Facts Laws Theories
(experimental (summary statements (simplified understanding Observations) of facts - prediction of of nature - tells how & why)
what will happen)
CHAPTER 1. FUNDAMENTAL CONCEPTS OF CHEMISTRY
A. Introduction
Chemistry is the area of science concerned with the properties of atoms and aggregates of atoms called molecules. The goals of chemical investigation are to develop a fundamental understanding of phenomena relating to the formation, properties, interactions and transformations of molecules, and to utilize this knowledge for the good of humankind. In general, scientific investigation follows a logical sequence of steps called the “scientific method”. The scientific
method consists of (a) characterizing the phenomenon of interest by making definitive experimental observations, (b) deducing laws which summarize a body of experimental facts and enable the prediction of what will happen when variables are changed, and (c) formulating theories which relate the observed behavior to the fundamental nature of matter.
and kinetics) to achieve our objective of “understanding” what is happening when a piece of paper burns.
All scientific languages require precise definitions of the properties to be investigated and quantitative scales and units by which they can be measured. Therefore, as the first topics of discussion, we shall embark on a brief review of common units of measurement and conversion factors.
B. Units of Measurement
Experimental observation requires measurement. To make a measurement one needs: 1. A precise definition of the property to be measured (i.e., length = straight-line distance
between two points).
2. A measurement standard (e.g., Pt-Ir bar at the National Bureau of Standards).
3. A measurement device or instrument (a ruler or meter stick that has been calibrated against the standard).
A wide variety of units are in common usage throughout the world. In the USA, we commonly use the English system of units for the various basic quantities listed in the table below.
Quantity Definition Common Units
Mass Quantity of matter kg, g, oz, lb, ton
Length Distance between two points km, m, cm, in, yd, ft, mi Time Interval between two events s, min, hr, yr
Temperature Heat intensity (kinetic energy) 0K, C, F, R0 0 0
dv dt d2x
dt2
dx dt
2
Once a set of units has been chosen for the fundamental quantities discussed above, units for other quantities may be derived from their functional dependence on the fundamental quantities. Examples of common derived units are given in the following table.
Derived Units
Volume (Length)3 m , cm , L (1 L=1000 cm )3 3 3
Force F = ma = m kg m/s = N (Newton)2
= m g cm/s = dyne2
Energy E = ½mv 2 kg (m/s) = J (Joule)2
= ½m g (cm/s) = erg2
Pressure P = F/A N/m = Pa (Pascal) (1 atm = 101.3 k Pa =2
14.7 psi; 1 bar = 100 k Pa)
C. Conversion Factors
Because we will be employing a variety of units in this course, it will be necessary for you to be able to easily convert from one to another. This can be accomplished by using conversion factors. A conversion factor is an equality in the sense that it relates two equal quantities. Examples of common conversion factors with which you should already be familiar are
l ft = l2 in l yd = 3 ft 1 mi = 5280 ft l lb = l6 oz l ton = 2000 lb 1 hr = 3600 s.
Three very important conversion factors relating length, mass, and volume between the metric and English systems of units are
1 in = 2.54 cm 1 lb = 454 g 1 qt = 0.946 L
12 in
1 ft 1 (but
12
1 1)
12 x
y 1
3 ft x 12 in
1 ft 36 in
36x1015 in. x 1 ft 12 in. x
1 mile 5280 ft
(36x1015)
(12)(5280) mile 5.7x10
12 mile
be unity;
.
The units are treated as algebraic quantities, the same as x and y in the equation
12 x = y
.
Since a conversion factor (such as the ratio 12 in/1 ft) is unity, multiplication by another quantity does not change the absolute magnitude of that quantity - only its units.
.
Both 3 ft and 36 in are expressions of the same length, only in different units! The procedure for unit conversion is simple:
1. Start with the quantity you want to convert.
2. Multiply it by an appropriate conversion factor arranged such that the old unit cancels algebraically and the new unit remains.
Examples: a). Convert 36x10 in. to miles.15
.
1 km x 1000 m
= mass/volume) may be regarded as a conversion factor that relates the mass of a substance to its volume. What volume in liters is occupied by 2 lb of iron if its density is 7.86 g/cm ?3
Exercises: a). Calculate the kinetic energy of a baseball having a mass of 4.0 oz and a speed of 60 mi/hr in SI units. (ANS = 41 J)
E = ½mv = 0.5[(4.0 oz)(1 lb/16 oz)(454 g/1 lb)(1 kg/1000 g)][(60 mi/1 hr)2
(5280 ft/1 mi)(12 in./1 ft)(2.54 cm/1 in.)(1 m/100 cm)(1 hr/3600 s)]2
= 41 J.
b). The density of silicon is 2.33 g/mL and the mass of a silicon atom is 4.65x10 23 g. Calculate the number of silicon atoms in a 1 in. cube of silicon. (ANS = 8.21x10 )23
1 in. Si(2.54 cm /1 in. )(1 mL/1 cm )(2.33 g Si/1 mL Si)(1 Si atom/4.65x103 3 3 3 3 23 g) = 8.21x10 .23
c). The density of ethanol is 0.794 g/mL at 25 C. What volume of ethanol should beo
measured out to obtain a sample weighing 0.33 lb? (ANS = l.9x10 mL)2
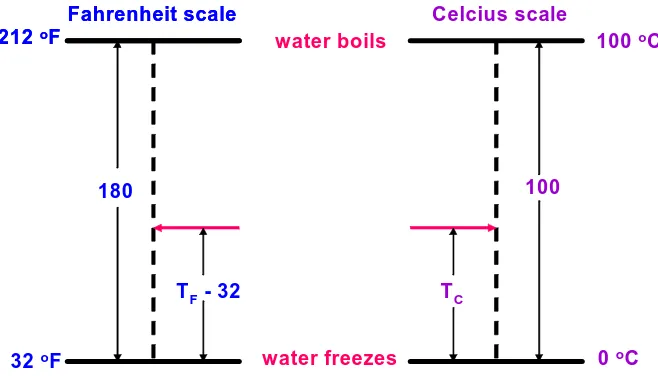
Fahrenheit scale Celcius scale
TF- 32 TC
100
180
water boils
water freezes 212 oF
32 oF
100 oC
0 oC
Fahrenheit scale 212 oF
TF 32 180
TC 100 D. Temperature Conversion
The English system temperature scale is the Fahrenheit scale while the metric system temperature scale is the Celsius scale. Temperatures that are measured on two different scales cannot be converted by simply multiplying by a conversion factor. Therefore, it is necessary to understand how the scales are defined:
Figure 1.1. Diagram illustrating the Fahrenheit and Celsius temperature scales.
With this in mind, conversion from one scale to the other is easily accomplished by setting up the following proportions;
;
so 100(T 32) = 180 TF C
hence T = (100/180)(T 32)C F
and T = (180/100)T + 32.F C
0.156 x 84.25 x 1.7854
1.6 15
E. Significant Figures
The result of a measurement should be expressed by numbers in which only the last digit is in doubt. The number 234.5, for example, implies that the measurement is accurate to ± 0.1 units. This number is said to have 4 significant figures.
Rules of significant figures:
1. The digit 0 is only significant if it is preceded by another number (other than 0). 1060 has 4 significant figures
0.0016 has 2 significant figures
2. Addition and subtraction: round off at the digit closest to the decimal point; 63.43 1.245 + 0.4652 = 62.65 (not 62.6502)
3. Multiplication and division: keep only the number of digits held by the number having the fewest significant figures;
(not 14.6659).
4. Terminal zeros should be expressed in exponential notation unless they are significant. 93,000,000 expressed to 3 significant figures is 9.30x107
Exercises: Carry out the following conversions observing the rules of significant figures. a). 12 oz to mg. (3.4x10 mg)5
b). 5 nickels to Swiss Francs where 1 SF = 0.36 $. (0.69 SF) c). 3x10 cm/sec to mi/hr. (7x10 )10 8
d). The density of Hg (13.6 g/cm ) to lb/in . (0.491 lb/in )3 3 3
F. Basic Constituents of Matter
Atoms are the basic building blocks of matter. They are, however, not the most fundamental particles of nature (which are quarks and leptons). Atoms are composed of the following particles:
Diffuse cloud containing six electrons circulating around a nucleus
nucleus
Diameter approx. 0.0000000002 m = 2x10-10m = 0.2 nm
Diameter approx. 0.000000000000006 m = 6x10-15m = 6 fm
b). Protons - particles having exactly the opposite charge of an electron, but possessing 1836 times more mass.
c). Neutrons - particles with slightly more mass than protons, but without charge.
The electrons of an atom are diffusely distributed about a very tiny nucleus in which the protons (in number equal to the number of electrons) and neutrons are tightly packed.
Anatomy of a carbon atom
Figure 1.2. Schematic illustration of the nominal sizes of the atom and its nucleus.
If an atom were the size of the earth, its nucleus would only be 200 ft in diameter. The mass and charge properties of neutrons, protons and electrons are summarized in the table on the next page. Important properties of atoms:
Particle Charge Mass Rest mass energy (m c )0 2
Proton +e* 1.673x10 27 kg 938.2 MeV
Neutron 0 1.675x10 27 kg 939.5 MeV
Electron e 9.109x10 31 kg 0.511 MeV
e = 1.602x10 Coulomb
* 19
The number of protons in an atom is called the atomic number and is designated by the letter Z.
The number of nucleons (i.e., protons and neutrons) in an atom is called the atomic mass number and is designated by the letter A.
The number of neutrons in the nucleus of an atom may be computed from the relationship
N = A Z.
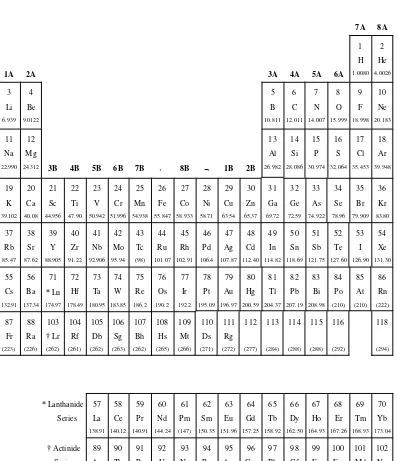
An element is composed of atoms of a particular Z (presently 116 elements are known). Each element is given a special name and chemical symbol. For example, the chemical symbols for aluminum, sodium, and tin are Al, Na, and Sn, respectively. The chemical symbols of all the elements are displayed in the periodic table shown in Fig. 1.3. In this table, the elements are ordered according to their atomic numbers and arranged in a manner that places elements having similar chemical properties in columns (called groups). Two series of elements (called the inner transition elements) have such similar chemical properties that they are removed from the main body of the table and displayed at the bottom. They are known as the lanthanide (or rare earth) and actinide elements. Notable groups among the representative elements are the alkali metals (grp 1A), the alkaline earth metals (grp 2A), the halogens (grp 7A), and the noble gases (grp 8A).
7A 8A
6.939 9.0122 10.811 12.011 14.007 15.999 18.998 20.183
11 12 13 14 15 16 17 18
Na Mg Al Si P S Cl Ar
22.990 24.312 3B 4B 5B 6B 7B 8B 1B 2B 26.982 28.086 30.974 32.064 35.453 39.948
19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
39.102 40.08 44.956 47.90 50.942 51.996 54.938 55.847 58.933 58.71 63.54 65.37 69.72 72.59 74.922 78.96 79.909 83.80
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
85.47 87.62 88.905 91.22 92.906 95.94 (98) 101.07 102.91 106.4 107.87 112.40 114.82 118.69 121.75 127.60 126.90 131.30
55 56 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
132.91 137.34 178.49 180.95 183.85 186.2 190.2 192.2 195.09 196.97 200.59 204.37 207.19 208.98 (210) (210) (222) * Lu
174.97
87 88 103 104 105 106 107 108 109 110 111 112 113 114 115 116 118
Fr Ra Rf Db Sg Bh Hs Mt Ds Rg
(223) (226) (261) (262) (263) (262) (265) (266) (271) (272) (277) (284) (288) (288) (292) (294) † Lr
138.91 140.12 140.91 144.24 (147) 150.35 151.96 157.25 158.92 162.50 164.93 167.26 168.93 173.04
† Actinide
Series
89 90 91 92 93 94 95 96 97 98 99 100 101 102
Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No
(227) 232.04 (231) 238.03 (237) (242) (243) (247) (247) (251) (254) (257) (258) (259)
Figure 1.3. The periodic table of the elements.
decay processes. The stability of an atom is primarily determined by the relative numbers of protons and neutrons it has. There are no stable atoms of Tc, Pm, and elements above Bi.
27
to be Al atoms, they must all have 13 protons. However, they contain different numbers of neutrons. The only stable kind of Al atom contains 14 neutrons.
Stable Al: Z = 13, N = 14, A = 27
Atoms of the same element having different numbers of neutrons are called isotopes. The isotope of aluminum having 14 neutrons may be completely specified using the following notation:
.
Sometimes the number of neutrons is included as a subscript on the right hand side; ,
but this is really not necessary since the number of neutrons can always be determined from the mass number (A) and the atomic number (Z). The 15 kinds of Al atoms are shown in the diagram below.
proton excess Stable neutron excess
The stable isotopes of low-Z elements, such as aluminum, have very nearly the same number of protons and neutrons. For example, the stable isotope of aluminum ( Al) has 13 protons and 1427
neutrons. The isotopes to the left of Al in the above diagram have a proton to neutron ratio greater27
than one (i.e., they have an excess of protons over neutrons) while the isotopes to the right have a proton to neutron ratio less than one. All of these isotopes are unstable and undergo a type of radioactive decay called beta decay. The particular types of beta decay processes by which proton excess isotopes decay (known as β and electron capture decay) convert a proton to a neutron;
+
p n + e (+ neutrino) where e is an antielectron (positron)+ + +
Beta decay of proton excess isotopes:
p + e n (+ neutrino) where e is an electron.+
Beta decay of neutron excess isotopes: n p + e (+ antineutrino). +
The end result of a beta decay process is to transmute the original isotope into an isotope of the adjacent element having atomic number Z 1 in the case of β and electron capture decay or Z
+
+ 1 in the case of β decay. For all of the beta decay processes, the product isotope (or daughter) has the same atomic mass number as the parent since one kind of nucleon has simply been replaced by the other. It is important to note that the daughter isotope has a proton to neutron ratio that is closer to the proton to neutron ratio of the corresponding stable isotope than that of the parent. Ultimately, all unstable isotopes decay step by step to form stable isotopes.
Example: Predict the type of beta decay the unstable isotope C undergoes and give the end14
product of the decay.
The atomic number of carbon is 6 (see periodic table) and so C has 8 neutrons. Therefore14
C is a neutron excess isotope and will undergo β decay producing the isotope N.
14 14
Exercise: Predict the type of decay and product isotope for the radioactive isotope Ca. 36
The atomic number of Ca is 20. Since Ca is a low-Z element, the stable isotopes of Ca should have nearly the same number of neutrons as protons (20). Therefore Ca is on the36
proton excess side of stability and will decay by β /electron capture to give K.
+ 36
G. Atomic Mass
In order to deal quantitatively with atoms, one must have a means of counting them. The most straightforward way to count atoms is to determine their masses. From a knowledge of the mass of a single atom and the mass of a sample, the number of atoms in the sample may be determined. Just as a convenient mass scale has been devised for dealing with macroscopic quantities, so also has a convenient mass scale been defined for dealing with atomic (microscopic) quantities. The isotope of carbon having 6 neutrons, C, has been chosen as the mass standard for the atomic mass12
scale. Its mass is arbitrarily taken to be exactly 12 atomic mass units (amu). The masses of all other elements may then be compared with the mass of a C atom to determine their relative masses on12
this scale. One method for doing this is by mass spectrometry.
1 g = 6.0221367x10 amu23
has three stable isotopes; Mg, Mg, and Mg. Any sample of magnesium atoms will contain fixed24 25 26
proportions of all three of these isotopes. In order to determine the average weight of a magnesium atom, which defines its atomic weight, it is necessary to know these fixed proportions or natural abundances as they are called. The natural abundances of the elements have been determined experimentally and for most elements they are amazingly constant from one sample to another. It is the variability of the natural abundances that limits the accuracy of most atomic weights. Lets determine the atomic weight of magnesium from the data given in the table below.
Isotope Atomic Mass (amu) Relative Abundance (%)
Mg 23.985042 78.99
24
Mg 24.985837 10.00
25
Mg 25.982594 11.01
26
In any mineral containing Mg atoms, 78.99% of them will be Mg, 10.00% will be Mg and 11.01%24 25
will be Mg. Hence the average atomic mass (atomic weight) of Mg is26
Atomic weight = (0.7899)(23.99) amu + (0.1000)(24.99) amu + (0.1101)(25.98) amu = 24.31 amu.
This is the value that appears for magnesium in periodic tables. (Note that the sum of the natural abundances equals 1 and that the accuracy of the atomic weight is limited by the number of significant figures in the natural abundances).
In order to convert the weights of atoms from the atomic mass scale to the metric scale, you must know the conversion factor:
The number 6.022x10 is called Avogadro’s number.23
1 atom Fe x 55.847 amu iron 1 atom Fe x
1 g Fe
6.0221x1023 amu Fe 9.274x10
23 g Fe.
1 mole Fe atoms x 6.0221x10
23 Fe atoms
From the periodic table, we find the atomic weight of Fe to be 55.847 amu.
A very special unit has been defined for use in atom counting. This unit is called the mole (mol).
1 mole of atoms = 6.0221367x1023
Example: What is the mass of 1 mole of Fe atoms?
This example shows explicitly that the definition of the mole has been chosen such that
Atomic weight = mass of 1 mole of atoms
Exercise: The radius of a nucleus is approximately given by the simple formula R = 1.4x10 13A1/3
cm. Calculate the density (in tons/cm ) of a nucleus of A1.3 27
Modern chemistry is founded upon the following principles which were first delineated by Dalton:
a). Atoms are the fundamental particles of which matter is composed.
b). Atoms of any particular element are identical in all their chemical properties.
c). Atoms of one element are different in mass and other properties from all other atoms (i.e. they are distinguishable).
d). Chemical change is the result of the union or separation of atoms.
mass Fe
that molecules must be composed of integer combinations of atoms was supported by the law of definite proportions. This law states that the masses of the different elements making up a particular compound (a substance consisting of two or more elements combined as molecules) are in definite and unchanging proportions. Take the water molecule, H O, for example. Since we now2 know that each molecule of water contains two atoms of hydrogen and one atom of oxygen, it is obvious that all water molecules, no matter what their origin, should exhibit the same mass ratio; (mass H)/(mass oxygen) = (2 amu)/(16 amu) =1/8. Another test of the hypothesis that molecules are composed of integer numbers of different atoms is provided by the law of multiple proportions: if two elements form more than one compound, the different weights of one which combine with the same weight of the other are in the ratio of small whole numbers. As an example of the application of the law of multiple proportions, consider two compounds formed from iron and oxygen atoms, FeO and Fe O ;2 3
Molecular formulas reveal the composition of a compound. They specify the exact number of atoms of each element that make up a single molecule. For example, the molecular formula of sulfuric acid is H SO . From this formula, one may deduce the following numerical relationships:2 4
One H SO molecule contains: 2 4
Therefore, the mass of 1 sulfuric acid molecule = 98 amu. Also
One mole of H SO molecules contains:2 4
2 moles of H [mass = 2(1) g] 1 mole of S [mass = 32 g] 4 moles of O [mass = 4(16) g].
Therefore, the mass of 1 mole of sulfuric acid = 98 g.
The mass of one molecule of a compound is its molecular weight. Hence, in the above example, the molecular weight of sulfuric acid is 98 amu.
Exercise: How many oxygen atoms are in 5.00 g of potassium dichromate (K Cr O )?2 2 7
The molecular weight of PD is 2(39.1) + 2(52.0) + 7(16.0) = 294.2 amu.
(5.00 g PD)(1 mol PD/294.2 g PD)(7 mol O/ 1 mol PD)(6.022x10 atoms O/1 mol O)23
= 7.16x10 atoms of O.22
The empirical formula of a compound only indicates the relative numbers of atoms of each element that makes up a molecule. An empirical formula differs from a molecular formula by an integer: Molecular Formula = n x (Empirical Formula) where n is an integer.
Exercise: What is the empirical formula of a compound containing 92.3% carbon and 7.69% hydrogen by weight?
In 100 g of compound there are 92.3 g C and 7.69 g H. (92.3 g C)x(1 mol C/12.0 g C) = 7.69 mol C
(7.69 g H)(1 mol H/1.00 g H) = 7.69 mol H
moles H/moles C = 1, therefore the empirical formula is CH.
Exercise: If the molecular weight of the compound in the preceding exercise is 78 amu, what is its molecular formula?
per molecule is 78/13 = 6 and hence the molecular formula is C H .6 6
J. Chemical Reactions
Chemical reactions are processes whereby one substance is transformed into another as a result of the combination or disassociation of atoms. A chemical equation can be written to describe the transformation both qualitatively and quantitatively. For example, the transformation of silver ions by reaction with molecules of hydrogen sulfide in aqueous solution into solid silver sulfide and aqueous hydrogen ions may be concisely expressed as
In a chemical reaction, the following must be conserved: a). Atoms - neither created nor destroyed
b). Charge
c). Energy + mass - the sum of the energy plus the energy-equivalent of the mass (mc ) remains2
constant in a chemical reaction. Consider the reaction
Na + OH NaOH + energy.+
It is easy to see that the first two conservation principles are obeyed, but what about the third? In the above example, where did the energy come from? Since energy and mass are related by the Einstein equation, E = mc , the energy + mass conservation principle means that2
In most chemical reactions, the energy involved is so small compared to the masses of the atoms that the conversion of mass into energy may be neglected and for most practical purposes, it may be assumed that mass alone is conserved. This is not a good assumption in nuclear reactions, however. Consider forming an Al atom by combining H atoms and neutrons. The mass + energy27
balance for this process is
13 H + 14 n Al + energy1 27
13(1.007825) amu + 14(1.008665) amu = 26.981535 amu + energy/c 2
energy = mc = (13.1017 + 14.1213 - 26.9815)amu x (931.5 MeV/amu) = 225.0 MeV.2
This energy is the binding energy that holds the Al nucleus together. The mass change per mole is27
0.242 g.
A complete chemical equation must be balanced; both the numbers of atoms of each element and the total charge must be the same on both sides of the equation. For most reactions, this may be done by trial and error. For example, the steps involved in balancing the reaction
NH + O NO + H O3 2 2 are:
1. Balance the hydrogens: 2 3 2. Balance the oxygens: 5/2 2 3. Clear all fractions: (multiply by 2)
_____________________ 4 NH + 5 O 4 NO + 6 H O.3 2 2
K. Chemical stoichiometry
The quantitative information contained in balanced chemical equations, in conjunction with a knowledge of atomic masses, allows one to determine the amounts of reactants that are used and the amounts of products that are formed in chemical reactions. This procedure is referred to as stoichiometry. The following examples illustrate typical stoichiometric calculations.
12.0 g CO x 1 mol CO
Step 2: use the relationships given by the chemical reaction to set up conversion factors for converting 12.0 g of CO to the equivalent mass of CO .2
What mass of oxygen was consumed?
Example: How many grams of the compound C H NO can be formed from 20.0 g C, 10.0 g H ,9 11 4 2
3.00 g N , and 5.00 g O ?2 2
In this type of problem, you must first identify the limiting reagent. It is likely that the quantities of the various substances listed above are not in the same relative proportions as required by the reaction stoichiometry. Therefore, one of these substances will be totally consumed before the others causing the reaction to end. This substance is called the limiting reagent. The easiest way to find the limiting reagent is to convert each of the starting substances to the equivalent number of moles of product. Then the substance that yields the least amount of product is the limiting reagent and the amount of product it yields is all that can be produced under the given conditions.
0.0781 mol cmpd x 197 g cmpd
1 mol cmpd 15.4 g cmpd
0.20 g Al(OH)3 x 1 mol Al(OH)3 78.0 g Al(OH)3 x
3 mol CH4 4 mol Al(OH)3 x 6.022x1023 molecules CH4
1 mol CH4 1.2x10
21 molecules CH 4
Since the available oxygen gives the smallest yield of the compound, it is the limiting reagent. Therefore the maximum amount of compound that could be produced is
.
How many grams of H are left after the reaction? (Ans. = 9.14 g)2
Exercises: a). How many molecules of CH will be produced when 0.20 g of Al(OH) are prepared4 3
by the reaction
A1 C + H O Al(OH) + CH ?4 3 2 3 4
(Ans. = l.2x10 )21
The balanced reaction is A1 C + 12 H O 4 Al(OH) + 3 CH . Therefore,4 3 2 3 4
b). How much Fe can be produced from the reaction of 1.00 ton C and 5.00 ton Fe O2 3
using the reactions:
C + O CO2
CO + Fe O Fe + CO ?2 3 2 (Ans. = 3.10 ton Fe)
The balanced reactions are 2 C + O 2 CO2
3 CO + Fe O 2 Fe + 3 CO .2 3 2
1.00 ton C x (1 ton-mol C/12 ton C) x (2 ton-mol CO/2 ton-mol C) x
(2 ton-mol Fe/3 ton-mol CO) x (55.8 ton Fe/1 ton-mol Fe) = 3.10 ton Fe.
c). A quantity of FeCO was converted to Fe O and CO . The CO escaped as a gas3 2 3 2 2
and the ratio of the weight of the remaining solid sample to the weight of the original FeCO was 0.754. What fraction was converted into Fe O and CO ?3 2 3 2
FeCO + O Fe O + CO .3 2 2 3 2 (Ans. = 0.791)
The balanced reaction is 4 FeCO + O 2 Fe O + 4 CO .3 2 2 3 2
Let X g = original weight of FeCO and Y g = weight of FeCO that reacted. Then the3 3
weight of Fe O formed was Y g FeCO x (1 mol FeCO /115.8 g FeCO ) x2 3 3 3 3
(2 mol Fe O /4 mol FeCO ) x (159.6 g Fe O /1 mol Fe O ) = 0.689Y g Fe O .2 3 3 2 3 2 3 2 3
The weight ratio is (X Y + 0.689Y)/X = 0.754, and so 1 Y/X + 0.689 Y/X = 0.754. Therefore, the fraction converted = Y/X = 0.246/0.311 = 0.791.
L. Solutions
A solution consists of a mixture of at least two substances in the liquid phase -the solute (dissolved substance) and the solvent (substance in which the solute is dissolved). In order to work quantitatively with solutions, the amount of the solute and the amount of either the solvent or the solution must be specified. This is done by defining various concentration units.
Exercises: a). Calculate the molarity of 32.6 mL of a solution containing 5.00g of NaCl. (Ans. = 2.62 M)
(5.00 g NaCl/32.6 mL soln) x (1 mol NaCl/58.5 g NaCl) x (1000 mL/1 L) = 2.62 mol NaCl/L soln.
b). Calculate the molarity and molality of 100 g of solution containing 10.0 g NaCl and having a density of 1.071 g/mL. (Ans: M = 1.83, m = 1.90)
(10.0 g NaCl/100 g soln) x (1 mol NaCl/58.5 g NaCl) x (1.071 g soln/1 mL soln) x (1000 mL/1 L) = 1.83 mol NaCl/L soln.
[(10.0 g NaCl) x (1 mol NaCl/58.5 g NaCl)]/[(100 g soln - 10.0 g NaCl) x (1 kg/1000 g)] = 1.90 mol NaCl/kg solvent.
c). Calculate the molarity and molality of a solution of ethanol (C H OH) in water if the2 5
mole fraction of ethanol is 0.050 and the density of the solution is 0.997 g/mL. (Ans: M = 2.57, m = 2.93)
Let X = mols eth and Y = mols water. Then X/(X+Y) = 0.050 so X/Y = (0.05)/(1 0.05) = 0.0526. In 1 L of solution there are 997 g of solution, so (Y mol water) x (18 g water/1 mol water) + (X mol eth) x (46.0 g eth/1mol eth) = 997, (18Y) + (46.0)(0.0526Y) = 996; Y = 48.8 mol water, X = 2.57 mol eth. Therefore M = 2.57 mol eth /L soln. Molality = (2.57 mol eth)/[(48.8 mol water) x (18 g water/1 mol water) x (1 kg /1000 g)
= 2.93 mol eth/kg water.
Dilution problems:
In the laboratory, it is frequently necessary to make a specified volume of solution having a particular concentration from a more concentrated stock solution. The following examples illustrate how this task may be accomplished.
Example: Describe how to make 1.00 L of 1.00 M HCl from a solution of 12.0 M HCl.
L. Therefore, 1.00 L of 1.00 M HCl may be obtained by measuring out 0.0833 L of 12.0 M HCl and diluting it with water to a total volume of 1.00 L.
Notice that the volume of 12.0 M HCl required in the above example is given by the simple dilution rule:
,
where M = concentration of soln. 1,1 V = volume of soln. 1,1 M = concentration of soln. 2, 2 V = volume of soln. 2.2
Neutralization reactions:
Neutralization involves the reaction of an acid with a base. The most common acids and bases are defined by the following properties (first elaborated by Arrhenius):
An acid is a substance that increases the H concentration in solution;+ A base is a substance that increases the OH concentration in solution.
In simple neutralization reactions, the net reaction that occurs when an acid and a base are mixed is H + OH H O.+
2
For example, when the strong acid HCl is mixed with the strong base NaOH, the reaction is
H + Cl + Na + OH H O + Na + Cl ,+ + + 2
N equivalents of acid or base liters of solution 1 mole H neutralizes 1 mole OH+ .
The above relationship provides the key to solving neutralization problems.
Example: How many moles of HCl does it take to neutralize 1 mole of Mg(OH) ?2
1 mole of Mg(OH) provides 2 moles of OH in aqueous solution, and so 2 moles of HCl2
would be required.
Sometimes it is more convenient to work neutralization problems in terms of equivalents rather than moles. An acid/base equivalent is defined as follows:
1 equivalent of acid = the quantity which gives 1 mole H ,+ 1 equivalent of base = the quantity which gives 1 mole OH . Therefore,
1 eq HCl = 1 mole HCl 1 eq H SO = 1/2 mole H SO2 4 2 4
1 eq NaOH = 1 mole NaOH
1 eq Mg(OH) = 1/2 mole Mg(OH) .2 2
Example: What is the equivalent weight of H SO ?2 4
1 eq H SO x (1 mol H SO /2 eq H SO ) x (98 g H SO /1 mol H SO ) = 49 g H SO .2 4 2 4 2 4 2 4 2 4 2 4
The reason that equivalents are convenient to use is that regardless of the reaction stoichiometry,
1 eq acid neuralizes 1 eq base.
Another commonly employed concentration unit is normality (N), defined as
100 mL Ba(OH)2 x 0.50 mol Ba(OH)2
Example: How many grams of HNO are needed to neutralize 100 mL of 0.50 M Ba(OH) ?3 2
Method 1: using moles -
Method 2: using equivalents
-Titration is a method for determining the concentration of an acid or a base. The concentration of an acid, for example, may be accurately determined by the stepwise addition of a base having a known concentration. The equivalence point is reached when the number of equivalents of base added exactly equals the initial number of equivalents of acid. Experimentally, this point may be determined by using an indicator - a substance that causes the solution to change color at the equivalence point.
Example: In a titration, 35.80 mL of 0.1000 M NaOH is needed to reach the equivalence point when added to 20.00 mL of an H SO solution of unknown concentration. Calculate the2 4
molarity and normality of the original H SO solution. 2 4
Solution using moles - Since the volume and concentration of the base are known, the number of moles of acid that were neutralized can be calculated using the stoichiometric coefficients of the balanced chemical reaction:
35.80 mL NaOH x 0.1000 mol NaOH
35.80 mL NaOH x 0.1000 mol NaOH 1000 mL NaOH x
Solution using equivalents - If equivalents are used, there is no need for a balanced reaction, but one has to take into account the fact that there are 2 equivalents of H SO per mole;2 4
Therefore,
Exercise: The concentration of an oxalic acid (H C O ) solution is to be determined by titrating2 2 4
Exercise: A student mixes 25.00 mL of 0.1500 M HCl with 40.00 mL of 0.0500 M Ca(OH) .2
What is the final concentration of each ion in this solution? HCl H + Cl+
Ca(OH) Ca + 2 OH2 2+
H + OH+ H O
2
The numbers of moles initially are:
H = 25.00 mL x (0.1500 mol/1000 mL) = 0.003750 mol+
Cl = same = 0.003750 mol
Ca = 40.00 mL x (0.0500 mol/1000 mL) = 0.00200 mol2+
OH = 2 x Ca = 0.00400 mol2+
After mixing, all of the H has reacted and 0.00400-0.00375 = 0.00025 mol OH remains.+
Therefore [H ] = 0, [Cl ] = 0.003750/0.06500 = 0.05769 M, [Ca ] = 0.00200/0.06500+ 2+
Review questions
1. The potential energy of a spring is given by the equation E = ½kx , where k is the forcepot 2
constant and x is distance. What are the SI units for the force constant?
2. An American traveling in Europe stops at a gasoline station to fill the tank of his American car. He knows the tank has a capacity of 20 gallons. How many liters should he expect the tank to hold?
3. The American in problem 2 now wishes to check the pressure of his tires, which should be inflated to 30 psi. What reading should he expect on a pressure gauge that reads in millibar? (Note: psi is not really a pressure unit, but rather the ratio m/A with m in lbs. Also, 1 bar = 1x105
Pa and a = 9.80 m/s where a is the acceleration of gravity).2
4. An Eskimo walks out of his igloo to read the temperature on an outdoor thermometer and finds that the reading is the same on both the Celsius and Fahrenheit scales. Should he put on his coat? (What is the temperature?)
5. How many neutrons does a nucleus of the isotope Am contain? What is the daughter isotope241
that is formed when Am undergoes alpha decay? (In alpha decay, the nucleus emits a particle241
consisting of a He nucleus). 4
6. Stable isotopes of heavy elements contain many more neutrons than protons. For example, the most stable isotope of lead, Pb, contains 82 protons and 126 neutrons. Propose a reason for208
this excess of neutrons over protons.
7. Given the information below concerning the stable isotopes of chromium, determine its atomic weight.
Isotope Mass (amu) Natural abundance (%)
Cr 49.946047 4.345
50
Cr 51.940511 83.79
52
Cr 52.940652 9.50
53
Cr 53.938884 2.365
54
8. The composition of calcium pyrophosphate is 25.3% Ca, 39.2% P, and 35.5% oxygen by weight. What is the empirical formula of calcium pyrophosphate?
9. How many atoms of carbon would it take to make 15.0 g of 2-chlorobutane (CH CHClCH CH )?3 2 3
How many grams of hydrogen would this sample contain?
10. A solution is formed by mixing 2.50 g of Ba(OH) in 600 mL of water. Calculate the molarity,2
molality, and normality of this solution. (Note: you may neglect the volume of the Ba(OH) .2 Also, the density of water is 1.00 g/mL).
11. What is the total volume of a solution originally having a volume of 35.0 mL and containing 40.0 mg of AgNO per mL after it has been diluted to a concentration of 16.0 mg/mL?3
12. What was the mass of thorium contained in a sample that required 35.0 mL of 0.0200 M H C O2 2 4
to completely precipitate the thorium as Th(C O ) ?2 4 2
13. A 0.250 g sample of a solid acid was dissolved in water and exactly neutralized by 40.0 mL of 0.125 N base. What is the equivalent weight of the acid?
14. What volume of 5.00 N H SO is required to neutralize a solution containing 2.50 g NaOH? 2 4 15. What are the concentrations of ions remaining in solution when 10.0 mL of 0.205 M HNO is3
added to 15.0 mL of 0.235 M NaOH? Answers
1. Kg/s = N/m2 14. 12.5 mL
2. 76 L 15. [OH ] = 0.0588 M, [Na ] = 0.141 M, +
3. 2069 mbar [NO ] = 0.0820 M3
4. 40 5. 146, Np237
7. 52.00 amu 8. Ca P O2 4 7
9. 3.91x10 C atoms, 1.46 g H23
10. M = 0.0243, m = 0.0243, N = 0.0487 11. 87.5 mL
THINGS YOU SHOULD KNOW
Conversion factors: English units of length, mass, time, and volume. English to metric conversion factors:
1 in. = 2.54 cm, 1 lb = 454 g, 1 qt = 0.946 L 1 g = 6.0221x10 amu23
1 mole = 6.022x1023
Mass of 1 mole = atomic weight expressed in grams
Definitions: Density = mass/volume; Pressure = force/area Atomic number
Atomic mass number Isotope
Ion
Beta decay
Celcius and Fahrenheit temperature scales Molecular formula
Empirical formula Limiting reagent Neutralization reaction Equivalent
Concentration units (M, m, N, wt %, mole fraction) Arrhenius acid and base
Equivalence point
Relationships: Significant figures M V = M V1 1 2 2
1 mole H neutralizes 1 mole OH +