability and nonflammable. For several cases, ionic liquids could be used as reusable solvent for biomass. Ionic liquids that contains a 1-butyl-3-methyl imidazolium could decrease the crystallinity index [2].
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
77
lignin, decreasing crystallinity index of cellulose and enhance material porosity [1]. Pretreatment using ionic liquids for Asclepias syriaca dan Poplar produced breaking off cellulose and lignin. Cellulose can be resulted from enzimatic hydrolysis and this research showed this enzymatic hydrolysis in more effective than that hydrolysis in absence of ionic liquid pretreatment [3].
Varma and Namboodiri (2001) used Microwave-Assisted Organic Synthesis to produce ionic liquids [4]. Ionic liquids usually synthesized on conventional reaction in up to 80 0C for several hours. This condition could be solved using microwave-assisted synthesis. Zhu et al. (2012) reported that 1-butyl-3-methyl imidazoliumbromide and 1-allyl-3-methyl imidazolium chloride synthesized by using microwave-assisted synthesis faster than conventional method [5]. This paper disclosed on the synthesizing ionic liquid i.e. 1-butyl-3-methyl imidazoliumbromide and investigate its application for dilute pretreatment of palm empty bunch.
EXPERIMENT
Palm empty bunch was taken from Pabrik Minyak Sawit, PTPN XIII Parindu at Kabupaten Sanggau, Kalimantan Barat, Indonesia. This sample was biomass waste of palm refinery for palm cooking oil. The sample has been cleaned, heated under sunlight, and milled at Forestry Laboratory, Universitas Tanjungpura. Sample in 40 mesh size was oven dried at 105 oC for 4 hours before used.
Synthesis of 1-butyl-3-methyl imidazoliumbromide
Synthesis of 1-butyl-3-methyl imidazolium bromide or [BMIM]bromide was carried out using two methods for the comparison, which are refluks and Microwave-assisted Synthesis (MAS) methods [4]. The domestic microwave oven (Sharp R-249IN (800 W) and modified Teflon based reactor were used in the synthesis using MAS method. The precursors in synthesizing [BMIM]bromide are N-methyl imidazole and butylbromide which are used stoichiometrically. The product was characterized using Fourir Transform Infra Red (Alpha Bruker FTIR at Physical Chemistry and Materials Research Laboratory, ITB), Proton and Carbon-Nuclear Magnetic Resonance (1H-NMR (500 MHz) and 13C-NMR (125 MHz) using JEOL NMR JNM ECA-500 at Puspiptek Kimia, LIPI Serpong, Indonesia and Liquid Chromatography-Mass Spectrometry ((LC-MS Mariner Biospectrometry Work Station with C-18 Column at Puspiptek Kimia, LIPI Serpong, Indonesia) [6].
Pretreatment of palm empty bunch
Pretreatment of PEB was carried out using refluks equipment at 100 oC and stirring method. ratio of [BMIM]bromide and water (1:1, v/v) were added to PEB (10:1). Scanning electron microscope was used to observ the change of its morphological surface. Palm empty bunch without pretreatment was measured as well for comparison. The decreasing of cellulose crystallinity index of PEB was examined by using Fourier Transform Infra Red.
Fermentation substrate
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
78 Extraction of cellulase enzyme
Substrate of fermentation added by tween 80 0.1%, shaked at 150 rpm for 2 h and centrifugated at 3000 rpm for 10 minutes. Supernatant resulted was used as crude extract enzyme and kept cool stored.
Activity assay of cellulase enzyme
Citrate buffer pH 4.8; 0.05 M; 1 mL and one strip of Whatman filter paper no. 1 size 1x6 cm was put into reaction tube, and heated at 50 oC for a minute. Crude extract of enzyme 0,5 mL was added to the tube and incubated at 50 oC for a hour in waterbath. Then, reaction tube was ice cooled and the whatman filter paper was emitted. This solution was added by 0.5 mL of phenol, vortexed and diluted with citrate buffer for 5 times. On this procedure, the reaction tube was wrapped by aluminium foil. Glucose concentration was evaluated on maximum wavenumber [13-14]. Cellulase enzyme activity counted as follows:
t Fp x G ) mL / U ( activity
Enzyme =
G is a glucose concentration in µmol/mL, Fp is dilution factor, and t is incubation time.
Preparation of yeast inoculum
Yeast inoculum was made by using Saccharomyces cerevisiae which precultured in 50 mL media. Composition of medium were 1 g KH2PO4, 1 g (NH4)2SO4, 0.1 g MgSO4.7H2O
and 1 ose Saccharomyces serevisiae in 1L water. Saccharomyces cerevisiae which has been precultured was incubated at 30 oC, 100 rpm for 24 h.
Simultaneous Saccharification and Fermentation
Palm empty bunch pretreated with [BMIM]bromide was put in fermentation bottle and added with 50 mL nutrient solution 1 g L-1 (NH4)2PO4 dan 0.05 g L-1 MgSO4.7H2O.
Buffer citrate pH 5 0.05 M was added to solution until pH 5 and was sterilized. Crude extract enzyme 10% inoculated to fermentation bottle then was added with 10% inoculums, and shaked on 124 rpm. Every 24, 48, 72 and 96 h, this compound was destilated at 76-80 oC. The destilate resulted was examined as its ethanol concentration by using gas chromatography.
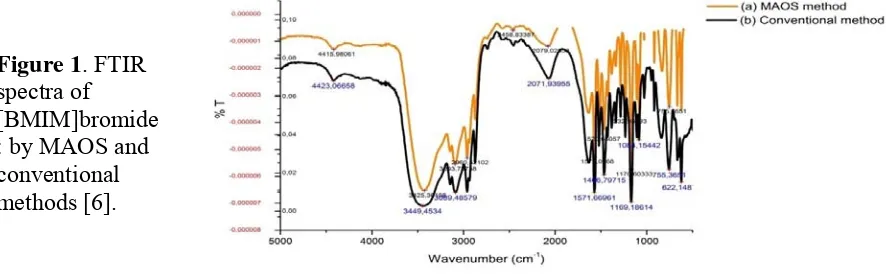
Figure 1. FTIR spectra of [BMIM]bromide : by MAOS and conventional methods [6].
RESULT AND DISCUSSION
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
79
methods (Figure 1). Chemical shifts of 1H-NMR and 13C-NMR of [BMIM]bromide were presented on Table 1 and 2, respectively. Furthermore, the molecular mass of [BMIM]bromide which has been synthesized using refluks and MAS were evaluated by using Liquids Chromatography-Mass Spectrometry is 139, which confirmed as the molecular mass of [BMIM]bromide. Contaminant which could be there was assumed from precursor i.e methyl imidazol. Overall, the synthesis of [BMIM]bromide using MAOS and conventional methods have been successfully performed.
Table 1. Chemichal shifts (in ppm) of 1H-NMR of [BMIM]bromide.
Subject 1H, s, NCHN
2H, m, CH3NCHCH
2H, t, NCH2(CH2)2CH3
3H, s,NCH
2H, NCH2CH2CH2CH3
2H, N(CH2)2CH2CH3
3H, N(CH3)2CH3 [BMIM]Br
MAS method 7.4 7.1 4.8 3.8 1.8 1.3 0.9
[BMIM]Br
reflux method 7.4 7.1 4.8 3.8 1.9 1.3 0.9
Table 2. Chemichal shifts (in ppm) of 13C-NMR of [BMIM]bromide.
Subject C1 C2 C3 C4 C5 C6 C7 C8
[BMIM]Br MAS Method 12.800 18.876 31.352 49.351 127.594 123.521 123.432 35.797 [BMIM]Br refluks Method 12.829 18.886 31.381 49.370 127.00 123.531 122.281 35.854
Figure 2.
Crystallinity index of palm empty bunch that showed in FTIR spectra [6]
Absorption wavenumber at 1433.7 cm-1 showed -CH2 bond of glucose in cellulose
structure. Strong band in this position describe the high of crystallinity index. Crystallinity index has also been screened by using lateral order index (LOI) as described on Table 3. Performance of crystal structur is characteristic at 1430 cm-1 and 897 cm-1 [2].
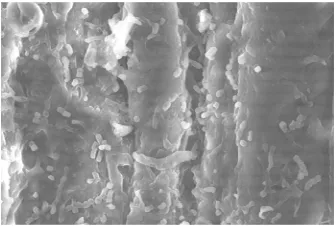
Figure 3. Morphologycal surface of palm empty bunch without pretreatment
Figure 4. Morphologycal surface of palm empty bunch pretreated with [BMIM]bromide
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
80
Investigation using scanning electron microscope at 3000x showed the morphologycal surface of palm empty bunch which was pretreated by[BMIM]bromide gives an open area, displayed more wide of residue surface than unpretreated palm empty bunch. It is figure out at Figure 3 and 4. Pretreatment of palm empty bunch for bioethanol production was used heating up to 100 0C for 1 hour.
Figure 5. Dissolution mechanism of cellulose in ionic liquids (modified from Pinkert et al., 2009 [10]).
Feng and Chen in Pinkert (2009) suggested dilute mechanism caused by ionic liquids. It can be explained, oxygen atoms and hydrogen of cellulose palm empty bunch forms a complex bonding which is called as electron donor – electron acceptor (EDA) with cation and anion of ionic liquid. In this interaction results filter out of hydroxyl group from cellulose ring and cause dissolution of cellulose in ionic liquid [10].
Synthesis of bioethanol was done by using Simultaneous Saccharification and Fermentation with Trichodermaviride and Saccharomycecerevisiae. Trichodermaviride has activity 0.11 U/mL in average. Fermentation was done for 24, 48, 72 and 96 hours and destilation process to separate ethanol at 78-80 oC. Trichoderma viride is a wellknown yeast that consist of cellulase enzyme. Berghem in Rose (1980) reported that Trichoderma viride
have three kinds of cellulase namely endo-β-1,4-glucanase, exo-β-1,4-glucanase and β -glucosidase or cellobiose [11]. Mechanism of these enzyme is firstly, endo-β-1,4-glucanase attack amorf cellulose region randomly and work area of exo-β-1,4-glucanase was opened. The second step is hydrolysis crystalline region of cellulose by exo-β-1,4-glucanase which release two units of glucoses or disaccharide. This disaccharide was hydrolysed by β- glucosidase and produce glucoses. Glucoses that performed will converted by
Saccharomyece cerevisiae into ethanol [12].
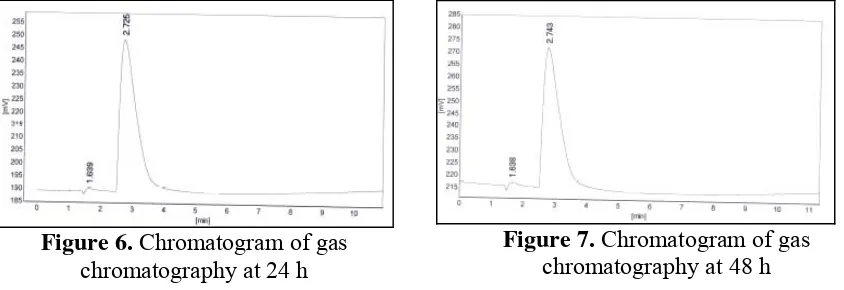
Figure 6. Chromatogram of gas chromatography at 24 h
Figure 7. Chromatogram of gas chromatography at 48 h
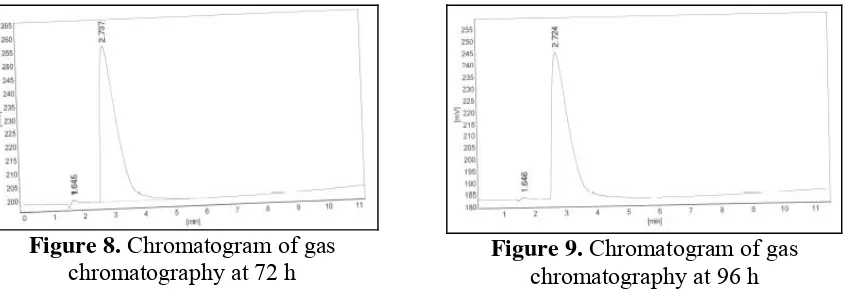
Chromatogram of gas chromatography showed, there is no differences between 24, 48, 72 and 96 h of SSF method. It can be seen on Figure 6 until Figure 9. Calculation using standard ethanol chromatogram showed that all of time fermentation has the same ethanol
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
81
concentration i.e 0.69%. The small of ethanol concentration caused by many factors such as ionic liquids toxic and inhibit yeast during Saccharification and fermentation, accurs side reaction during process etc.
Figure 8. Chromatogram of gas chromatography at 72 h
Figure 9. Chromatogram of gas chromatography at 96 h
Cation and anion’s of ionic liquids is an urgent factor in synthesis which correlated to enzyme and microbe. Not all of ionic liquids adaptable for enzymatic reaction. Although [BMIM]bromide has big impact to decrease crystallinity of cellulose from palm empty bunch however [BMIM]bromide assumed still in trapped in cellulose structure.
CONCLUSION
Ionic liquids synthesis namely 1-butyl-3-methyl imidazoliumbromide or [BMIM]bromide has successfully performed by microwave-assisted synthesis. Palm empty bunch that pretreated by [BMIM]bromide gives broad morphological surface than untreated palm empty bunch. Crystallinity index of pretreated palm empty bunch changes from crystalline into amorph. However it is not enough to convert cellulose’s palm empty bunch into bioethanol better.
ACKNOWLEDGMENT
This research was done in 2012 – 2013 periods and financially supported by Kementerian Riset dan Teknologi, Republik Indonesia. We gratefully acknowledge for this support.
REFERENCES
[1] Sun, Y., Cheng, J., Bioresour.Technol., 2002, 83,1-11. [2] Datta, R. Biotechnol.Bioeng.,1981, 23 (9): 2167-2170.
[3] Spiridon, J., Teaca, C. dan Bodirlau, R., BioResources,2010, 6 (1), 400-413. [4] Varma, R.S., Namboodiri, V.V., Pure Appl. Chem., 2001, 73, 8, 1309-1313. [5] Zhu, H., Li, Y., Wan, X., Adv. Mater. Res., 2012, 393-395, 1471-1474.
[6] Arianie, L., Wahyuningrum, D., Idiawati, N., Nurrachman, Z., Natalia, D., Prosiding Seminar Nasional Teknik Kimia – Teknologi Oleo dan Petroleum Indonesia, Universitas Riau. 2012.
The journal homepage www.jpacr.ub.ac.id ISSN : 2302 ‐ 4690
82
[8] Arianie, L., Idiawati, N and Wahyuningrum, D., Hidrolisis tandan kosong kelapa sawit menggunakan cairan ionik dan selulase, Prosiding seminar nasional Insinas Ristek, Jakarta, Indonesia. 2013.
[9] Arianie, L., Wahyuningrum, D., Nurrachman, Z., Natalia, D., Proceeding of International Conference on Mathematics and Natural Sciences, Bandung, Indonesia. 2012.
[10] Pinkert, A., Marsh, K., Pang, S., dan Staiger, M., Chem. Rev.2009, 109, 6712-6728. [11] Rose. A.H. 1987. Microbial Enzyme and Bioconversions. Academic Press. London. [12] Samsuri, M., Gozan, M., Hermansyah, H., Mardias, R. M., Nasikin, M., Presetya, B.
dan Wijanarki, A., Makara Teknologi, 2007, 11(1),17-24.
[13] Adney, B.; Baker, J. (1996), Measurement of cellulose activities: chemical analysis and testing task. Laboratory analytical procedure. Available from: http://cobweb.ecn.purdue.edu/~lorre/16/research/LAP-006.pdf. Accessed: June 20, 2007