Review
myo
-Inositol metabolism in plants
Frank A. Loewus
a,*, Pushpalatha P.N. Murthy
baInstitute of Biological Chemistry,Washington State Uni
6ersity,Pullman,WA99164-6340,USA
bDepartment of Chemistry,Michigan Technological Uni
6ersity,Houghton,MI49931,USA Received 3 June 1999; received in revised form 19 July 1999; accepted 19 July 1999
Abstract
The multifunctional position supplied bymyo-inositol is emerging as a central feature in plant biochemistry and physiology. In this critique, attention is drawn to metabolic aspects and current assessment is made of manifold ways in whichmyo-inositol and its metabolic products impact growth and development. The fact that a unique enzyme, common to all eukaryotic organisms where such assessment has been undertaken, controls conversion ofD-glucose-6-P to 1L-myo-inositol-1-P provides a useful point
of departure for this brief metabolic survey. Some aspects such as biosynthesis, phosphate and polyphosphate ester hydrolysis, and O-methylation of myo-inositol have captured the consideration of molecular biologists, yet other aspects including oxidation, conjugation, and transfer to phospholipids remain virtually untouched from this viewpoint. Here, an attempt is made to enlist new interest in all facets ofmyo-inositol metabolism and its place in plant biology. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords: Galactinol; Galactopinitol; Glycosylphosphatidylinositol; Glycosylinositolphosphorylceramide; IAA-MI conjugates; myo-Inositol; 1L-myo-Inositol-1-P; Ins(3)P1synthase; MI kinase; MI monophosphatase; MI oxidation pathway;O-Methyl Inositols; Ononitol;
Phosphatidyli-nositols; Phytic acid; Pinitol; Raffinose
www.elsevier.com/locate/plantsci
1. Introduction
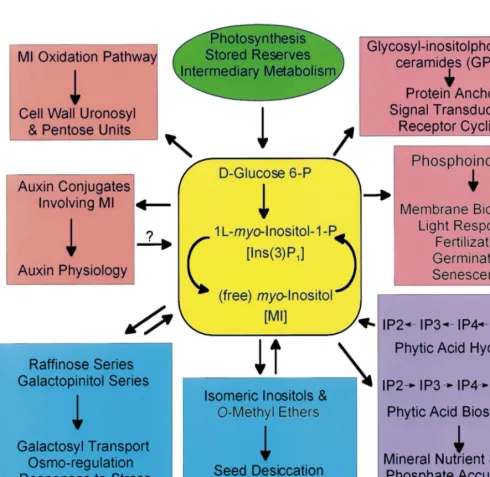
Isolation of myo-inositol (MI) from muscle ex-tract by Scherer in 1850 led to 80 years of intense interest on the natural occurrence, properties, derivatives, and stereoisomers of the cyclitols. Then, discovery that MI functioned as a growth factor for certain microorganisms and as a re-quirement for growth of certain mutant forms of yeast prompted fresh interest in its biochemical and biological features. Soon it became apparent that MI played a central role in growth and
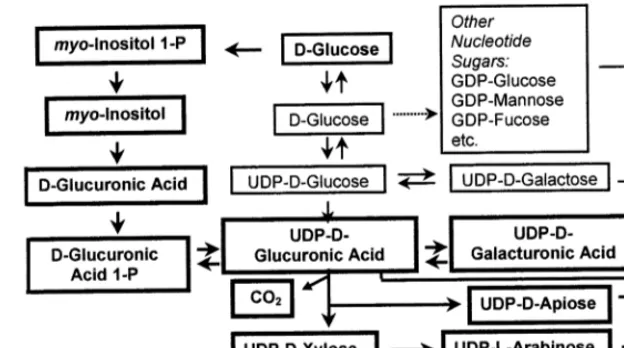
devel-opment [1]. This was especially true in plant biol-ogy where molecular entities containing or utilizing MI were involved in structure and func-tion [2]. Fig. 1 summarizes this informafunc-tion by categorizing specific products of MI metabolism with particular interest to plant biologists and by identifying avenues of inquiry which may lead to a better appreciation of this unique molecule and its position in plant science. Colored backgrounds in Fig. 1 attempt to provide a sense of related func-tions while avoiding the confusion of ‘metabolic mapping’.
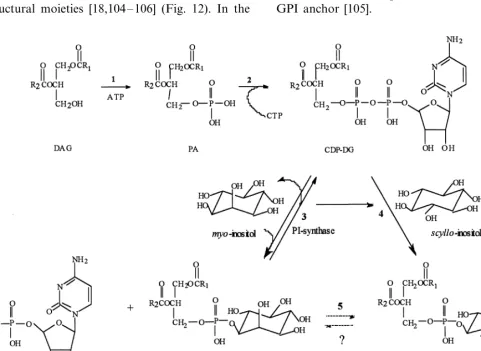
Conversion of D-glucose-6-P to 1L-MI-1-P
con-stitutes the first committed step in MI biosynthesis [1 – 3]. Metabolic processing of MI beyond biosyn-thesis produces other stereo-forms of inositol and leads to a host of functional roles, all of which require this unique cyclitol. These include:
Abbre6iations: ABA, abscisic acid; IAA, indole-3-acetic acid; MI,
myo-inositol; Ins(3)P1, 1L-myo-inositol-1-P; InsP6 or MI-P6, phytic
acid; MIOP, myo-inositol oxidation pathway; SNOP, sugar nucle-otide oxidation pathway.
* Corresponding author. Tel.:+1-509-335-3413; fax:+ 1-509-335-7643.
E-mail address:[email protected] (F.A. Loewus)
Cycling of1L-MI-1-P and free MI by MI
phos-phatase and MI kinase [4,5].
Oxidation of free MI toD-glucuronic acid with
its subsequent role in biogenesis of uronosyl and pentosyl units of pectin, hemicelluloses, and related structures in plant cell walls [1,2,6].
Esterification of MI to form auxin (IAA) esters
and their glycosides [7,8].
Conjugation of free MI with UDP-D-galactose
to form galactinol, the galactosyl donor for
biosynthesis in the raffinose and galactopinitol series of oligosaccharides [9,10].
Isomerization and methylation of MI and other
isomeric (scyllo-,chiro-,muco-, andneo-) inosi-tols to form O-methyl inositols (sequoyitol, bornesitol, quebrachitol, pinitol, ononitol, etc.) which participate in stress-related responses, storage of seed products, and production of inositol-glycosides such as pinitol-galactosides [9 – 13].
Fig. 2.myo-Inositol.
If one takes biosynthesis as a basis of assign-ment and traces the origin from D-glucose
6-phos-phate, then clockwise assignment preserves biosynthetic relatedness. Unhappily, recent ad-vances involving key roles for MI polyphosphates involved in signal transduction (where addition or loss of a single phosphate may alter assignment fromD- toL- or vice-versa) have created confusion
for those unfamiliar with rules of cyclitol nomen-clature. The upshot is a tentative agreement by the International Union of Biochemistry to relax rules of nomenclature so that1L-MI-1-P, the product of myo-inositol-1-phosphate synthase, may be desig-nated 1L-MI(1)P1, 1D-MI(3)P1, or simply, Ins(3)P1
where the symbol Ins signifies MI with counter-clockwise numbering from 1D. Thus, 1D
-MI(1,4,5)P3, an important physiological signal
generated during phosphatidylinositol-4,5-bisphos-phate metabolism, becomes simply Ins(1,4,5)P3.
More detailed discussion of the stereochemistry of MI and its phosphate esters is found in [14] and on the Internet at http://www.chem.qmw.ac.uk/
iubmb/nomenclature/.
3. MI biosynthesis
3.1. E6idence for cyclization of D-glucose to MI
Although the D-gluco configuration inherent in
MI was recognized by Maquenne as early as 1887 and the proposition that D-glucose-6-phosphate
cyclized to form Ins(3)P1 enzymatically was
ad-vanced by H.O.L. Fischer in 1945, unequivocal evidence for conservation of the 6-carbon chain of
D-glucose during cyclization to MI did not appear
until 1962 [20,21].
The experimental approach involved recovery of labeled MI from aD-[1-14C]glucose-labeled parsley
leaf followed by administration of this labeled MI to detached immature strawberry fruits where it was utilized as a carbon source for pectin biosyn-thesis [20,22]. Carbon-14 was recovered inD
-galac-turonosyl and L-arabinosyl residues of pectin
which upon radioanalysis revealed 79% of the label in carbon 1. Distribution of 14C in
sucrose-derived D-glucose, pectin-derived D-galacturonate
and L-ascorbic acid from the parsley leaf also had
\80% of their 14C in carbon 1. In other words,
about 80% of the 14C in these products of D -(1-14C)glucose metabolism remained at the original
Fig. 3. Conventions for numbering substituents inmyo -inosi-tol.
Biosynthesis of phytic acid (MI-P6) and phytic
acid pyrophosphates [14 – 16].
Metabolic recycling of products of phytic acid
hydrolysis during phytase-mediated phytic acid dephosphorylation [14 – 16].
Biosynthesis of phosphatidylinositol, its
poly-phosphates, and precursors of MI polyphos-phate-specific signal transduction [1,2,14,17].
Glycosylated-phosphatidylinositol and
glycosy-lated-inositolphosphorylceramide [18,19].
2. Nomenclature
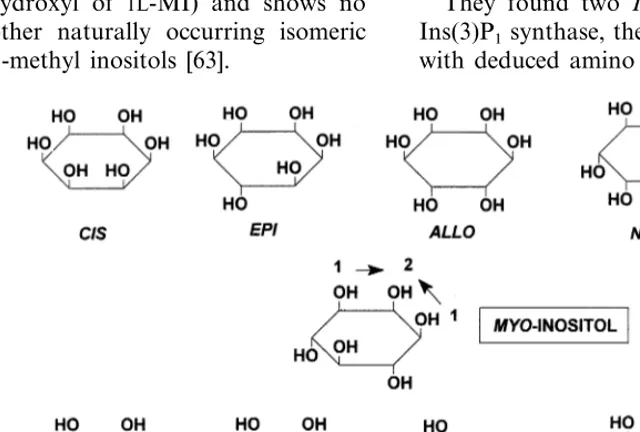
The nomenclature of inositols has been an on-going source of confusion and conflict for decades. MI is ameso compound with a plane of symmetry that rotates the structure about C2 and C5 as fixed positions (Fig. 2). The remaining four carbon atoms consist of two prochiral pairs, C1=C3 and C4=C6. If the carbon ring is numbered clock-wise, as shown by numbers inside the ring, assign-ment of a single substituent on carbon 1 is 1L.
Conversely, if the carbon ring is numbered coun-terclockwise as shown by numbers external to the ring, assignment is 1D. 1L-MI-1-P, the product of 1L-myo-inositol-1-phosphate synthase (E.C.5.5.
site of labeling. Some redistribution of 14C
be-tween terminal carbons is normal during triose/
hexose phosphate metabolism.
This analytical approach provided profound in-sight into three aspects of MI metabolism, namely MI biosynthesis, the MI oxidation pathway, and phytic acid biosynthesis. It provided evidence for cyclization of the carbon chain of D-glucose to
form MI. It revealed an alternative biosynthetic pathway to uronic acid and pentose constituents of plant cell wall polysaccharides quite indepen-dent of the one involving UDP-D-glucose
dehy-drogenase. Finally, it supplied stereochemical evidence for the putative initial phosphorylated intermediate leading to phytic acid, a major form of phosphate storage in plants.
3.2. Mechanism of MI biosynthesis
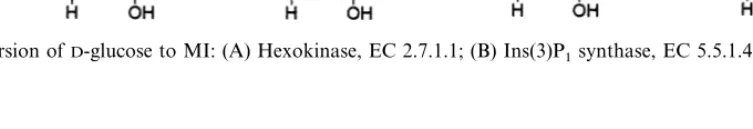
Biosynthetic conversion of D-glucose to free MI
involves three enzymatic steps (Fig. 4). Step B, cyclization ofD-glucose-6-P to Ins(3)P1, is the first
committed step in MI biosynthesis. Step C, loss of phosphate, releases free MI. Overall, this scheme constitutes the sole pathway of MI biosynthesis in cyanobacteria, algae, fungi, plants, and animals and occupies a central role in their cellular metabolism.
Cyclization of D-glucose-6-phosphate to
Ins(3)P1is irreversible. This process also highlights
a dilemma in nomenclature since D-glucose is
numbered clockwise about the pyranose ring while conventional numbering of MI inverts the num-bering of its inherent D-gluco configuration.
Ins(3)P1 synthase appears to be a highly
con-served enzyme [23]. Functionally, this conversion of D-glucose 6-P to Ins(3)P1 involves three
sub-steps (Fig. 5):
NAD+-coupled oxidation of carbon 5 of D
-glucose-6-P.
Aldol condensation between carbon 1 and
car-bon 6 of 5-keto-D-glucose-6-P (D-xylo
-5-hexulose-6-P).
NADH-catalyzed reduction of 2-myo
-inosose-1-P (D-2,4,6/3,5-pentahydroxy-cyclohexane-2-P
to yield Ins(3)P1.
Specific points of interest regarding Ins(3)P1
syn-thase include:
Preference for the b-anomeric form of
glucose-6-P.
Enzyme-bound NAD (removable by charcoal
treatment to generate an inactive apo-enzyme).
NAD+-catalyzed oxidation at carbon 5 of
glu-cose-6-P (substep 1) to yield an enzyme-bound 5-keto-glucose-6-P with hydride ion transfer from glucose-6-P to the pro-S position of car-bon 4 on the nicotinamide moiety of NAD+ (Kinetic studies indicate a sequential reaction with NAD+ adding first. There is a distinct isotope effect in removal of hydrogen from carbon 5 of glucose-6-P.).
Base-catalyzed cyclization (substep 2). —
Oxy-gen at carbon 5 is retained. The pro-R hydro-gen is removed from carbon 6 while the pro-S
hydrogen is retained. The second intermediate within brackets is 2-myo-inosose-1-P (D-2,4,6/
3,5-pentahydroxycyclohexanone-2-P). For addi-tional details, see [24].
Fig. 4. Conversion ofD-glucose to MI: (A) Hexokinase, EC 2.7.1.1; (B) Ins(3)P1synthase, EC 5.5.1.4; (C) MI monophosphatase, EC 3.1.3.25.
Stereospecific oxidation of enzyme-bound
NADH (substep 3) including transfer of its pro-Shydride ion to thesi face of the carbonyl group to generate Ins(3)P1.
An undisturbed phosphate – carbon bond.
Hydrolysis of Ins(3)P1 by a specific MI
monophosphatase [4] completes overall conversion of D-glucose to free MI.
3.3. Biochemical and physiological aspects of
Ins(3)P1 synthase
The structural gene INO1 for Ins(3)P1 synthase
was first isolated from the yeast Saccharomyces
cere6isiaeby Donahue and Henry [25]. Subsequent
studies involvingino1 mutations in MI auxotrophs provided insight into regulatory control processes [3,26]. Transcripts with homology to this gene have been obtained from several plant sources and are summarized in these cited references [3,23]. The first such plant gene for Ins(3)P1 synthase to
be characterized was tur1, a cDNA from the duck-weed,Spirodela polyrrhiza, which was rapidly and spatially up-regulated during an ABA-induced morphogenic response [27]. This effect was local-ized to stolon tissue that connects the developing turion to the node of the mother frond. The authors considered several possible scenarios in which MI synthesis might play a role. These in-cluded phytic acid accumulation, lipid synthesis, an ABA signal transducing mechanism involving phosophoinositide metabolites, altered flux of MI metabolites into the cell wall and/or an auxin-linked cell elongation involving auxin conjugates, and induced response to stress involving methyl ethers of inositol. Of these possibilities, an effect on the nature of cell wall structures appeared most interesting. Since S. polyrrhiza was not yet trans-formable at the time of these studies, Smart and Flores [28] generated transgenic Arabidopis plants over-expressing Ins(3)P1 synthase TUR1 cDNA
from S. polyrrhiza and found these plants to
con-tain elevated Ins(3)P1 synthase activity with
con-comitant four-fold increase in endogenous MI. Comparison of transgenic to wild-type plants re-vealed no significant differences in whole plant growth habit, expansion growth, germination rate, flowering time, stem thickness, in vitro root growth and germination/survival on high salt or low temperature regimes. A four-fold increase in endogenous MI may have been insufficient to
trigger gross differences in growth or development although compositional differences have been ob-served in lily pollen germinated in media that was supplemented with MI ranging from 0.3 to 2.8 mM [29].
In algae and plants, both cytosolic and chloro-plastic forms of Ins(3)P1 synthase have been
iso-lated and characterized [30]. Although the biochemical and kinetic parameters of these two forms do not differ significantly between each other or from other cytosolic Ins(3)P1 synthases
previously described [3], the native cytosolic form is homotrimeric while the native chloroplastic form is homotetrameric. Interestingly, a cyanobac-terium, Spirulina platensis, included in the cited study, contained only one cytosolic homote-trameric form as anticipated by the endosymbiont theory for a cyanobacterial origin of plastids [3,31,32].
Analysis of an INO1-like transcript (termed INPS1 by the authors [23]) from salt-stressed
Mesembryanthemum crystallinum (ice plant)
re-vealed a diurnal fluctuating increase in mRNA during the light period that could be coordinated with the gene encoding MI-O-methyltransferase, an enzyme methylating MI to D-ononitol which is
epimerized to D-pinitol. D-Pinitol accumulates in
salt-stressed M. crystallinum plants and is consid-ered to be the principal osmoregulator. Compara-ble experiments with Arabidopsis thaliana
transcripts failed to produce this effect and the authors conclude that there is probably no stress-mediated induction of Inps1 mRNA in Arabidop
-sis, an observation similar to that made by Smart and Flores [28]. Salt-tolerant varieties of rice grown in a NaCl environment exhibited a photore-sponsive enhancement of chloroplast and cytosolic Ins(3)P1 synthase activity [33]. The authors
specu-late on the possible role of free MI as an osmolyte in the chloroplast through coordinate activation and/or induced expression of Ins(3)P1 synthase
and MI monophosphatase.
Keller et al. [34] obtained a full-length cDNA from potato epidermal tissue that encoded Ins(3)P1 synthase (termed StIPS-1). RNA blot
analysis revealed the highest StIPS-1 transcript levels in photosynthetic tissues but much lower levels in roots and tubers. Light greatly elevated
StIPS-1 transcript levels but drought stress had no
strongly reduced levels of MI, galactinol, and raffinose. These plants also showed distinct mor-phological aberrations including decreased overall tuber yield. These findings highlight broad bio-chemical and physiological effects brought on by altering Ins(3)P1 synthase activity in potato and
possibly most other plant species.
Quite recently, Yoshida and co-workers [35] isolated a cDNA clone, pRINO1, from rice (Oryza sati6a L.) callus suspension cultures that is highly
homologous to Ins(3)P1 synthase from yeast and
plants. Its transcript appears in the apical region of globular-stage embryos 2 days after anthesis and strong signals were detected in the scutellum and aleurone layer after 4 days. Phytate-contain-ing particles or globoids appeared in the same tissues at 4 days, coinciding with the RINO1 transcript. This study demonstrates that Ins(3)P1
synthase is probably the first committed step in phytic acid biosynthesis although a complemen-tary process involving salvage of MI by MI kinase remains untested.
In studies just reviewed, genes encoding Ins(3)P1
synthase are variously identified as INO1 (Saccha
-romyces cere6isiae), TUR1 (Spirodela polyrrhiza),
StIPS-1 (Solanum tubersum), INPS1 (Mesem
-bryanthemum crystallinum), pRINO1 (Oryza sa
-ti6a), etc. Deduced amino acid sequences obtained
from these plant-derived sources are quite similar. In the interest of consistency, a common term like INPS1 as proposed by Ishitani et al. [23] seems desirable.
3.4. Concerning free MI
Dephosphorylation of Ins(3)P1 constitutes the
sole de novo route to free MI in plants. All other sources derive from salvage mechanisms involving recovery of free MI from other metabolic MI-con-taining products. Free MI is generally regarded as a ubiquitous constituent of plant tissues and in some species, notablyActinidia arguta, (kiwifruit), MI is the major ‘sugar’ constituent (60 – 65%) dur-ing the first 20 – 30 days after anthesis [36]. Stress-related aspects of MI accumulation have been noted repeatedly [1,2,6,23,37,38] but specific bio-chemical and molecular details are needed. In fact, accumulation of free MI may be a more universal phenomenon in life forms than generally realized. In overwintering ladybird beetles (Creatomegilla
undecimnotata), free MI, functioning as a possible
cryoprotectant, increases more than four-fold (from 2.5 to 11 mg/mg wet weight) during winter months [39].
A relatively specific alkaline, magnesium-depen-dent phosphatase (MI monophosphatase, EC 3.1.3.25) from lily pollen hydrolyzes Ins(3)P1, its
enantiomer Ins(1)P1, and at a somewhat lower
rate, Ins(2)P1, to free MI [40,41]. Animal tissues
contain a similar MI monophosphatase [42] but Ins(2)P1 which is substituted in the axial position
is not a substrate although it does act as a compet-itive inhibitor in the case of bovine brain enzyme. There is need to revisit this matter of substrate specificity since most plant studies are dated in this regard.
Gillaspy et al. [4] cloned three MI monophos-phatase activities from tomato (LeIMP). All iso-forms were lithium ion-sensitive over a concentration range similar to that exhibited by human MI monophosphatase. When labeled anti-sense RNA probes were used to follow mRNA accumulation of these isoforms at different devel-opmental stages and in different organs, LeIMP1 mRNA accumulation was greatest in light-grown seedlings, flowers, young and mature green fruit (decreasing as fruit proceeded to the breaker stage), and callus tissue. LeIMP3 mRNA was detected in the same tissues and had a much higher response in the shoot apex. LeIMP2 mRNA levels were significantly lower than the other two isoforms. Consideration of apparent differences in the expression patterns of these three isoforms, prompted the suggestion that their activ-ities functioned within different cell types or spa-tially distinct cellular compartments. Evidence to support spatially regulated expression has since been presented [43]. Given the diverse demands for free MI as outlined in Fig. 1 and the ancestral history of plant organelles, such a suggestion is quite possible. Clearly, there is need for more information on localization of MI monophos-phatase within the plant cell.
Inhibition of MI monophosphatase by lithium ions is especially noteworthy. At 0.1 mM Li+, bacterial protein extracts, each expressing one of
the LeIMP gene products, had less than 20% of
Fig. 6. Cell wall polysaccharide biogenesis via themyo-inositol oxidation pathway (bold font) and the sugar nucleotide oxidation pathway.
MI monophosphatase, little is known regarding its impact on availability of free MI for numer-ous biosynthetic and regulatory requirements (Fig. 1). Lithium ion delayed initiation of DNA synthesis and cell division when introduced into synchronized Catharanthus roseus cell cultures, a condition largely prevented when MI was in-cluded in the medium. Preparations of MI monophosphatase from C. roseus were inhibited 80% by 10 mM Li+ [45]. Use of Li+ inhibition as a tool for studying modulation of free cellular MI appears to be a viable option, one which may also alter other metabolic pathways con-nected to a demand for free MI such as its role as substrate for MI kinase (EC 2.7.1.64). Curi-ously, little attention has been given to this latter enzyme which is present in plants, animals and microorganisms [46]. Its product, Ins(3)P1, has
the same configurational structure as that pro-duced by Ins(3)P1 synthase [5]. While one might
regard this recycling of MI back into a pool of Ins(3)P1 as a salvage mechanism, it fails to take
into consideration any localization of these en-zymic activities or temporal demands during de-velopment. Together, Ins(3)P1 synthase and MI
kinase constitute ways in which Ins(3)P1 is
formed from D-glucose-6-P or free MI in plants.
The former enzyme is biosynthetic while the lat-ter must rely on sources that generate free MI from MI monophosphatase or other MI-conju-gated forms. Unresolved are temporal and spa-tial patterns of synthase and kinase during growth and development.
4. myo-Inositol oxidation pathway
Substantial experimental support for a MI oxidation pathway (MIOP) in plants has accumulated during the past 35 years since this pathway was first proposed [22] with over 50 papers from Loewus’s laboratory alone addressing this topic. The presence of a MIOP has been demonstrated in a wide variety of plant tissues including strawberry fruit, parsley leaf, lily floral parts and pollen, pear pollen, cultured sycamore and rice callus, corn root-tip, duckweed, germinating and developing wheat, pine pollen, rubber latex serum, and algae.
The MIOP (Fig. 6) involves cyclization of
D-glucose-6-P to Ins(3)P1, loss of phosphate to form
MI, oxidation of MI to D-glucuronic acid,
phosphorylation at carbon 1 (aconfiguration), and conversion by uridylyl transferase to UDP-D
-glucuronic acid. Alternatively, D-Glucose-6-P is
converted to UDP-D-glucose, which undergoes
oxidation to UDP-D-glucuronic acid, a process
termed the sugar nucleotide oxidation pathway (SNOP) [1,2,6,21]. In Fig. 6, bold borders contrast steps of the MIOP and its metabolic products from those of the SNOP.
Both UDP-D-glucuronic acid and its product of
decarboxylation, UDP-D-xylose, strongly inhibit
NAD+-dependent
UDP-D-glucose dehydrogenase
[47]. The requirement for NAD+ as well as kinetic restraints imposed by product inhibition are important considerations when invoking the SNOP for UDP-D-glucuronic acid metabolism. Neither of
UDP-D-glucose oxidation will leave the MIOP as the
principal pathway to UDP-D-glucuronate and its
products. Relative fluxes in demands on UDP-D
-glucose coupled to the inhibitory effect of UDP-D
-xylose on oxidation of UDP-D-glucose may well
allow MIOP to play a major role in hexose/ pen-tose metabolism, cell wall pectin and hemicellulose formation, and starch synthesis [48 – 50]. When the hydrogen isotope effect, which occurs during Ins(3)P1 synthase-catalyzed conversion of D -[5-3H]glucose-6-P to [2-3H]MI, was utilized to
com-pare the functional role of the sugar nucleotide and MI oxidation pathways in germinating lily pollen [51], 3H/14C ratios of glucosyl and
galactur-onosyl residues from amyloglucosidase/pectinase hydrolysates strongly supported the view that con-version of glucose into galacturonic acid residues of pectin used the MIOP. Recently, UDP-D
-glu-cose dehydrogenase has been isolated and purified from soybean nodules [52] and cloned from soy-bean cell suspension cultures [53]. The expression pattern of the latter was studied at selected devel-opmental stages. Results suggest that this enzyme has a key regulatory role in production of hemicel-lulosic precursors. Access to this cloned gene for UDP-D-glucose dehydrogenase together with
those for Ins(3)P1 synthase and MI
monophos-phatase should provide the tools needed in future studies to dissect expression patterns of the two pathways at critical stages of growth and development.
5. Indole-3-acetic acid (IAA) conjugates of MI and its glycosides
Within the family of IAA-conjugates found in plants are a number of IAA-esters that include IAA-MI and IAA-MI with galactosyl or ara-binosyl substituents [7,8]. Biosynthesis of IAA-MI is a two step reaction mechanism involving UDP-glucose and free MI as substrates:
IAA+UDP-glucoseB.
\1-O-IAA-glucose+UDP 1-O-IAA-glucose+myo-inositolB.
\IAA-myo
-inositol+glucose
Addition of sugar residues as 5-b-O- sub-stituents follows esterification. These esters are generally regarded as inactive ‘storage’ forms whereby plants cope with excess auxin production.
Alternately, such structures might facilitate trans-port of auxin within the plant. Apart from the fact that free MI is required for IAA-MI production, little is known regarding the metabolic fate of this moiety. During tropic response of a plant to an asymmetric stimulus, there is asymmetric distribu-tion of IAA. Assuming that the source of this hormone is an IAA conjugate such as IAA-MI, then the fate of MI may well be related to its capacity to undergo oxidation via the MIOP and enter into cell wall constituents of pectin and/or hemicellulose. Such studies have yet to be undertaken.
6. Inositols and their methyl ethers
6.1. Galactosyl inositols (raffinose and
galactopinitol series)
There are nine isomeric structures for inositol (Fig. 7). Eight isomers are diastereomeric, one of which is enantiomeric. Naturally occurring inosi-tols and/or their methyl ethers in plants include
myo-,scyllo-, muco-, neo-, +chiro-, and −chiro
-inositol [54 – 56]. Onlymyo-inositol (MI) is synthe-sized de novo from hexose phosphate by MI 1-phosphate synthase and MI monophosphatase. Production of other isomeric inositols involves metabolic processing of MI. For many years fol-lowing their isolation and characterization, inter-est in these inositols was limited largely to their chemistry [57] and to their occurrence, which in some cases appeared to be species-specific [58]. An important physiological role for MI emerged with the discovery that galactinol (O-a-D
-galactopyra-nosyl (13)-Ins) functioned as galactosyl donor for biosynthesis of the trisaccharide, raffinose and for higher galactosyl homologues that are involved in phloem transport, seed development, seed desic-cation, and numerous stress-related responses of plants [9 – 12,59]. Subsequent studies uncoveredO -methyl inositol-based galactosides containing ononitol (4-O-methyl Ins) and pinitol (1D-4-O
Stachyose synthase (galactinol donor),
raffinose+galactinolstachyose+myo- ino-sitol
An exchange reaction, myo-[3H]inositol+
galactinol[3H]galactinol+myo-inositol
Stachyose synthase (galactosylononitol donor),
raffinose+ galactosylononitol stachyose +
ononitol
Galactosylononitol synthase – ononitol+
galac-tinolgalactosylononitol+myo-inositol Re-analysis of the structure of a galactosylonon-itol previously isolated from adzuki bean [61] re-veals it to be identical to the same galactosyl-ononitol involved in raffinose and stachyose biosynthesis [62].
Galactinol synthase utilizes UDP-D-galactose as
its galactosyl donor. In enzyme preparations of rice bean (Vigna umbellata), galactinol is a re-quired galactosyl donor for galactosylononitol and this, in turn, donates galactosyl moities to raffinose and stachyose. Only galactinol functions as galactosyl donor to galactosylononitol [12]. A study of ononitol biosynthesis and accumulation
in V. umbellata during drought stress found that
methylation of MI appears to correlate with stem-localized methyltransferase activity and a signifi-cant rise in ononitol in the leaves [63]. The methyltransferase is specific for the 4-hydroxyl of Ins (a.k.a., 6-hydroxyl of 1L-MI) and shows no
activity with other naturally occurring isomeric inositols and O-methyl inositols [63].
6.2. Stress-related responses in6ol6ing inositol or
O-methyl inositol
A broad variety of naturally occurring plant constituents contribute to osmotic regulation in plants that have been exposed to stressful environ-ments [64]. Included among these are the inositols and theirO-methyl ethers. The term ‘stress’ is used here to refer to such abiotic factors as drought, heat, cold, salinity, pollutants, and reactive oxygen species. Brief overviews are found in several stud-ies involving inositols and stress tolerance [9 – 11,23,33,35,37,38,56,65,66].
The halophytic ice plant (Mesembryanthemum
crystallinum) is a model for studies involving
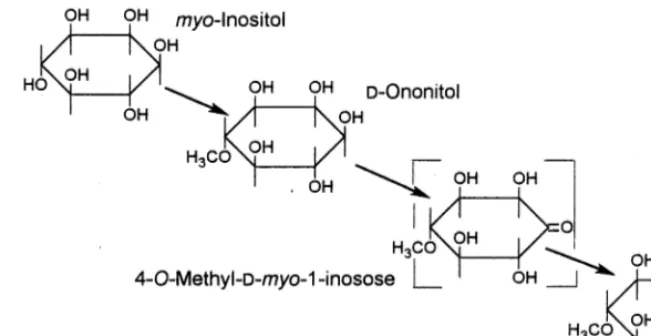
in-duced crassulacean metabolism. Plants irrigated with 400 mM sodium chloride accumulate pinitol which eventually constitutes over two-thirds of the soluble carbohydrate fraction and approximately 10% of the dry weight [67]. The osmotic adjust-ment of this plant is generally attributed to its high level of pinitol. Bohnert and his colleagues [11] have studied the biosynthetic pathway that leads to pinitol accumulation with the hope that this effort will lead to a better understanding of drought and salt tolerance. The pathway involves three enzymic steps beyond hexose phosphate (Fig. 8).
They found two Inps-like transcripts encoding Ins(3)P1synthase, the first enzyme in the pathway,
with deduced amino acid sequences nearly
Fig. 8. Biochemical conversion ofmyo-inositol to pinitol. Bracket indicates theoretical intermediate.
cal to Ins(3)P1 synthase from other plants. Under
salinity stress, theInps1RNAup-regulated five-fold or more and free MI accumulated ten-fold [23]. The second step, an O-methyl transferase, methy-lates C4 of MI. Transgenic tobacco plants bearing IMT1, the O-methyl transferase from ice plant, were found to accumulate ononitol and provide better protection under drought and salt-stress conditions than wild-type plants [68]. The enzyme catalyzing step three, epimerization of C1 of onon-itol, has yet to be examined but it is assumed that this step is not rate-limiting. Immunocytology and solute measurements [69] led to discovery of in-creased phloem transport of MI accompanied by increased transport of Na+ and inositol to leaves of ice plant under stress. It was found that seedlings of ice plants, which are not salt-tolerant, developed patterns of gene expression and polyol accumulation observed in mature salt-tolerant plants and that MI-enhanced Na+ uptake and transport increased [38]. From these data it is proposed that a Na+/MI symport might exist that promotes Na+ uptake in the ice plant. This is a novel idea which, as the authors point out, may be a general mechanism of controlling Na+uptake in glycophytes. It also offers new clues regarding the osmoregulatory role of O-methyl inositols.
It is safe to assume that research on inositol-linked, stress-related processes in plants is still in its pioneering stages. There is an interesting obser-vation that sap collected during the dormant pe-riod (winter and early spring) from sugar maple
(Acer saccharum) is rich in quebrachitol (1L-2-O
-methyl-chiro-inositol), ranging from 4 – 6% of total
solids. Yet when trees break dormancy virtually no quebrachitol remains in the sap [70]. Current studies on the role of cyclitols as stable organic osmolytes in trees may be one explanation [65].
7. Structure and conformation of phytic acid
In 1872, Pfeffer showed that subcellular parti-cles in wheat endosperm contained a calcium/ mag-nesium salt of organic phosphate. Two structures were proposed for phytic acid and it took over 50 years to resolve the structural issue. In 1908, Neu-berg proposed a structure that contained three cyclic pyrophosphate moieties and in 1914, Ander-son proposed a structure in which the six hydroxyl groups on MI are esterified with orthophosphate moieties [15,57,71 – 73]. NMR spectroscopy finally resolved the choice in favor of the latter in 1969 [74].
Cellular roles proposed for phytic acid including inhibition of protein phosphatases and subsequent modulation of calcium channel activity, attenua-tion of endocytosis, and inhibiattenua-tion of clathrin assembly [78,79]. Here, further work is needed to fully confirm these functions.
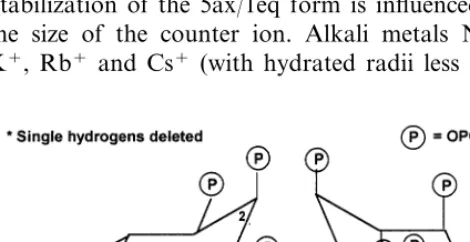
Although the orientation of the phosphate groups in phytic acid was established, there was much debate about the conformation adopted [71,80]. 1ax/5eq and 5ax/1eq (Fig. 9) differ signifi-cantly in overall molecular shape and the orienta-tion of polar groups. Consequently, the two conformations exhibit significant differences in chelating ability, including interaction with proteins. Therefore, the conformational preference of phytic acid in different environments is critical to understanding the chemistry and biochemistry of phytic acid. Assignment of conformation(s) for phytic acid [71] has only recently been fully re-solved. 1H-NMR spectroscopic methods [80]
en-abled Barrientos and Murthy [81] to show that MI adopts the sterically favorable 1ax/5eq conforma-tion (one phosphate axial and five equatorial) in pH range 1.0 – 9.0 and the sterically hindered 5ax/
1eq conformation above pH 9.5 (Fig. 9). Between pH 9.0 – 9.5 (the pKa region of the three least acidic protons) both conformations are in dynamic equilibrium. Dynamic NMR indicates that the activation energy for the conformational inversion process is 54.890.8 kJ/mole (compared to that for ring inversion of cyclohexane, about 45 kJ/
mole) [82]. Inversion to the 5ax/1eq form is facili-tated by complexation with metal ions which reduces electrostatic repulsion and thereby stabi-lizes the sterically hindered, dodeca-anionic form. Stabilization of the 5ax/1eq form is influenced by the size of the counter ion. Alkali metals Na+, K+, Rb+ and Cs+ (with hydrated radii less than
or equal to 2.76 A, ) stabilize the 5ax/1eq form whereas Li+ ion (hydrated radii 3.4 A, ) does not stabilize that form. In the presence of larger coun-ter ions such as tetramethylammonium and tetra-butylammonium ions, the presence of the sterically hindered 5ax/1eq form is not observed, thus indi-cating that complexation with counter ions is es-sential for ring inversion of phytic acid [82].
NMR spectroscopy also indicated that the con-formational preferences of individual isomers at different pH’s are dictated by structural features unique to the isomer such as the number of phos-phate moieties and the regiochemical and stereo-chemical arrangement of the phosphates [81]. Ins(1,2,3,4,6)P5adopts the 1ax/5eq form in the pH
range 1.0 – 9.0 and above 9.5 both 1ax/5eq and 5ax/1eq forms exist in dynamic equilibrium; the exclusive presence of the 5ax/1eq form is not observed. Inositol phosphates containing less than 5 phosphates showed no proclivity to undergo ring inversion to the sterically hindered form [81].
Molecular modeling studies were carried out using ab initio, semi-empirical and force field methods (MacroModel V6.0 and Gaussian 94), for both the 1ax/5eq and 5ax/1eq conformations of phytic acid in the fully protonated and dodeca-an-ionic state [82]. Molecular modeling calculations were consistent with NMR results in aqueous so-lution. Interestingly, calculations predicted that the relative stability of the two conformations is the same in vacuum and aqueous solution, namely, in the fully protonated state the sterically favourable 1ax/5eq form of phytic acid is more stable than the 5ax/1eq form and that in the dodeca-anionic state the sterically hindered 5ax/
1eq form is more stable than the 1ax/5eq form [82].
8. Biosynthesis of Phytic Acid
Although the presence of phytic acid in plant cells has been known for over a century, attempts to determine its biosynthesis in whole plants, plant organs, subcellular organelles, and cell cultures [14,77,78,83], have only recently seen signs of pro-gress. Evidence suggests that phytic acid biosyn-thesis occurs in cisternal endoplasmic reticulum and the product is subsequently deposited in phytin granules [84]. Investigations in duckweed,
Spirodela polyrhiza [16] and the slime mold, Dic
tyostelium [85,86], provided strong evidence for sequential phosphorylation of Ins(3)P1.
Spirodela polyrrhiza:
Ins(3)P1Ins(3,4)P2Ins(3,4,6)P3
Ins(3,4,5,6)P4Ins(1,3,4,5,6)P5
InsP6
Dictyostelium:
Ins(3)P1Ins(3,6)P2Ins(3,4,6)P3
Ins(1,3,4,6)P4Ins(1,3,4,5,6)P5
InsP6
Both pathways begin with Ins(3)P1which makes
biosynthetic sense in that Ins(3)P1 is product of
both Ins(3)P1 synthase and MI kinase. The close
relationship between Ins(3)P1 formation and
phytic acid biosynthesis in developing seeds is revealed in the elegant work of Yoshida et al. [35]. As discussed earlier in regard to Ins(3)P1
biosyn-thesis, in situ hybridization of developing rice grains seeds showed that the transcript for Ins(3)P1 synthase (RINO1) first appeared in the
upper half of the embryo two days after anthesis, increased for the next five days and then gradually decreased. Phytin-containing globoids first ap-peared in the scutellum and the aleurone layer four days after anthesis and increased gradually in both tissues. The appearance of globoids coincided with the localization of RINO1 transcripts thereby suggesting that enhanced Ins(3)P1 formation
drives phytic acid biosynthesis. It is interesting that phosphorylation at the 1 position of Ins(P)n
occurs quite late in both biosynthetic pathways suggesting that the second messenger Ins(1,4,5)P3
pathway and the phytic acid biosynthetic pathway do not intersect in these tissues. In contrast, phos-pholipase C-triggered production of Ins(1,4,5)P3
and subsequent conversion to phytic acid, which is involved in mRNA transport, have been shown in yeast [87]. In all pathways proposed, the 2 position of Ins(P)n is the last position to be
phosphorylated.
9. Hydrolysis of phytic acid:
The enzymes responsible for phytic acid hydrol-ysis are phytases, a special class of phosphatases that catalyze the sequential hydrolysis of phytic acid [14,15]. The sequence of hydrolysis by acid phytases was first established by Tomlinson and
Ballou [88,89]. Their methods for determining the structures of intermediate inositol phosphates are still widely used. Based on the position of the first phosphate hydrolyzed, two classes of acid phytases are recognized, the 6-phytase (EC 3.1.3.26) and the 3-phytase (EC 3.1.3.8) [15]. Both hydrolyze phytic acid to Ins(2)P1. The X-ray crystal structure
of a 3-phytase from Aspergillus niger has been determined [90]. The structure consists of a large
a/b-domain, which shows structural similarity to a high molecular weight acid phosphatase in rats, and a smaller a-domain. The active site contains the amino acid sequence, RHGXRXP, common in many acid phosphatases, and a cluster of basic amino acid residues that could facilitate binding of the negatively charged phytic acid at the active site. The enzyme did not contain bound substrate; therefore a model for substrate binding and cata-lytic activity was proposed by the authors. In the model, the conserved histidine (His 59) was in-volved in nucleophilic attack at the 3-phosphate. An unusual constitutive alkaline phytase that is present in lily pollen and seeds [91,92] initiates action by first removing the 5-phosphate of phytic acid. Subsequent hydrolytic steps remove phos-phate from the 4 and 6 position to yield Ins (1,2,3)P3 as the final product. This final product,
Ins(1,2,3)P3, has been shown to inhibit
iron-cata-lyzed free radical formation by chelating iron [93 – 95]. The presence of multiple phytases with differing specificity, pH optima, and biochemical properties in wheat bran and lily pollen suggests that hydrolysis of phytic acid is under the control of multiple phytases. We are probably entering a new phase of research which will involve under-standing the physiological importance of the mul-tiple phytases and the biological roles of inositol phosphates produced by them.
10. Pyrophosphorylated inositol phosphates
Recently, inositol phosphates containing one or more pyrophosphate groups, have been detected in slime mold and mammalian cells [78,79]. Com-pounds with a pyrophosphate group at the 1 or 5 position of phytic acid (pyrophosphoinositol pen-takisphosphate, PP-InsP5) or two pyrophosphate
groups tentatively placed at 1 and 5 or at 5 and 6 positions (bispyrophosphoinositol tetrakis phos-phate, [PP]2-InsP4) have been identified (Fig. 10).
phos-Fig. 10. Pyrophosphorylated inositol polyphosphates. (A) 5-PP-InsP5and (B) 1,5-[PP]2-InsP4.
phate group at the 5 position to a high energy pyrophosphate group, and a PP-InsP5 kinase,
which can convert a second phosphate to a py-rophosphate group, have been isolated [96]. These high-energy pyrophosphates are not metabolically static. They exhibit rapid turnover through the action of phosphatases and kinases. Estimates are that 30 – 50% of the cellular phytic acid cycles through these pyrophosphorylated derivatives ev-ery hour [97]. The potential involvement of py-rophosphorylated inositol phosphates in signal transduction and calcium metabolism is suggested by the observation that in a muscle cell line, the concentration of [PP]2-InsP4 declined rapidly
(70%) by activation of the cAMP-dependent path-way and that sigargin, which increases cytoplasmic calcium by depleting calcium stores in endoplas-mic reticulum, reduced the concentration of [PP]-InsP5and [PP]2-InsP4by 50% (reviewed in Shears
[79]). Such tantalizing bits of information indicate that these metabolically-active, high energy phos-phates could play multiple roles in phosphate and calcium metabolism and suggest that further work is necessary for a better understanding of their cellular roles.
11. Phosphatidylinositol and its polyphosphates
Due to the critical role that phosphoinositides play in signal transduction, this area of inositol chemistry has been actively investigated and its current status in both animals and plants has been comprehensively reviewed [98]. Phosphatidylinosi-tol (PtdIns) and its phosphorylated derivatives, PtdIns-4-phosphate (PtdInsP) and PtdIns-4,5-bis-phosphate (PtdInsP2), are present in plant tissues
[98] but their functions as extracellular signals remains an equivocal issue [99]. (As used in this review, the abbreviation PtdIns refers to PtdIns with a MI head group. If the head group is other than MI, the isomeric inositol will be specified.) In
plants, the biosynthesis of PtdIns via the CDP-dia-cylglycerol pathway is well established [98] (Fig. 11), as is subsequent phosphorylation of PtdIns by specific kinases to PtdInsP and PtdInsP2. More
recently, the presence of the 3-substituted polyphosphoinositides, PtdIns(3)P1 and
Pt-dIns(3,4)P2, in plant cells has been documented
[98].
Besides variability in the position and number of phosphate groups on the inositol ring, another source of structural heterogeneity in phosphoinosi-tides has been the presence of isomeric inositols. Although MI is the predominant form in the majority of inositol-containing compounds, the presence of scyllo-inositol-containing PtdIns (des-ignated PtdscylloI) has been found in plant cells
and chiro-inositol-containing PtdIns (designated
PtdchiroI) in animal cells. Isomeric
12. Glycosyl-phosphatidylinositol and Glycosyl-inositolphosphorylceramide
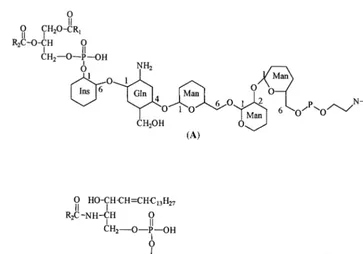
The discovery that glycosylated lipid molecules anchor proteins to cell membranes and that both the lipid anchors and the proteins are involved in cellular response to environmental stimuli has opened up an exciting area of research [104 – 106]. In plant cells, reports of the involvement of two such anchors, a glycosyl – PtdIns (GPI)-anchored nitrate reductase in blue light-stimulated nitrate uptake [107,108] and stimulation of glycosyl – ce-ramide-anchored alkaline phosphatase in low-phosphate medium [18,109], suggest that they may play important roles in plants as well.
The structure of the lipid moiety that anchors enzymes to the hydrophobic membranes has been investigated in a number of cells and structural information gleaned from these studies reveals that the GPI anchors have conserved and variable structural moieties [18,104 – 106] (Fig. 12). In the
conserved core structure, the 6-hydroxyl of the Ins moiety is glycosylated with a tetrasaccharide chain containing one glucosamine and three mannose units. The third mannose is connected to a phos-phorylated ethanolamine, the amine group of which forms an amide bond with the C-terminal end of the protein. Variability in the lipid portion includes the presence of a 1-alkyl-2-acyl glycerol or a ceramide unit in place of the diacylglycerol (see below) and variation in the fatty acid compo-sition. Structural heterogeneity in the hydrophilic portion of the molecule includes esterification of a hydroxyl at carbon 2 on the MI moiety with a long chain fatty acid, the presence of chiro -inosi-tol, galactose molecules glycosidically linked to the core mannose groups, and two or three ethanolamine molecules. Biosynthesis of GPI an-chors involves the sequential glycosylation of Pt-dIns in a step-wise manner and post translational modification of the protein with the preformed GPI anchor [105].
Fig. 12. Structures of (A) glycosyl-phosphatidylinositol and (B) glycosyl-inositolphosphorylceramide.
Employing methods developed for animal cells, Tischner and his colleagues showed that the inosi-tol-containing lipid anchor of nitrate reductase includes a diacylglycerophosphatidyl-MI moiety [107,108]. These methods include (i) release of the protein from membrane vesicles by the action of PtdIns-specific phospholipase C, (ii) decrease in the hydrophobicity of the released protein, (iii) in vivo labeling of the anchored protein by [3H]ethanolamine, and (iv) cross reactivity with
monoclonal antibody raised against the
GPI-an-chor of Trypanosoma GPI-anchored protein
[107,108]. However, the lipid anchor of alkaline phosphatase inSpirodelaexhibits chemical reactiv-ity that is not consistent with the presence of a GPI unit [18,109]. These characteristics include, incorporation of myo-[3H]inositol, [3
H]ethano-lamine, [3H]myristic and [3H]palmitic acid into
lipid-bound enzyme, resistance to cleavage of protein by PtdIns-specific phospholipase C, and hydrolysis of fatty acid units under strong acid and alkaline conditions but not under mild alkali. On the basis of these chemical characteristics, the authors have tentatively identified that the lipid moiety is a ceramide [18,109]. Thus the parent lipid must be a glycosylated inositolphosphorylce-ramide, also called glycophosphosphingolipid [19] (Fig. 12). The presence of glycophosphosphin-golipid, namely lipids containing the
inositolphos-phorylceramide group, in seeds from cotton, peanut, corn and soybeans has been known since the 1960s as a result of the pioneering work by Carter et al. [19]. However, our knowledge of the biosynthesis, localization, and biological roles of sphingolipids is limited. In light of the continually expanding role of lipids in signal transduction and the realization that sphingolipids mediate cell growth and differentiation in animal cells, the recent discoveries [18,104] of the involvement of inositol-containing glycosyl-lipids in nutrient as-similating processes has opened up an exciting area for future research [110].
13. Concluding Remarks
A beguiling simplicity shrouds a staggering complexity of reactions, pathways and physiologi-cal consequences arising from the unique biochem-ical properties of MI. Inherently, MI bears the
D-gluco configuration. Conformational responses
understand the central position of MI in plant metabolism and its multifunctional role. To reach such goals requires more knowledge of the regula-tion and control of MI biosynthesis, the selective nature of substituent groups, the interplay of iso-meric forms, the phosphorylative process, cellular deposition of fully phosphorylated MI as phytate, recall of phytic acid during critical stages of seed germination and seedling growth as well as infor-mation regarding metabolic redistribution of the energy and chemical fragments released during catabolism of phytic acid. It also entails a much greater effort to explore the properties of phos-phatidylinositols and their polyphosphates as po-tential signals in plant cells. Here, qualities common to plant and animal systems must be sorted out from those expressly unique to plants. Last, but certainly not least, the relationship be-tween oxidation of MI and cell wall biogenesis needed to be revisited. As Trethewey, Krotzky & Willmitzer [111] point out, ‘‘…the time is ripe for metabolic profiling and…this will emerge as a central aspect of plant functional genomics in the next decade.’’. The task of garnering this informa-tion lies ahead.
Acknowledgements
For many years, studies in FAL’s laboratory have been supported by grants from the National Institutes of Health. The authors also wish to acknowledge Dr. Søren Rasmussen, Risø National Laboratory, Denmark, whose 1997 Workshop on Phytate and Phytases in Plants provided the im-petus for this review. The authors thank those who reviewed this manuscript for their useful comments.
References
[1] F.A. Loewus, M.W. Loewus, myo-Inositol: Its biosyn-thesis and metabolism, Annu. Rev. Plant Physiol. 34 (1983) 137 – 161.
[2] D.J. Morre´, W.F. Boss, F.A. Loewus (Eds.), Inositol Metabolism in Plants, Wiley – Liss, 393 p, New York, 1990.
[3] A.L. Majumder, M.D. Johnson, S.A. Henry, 1L -myo-Inositol-1-phosphate synthase, Biochim. Biophys. Acta 1348 (1997) 245 – 256.
[4] G.E. Gillaspy, J.S. Keddie, K. Oda, W. Gruissem, Plant inositol monophosphatase is a lithium-sensitive enzyme
encoded by a multigene family, Plant Cell 7 (1995) 2175 – 2185.
[5] M.W. Loewus, K. Sasaki, A.L. Leavitt, L. Munsell, W.R. Sherman, F.A. Loewus, Enantiomeric form of
myo-inositol-1-phosphate produced by myo -inositol-1-phosphate synthase and myo-inositol kinase in higher plants, Plant Physiol. 70 (1982) 1661 – 1663.
[6] F. Loewus (Ed.), Biogenesis of Plant Cell Wall Polysac-charides, Academic Press, New York, 1973, p. 379. [7] J.P. Slovin, R.S. Bandurski, J.D. Cohen, Control of
hormone synthesis and metabolism 1. Auxins, in: P.J.J. Hooykaas (Ed.) Biochemistry and Molecular Biology of Plant Hormones, Elsevier, Amsterdam (in press). [8] J.D. Cohen, J.P. Slovin, Recent research advances
con-cerning indole-3-acetic acid metabolism, in: K. Palme, R. Walden, J. Schell (Eds.), Mechanism of Action of Plant Hormones, Springer, Berlin (in press).
[9] M. Horbowicz, R.L. Obendorf, Seed desiccation toler-ance and storability: Dependence on flatulence-produc-ing oligosaccharides and cyclitols (review and survey, Seed Sci. Res. 4 (1994) 385 – 405.
[10] R.L. Obendorf, Oligosaccharides and galactosyl cycli-tols in seed desiccation (review update), Seed Sci. Res. 7 (1997) 63 – 74.
[11] H. Bohnert, R.G. Jensen, Strategies for engineering water-stress tolerance in plants, Trends Biotechnol. 14 (1996) 89 – 97.
[12] T. Peterbauer, M. Puschenreiter, A. Richter, Metabolism of galactosylononitol in seeds of Vigna umbellata, Plant Cell Physiol. 39 (1998) 334 – 341. [13] T. Peterbauer, A. Richter, Galactosylononitol and
stachyose synthesis in seeds of Adzuki bean, Plant Physiol. 117 (1998) 165 – 172.
[14] P.P.N. Murthy, Insoitol phosphates and their metabolism in plants, in: B.B. Biswas, S. Biswas (Eds.),
myo-Inositol Phosphates, Phosphoinositides, and Signal Transduction, Subcellular Biochemistry Series, vol. 26, Plenum Press, New York, 1996, pp. 227 – 255.
[15] D.J. Cosgrove, Inositol Phosphates: Their Chemistry, Biochemistry and Physiology, Elsevier, Amsterdam, 1980, p. 191.
[16] C.A. Brearley, D.E. Hanke, Metabolic evidence for the order of addition of individual phosphate esters to the
myo– inositol moiety of inositol hexaphosphate in the duckweed Spirodela polyrhiza, Biochem. J. 314 (1996) 227 – 233.
[17] G.G. Cote´, R.C. Crain, Biochemistry of phosphoinosi-tides, Annu. Rev. Plant Physiol. Mol. Biol. 44 (1993) 333 – 356.
[18] H. Nakazato, T. Okamoato, M. Nishikoori, K. Washio, N. Morita, K. Haraguchi, G.A. Thompson Jr., H. Okuyama, The glycosylphosphatidylinositol-anchored phosphatase from Spirodela oligorrhizais a purple acid phosphatase, Plant Physiol. 118 (1998) 1015 – 1020. [19] R.A. Laine, T.C.-Y. Hsieh, Inositol-containing
sphin-golipids, Methods Enzymol. 138 (1987) 186 – 195. [20] F.A. Loewus, S. Kelly, Conversion of glucose to
inosi-tol in parsley leaves, Biochem. Biophys. Res. Commun. 7 (1962) 204 – 208.
[22] F.A. Loewus, S. Kelly, E.F. Neufeld, Metabolism of
myo-inositol in plants: Conversion to pectin, hemicellu-lose, D-xylose and sugar acids, Proc. Natl. Acad. Sci. USA 48 (1962) 421 – 425.
[23] M. Ishitani, A.L. Majumder, A. Bornhouser, C.B. Michalowski, R.C. Jensen, H.J. Bohnert, Coordinate transcriptional induction of myo-inositol metabolism during environmental stress, Plant J. 9 (1996) 537 – 548. [24] M.E. Migaud, J.W. Frost, Elaboration of a general strategy for inhibition ofmyo-inositol 1-phosphate syn-thase: Active site interactions of analogues possessing oxidized reaction centers, J. Am. Chem. Soc. 118 (1996) 495 – 501.
[25] T.F. Donahue, S.A. Henry, myo-Inositol-1-phosphate synthase: Characteristics of the enzyme and identifica-tion of its structural gene in yeast, J. Biol. Chem. 256 (1981) 7077 – 7085.
[26] M.J. White, J.M. Lopes, S.A. Henry, Inositol metabolism in yeasts, Adv. Microbial Physiol. 32 (1991) 1 – 51.
[27] C.C. Smart, A.J. Fleming, A plant gene with homology to D-myo-inositol-3-phosphate synthase is rapidly and spatially up-regulated during an abscisic-acid-induced morphogenic response inSpirodela polyrrhiza, Plant J. 4 (1993) 279 – 293.
[28] C.C. Smart, S. Flores, Overexpression ofD-myo -inosi-tol-3-phosphate synthase leads to elevated levels of inositol inArabidopis, Plant Mol. Biol. 33 (1997) 811 – 820.
[29] M. Chen, F.A. Loewus, myo-Inositol metabolism in
Lilium longiflorumpollen, Plant Physiol. 59 (1977) 653 – 657.
[30] A. RayChaudhuri, N.C. Hait, S. DasGupta, T.J. Bhaduri, R. Deb, A.L. Majumber, l-myo-Inositol 1-phosphate synthase from plant sources: Characteristics of the chloroplastic and cytosolic enzymes, Plant Phys-iol. 115 (1997) 727 – 736.
[31] D. Bhattacharya, L. Medlin, Algal phylogeny and the origin of land plants, Plant Physiol. 116 (1998) 9 – 15. [32] W.F. Doolittle, A paradigm gets shifty, Nature 392
(1998) 15 – 16.
[33] A. RayChaudhuri, A.L. Majumber, Salinity-induced enhancement of l-myo-inositol 1-phosphate synthase in rice (Oryza sati6a L.), Plant Cell Environ. 19 (1996)
1437 – 1442.
[34] R. Keller, C.A. Brearley, R.N. Trethewey, B. Mu¨ller-Ro¨ber, Reduced inositol content and altered morphol-ogy in transgenic potato plants inhibited for 1D-myo-inositol 3-phosphate synthase, Plant J. 16 (1998) 403 – 410.
[35] K.T. Yoshida, T. Wada, H. Koyama, R. Mizobuchi – Fukuoka, S. Naito, Temporal and spatial patterns of accumulation of the transcript of myo -inositol-1-phos-phate synthase and phytin-containing particles during seed development in rice, Plant Physiol. 119 (1999) 65 – 72.
[36] K. Klages, H. Donnison, H. Boldingh, E. MacRae,
myo-Inositol is the major sugar in Actinidia arguta
during enarly fruit development, Aust. J. Plant Physiol. 25 (1998) 61 – 67.
[37] J. Bu¨cker, R. Giderian, Accumulation of myo-inositol inPopulusas a possible indication of membrane disinte-gration due to air pollution, J. Plant Physiol. 144 (1994) 121 – 123.
[38] D.E. Nelson, M. Koukoumanos, H.J. Bohnert, myo -Inositol-dependent sodium uptake in ice plant, Plant Physiol. 119 (1999) 165 – 172.
[39] V. Kosˇta´l, O. Nedved, P. S&imek, Accumulation of high concentrations of myo-inositol in the overwintering la-dybird beetleCreatomegilla undecimnotata, Cryo Letters 17 (1996) 267 – 272.
[40] M.W. Loewus, F.A. Loewus, myo -Inositol-1-phos-phatase from the pollen of Lilium longiflorum Thunb, Plant Physiol. 70 (1982) 765 – 770.
[41] S.C. Gumber, M/W. Loewus, F.A. Loewus,@ Further studies on myo-inositol-1-phosphatase from the pollen of Lilium longiflorumThunb., Plant Physiol. 76 (1984) 40 – 44.
[42] L. Parthasarathy, R.E. Vadnal, R. Parthasarathy, C.S. Shyamala Devi, Biochemical and molecular properties of lithium-sensitive myo-inositol monophosphatase: A minireview, Life Sci. 54 (1994) 1127 – 1142.
[43] J.A. Styer, W. Gruissem, G.A. Gillaspy, Spatially regu-lated expression of tomato inositol monophosphatase genes, Plant Physiol. ASPP Meeting Suppl. (1999) Abst.149.
[44] C.E. Anderson, Lithium in plants, in: R.O. Bach, V.S. Gallicchio (Eds.), Lithium and Cell Physiology, Springer – Verlag, New York, 1990, pp. 24 – 46. [45] T. Nishida, H. Kodama, A. Komamine, The effects of
lithium chloride on the induction of proliferation of cells in suspension cultures of Catharanthus roseus, J. Plant Physiol. 142 (1993) 184 – 190.
[46] P.D. English, M. Deitz, P. Albersheim, Myoinositol kinase: partial purification and identification of product, Science 151 (1966) 198 – 199.
[47] D.S. Feingold, Aldo (and keto) hexoses and uronic acids, in: F.A. Loewus, W. Tanner, (Eds.) Plant Carbo-hydrates I, Encyclopedia of Plant Physiology, New Series, Vol. 13A, Springer Verlag, Berlin, 1982, pp. 3 – 76.
[48] C-L. Rosenfield, C. Fann, F.A. Loewus, Metabolic studies on intermediates in the myo-inositol oxidation pathway inLilium longiflorum pollen: I. Conversion to hexoses, Plant Physiol. 61 (1978) 89 – 95.
[49] C.L. Rosenfield, F.A. Loewus, Metabolic studies on intermediates in the in themyo-inositol oxidation path-way in Lilium longiflorum pollen: II. Evidence for the participation of UDP-xylose and free xylose as interme-diates, Plant Physiol. 61 (1978) 96 – 100.
[50] C.L. Rosenfield, F.A. Loewus, Metabolic studies on intermediates in the in themyo-inositol oxidation path-way in Lilium longiflorum pollen: III. Polysaccharidic origin of labeled glucose, Plant Physiol. 61 (1978) 101 – 103.
[51] M.W. Loewus, F.A. Loewus, The C-5 hydrogen isotope effect in myo-inositol 1-phosphate synthase as evidence for the myo-inositol oxidation pathway, Carbohydr. Res. 82 (1980) 333 – 342.
[53] R. Tenhaken, O. Thulke, Cloning of an enzyme that synthesizes a key nucleotide-sugar precursor of hemicel-lulose biosynthesis from soybean: UDP-glucose dehy-drogenase, Plant Physiol. 112 (1996) 1127 – 1134. [54] R. Mukherjee, E.M. Axt, Cyclitols fromCroton celtidi
-folius, Phytochemistry 23 (1984) 2682 – 2684.
[55] F.A. Loewus, Structure and occurrence of inositols in plants: see Ref. 2, pp. 1 – 11.
[56] M. Popp, N. Smirnoff, Polyol accumulation and metabolism during water deficit, in: N. Smirnoff (Ed.), Environment and Plant Metabolism, BIOS Sci. Publ, Oxford, UK, 1995, pp. 199 – 215.
[57] T. Posternak, The Cyclitols, Holden-Day, San Fran-cisco, 1965, p. 431.
[58] V. Plouvier, Distribution of aliphatic polyols and cycli-tols, in: T. Swain (Ed.), Chemical Taxonomy, Academic Press, New York, 1963, pp. 313 – 336.
[59] O. Kandler, H. Hopf, Oligosaccharides based on su-crose (sucrosyl oligosaccharides), in: F.A. Loewus, W. Tanner (Eds.), Encyclopedia of Plant Physiology, New Series, vol. 13A, Springer Verlag, 1982, pp. 349 – 383. [60] T.M. Kuo, C.A. Lowell, T.C. Nelson, Occurrence of
pinitol in developing soybean seed tissues, Phytochem-istry 45 (1997) 29 – 35.
[61] T. Yasui, Identification of a new galactosyl cyclitol from seeds ofVigna angularis Ohwi et Ohashi (adzuki bean), Agric Biol. Chem. 44 (1980) 2253 – 2255. [62] A. Richter, T. Peterbauer, I. Brereton, Structure of
galactosylononitol, J. Nat. Prod. 60 (1997) 749 – 751. [63] W. Wanek, A. Richter, Biosynthesis and accumulation
ofD-ononitol inVigna umbellatain response to drought
stress, Planta 197 (1995) 424 – 427.
[64] B.N. Timmerman, C. Steelink, F.A. Loewus (Eds.), Phytochemical Adaptations to Stress, Recent Advances in Phytochemistry, vol. 18, Plenum Press, New York, 1983.
[65] M. Popp, W. Leid, U. Bierbaum, M. Gross, T. Grosse-Schulte, S. Hams, J. Oldenettel, S. Schu¨ler, J. Wiese, Cyclitols, stable osmotica in trees, in: H. Rennenberg, W. Eschrich, H. Ziegler (Eds.), Contributions to Mod-ern Tree Physiology, SPB Academic Publ, The Hague, 1997, p. 270.
[66] N. Smirnoff, Q.J. Cumbes, Hydroxyl radical scavenging activity of compatible solutes, Phytochemistry 28 (1989) 1057 – 1060.
[67] M.J. Paul, W. Cockburn, Pinitol, a compatible solute in
Mesembryanthemum crystallinum L.?, J. Exper. Bot. 40 (1989) 1093 – 1098.
[68] E. Sheveleva, W. Chmara, H.J. Bohnert, R.G. Jensen, Increased salt and drought tolerance by D-ononitol
production in transgenic Nicotiana tabacum L, Plant Physiol. 115 (1997) 1211 – 1219.
[69] D.E. Nelson, G Rammesmayer, H.J. Bohnert, Regula-tion of cell-specific inositol metabolism and transport in plant salinity tolerance, Plant Cell 10 (1998) 753 – 764. [70] E.E. Stinson, C.J. Dooley, J.M. Purcell, J.S. Ard,
Quer-bachitol (a new component of maple sap and syrup, Agric. Food Chem. 15 (1967) 394 – 397.
[71] R. Lasztity, L. Lasztity, Phytic acid in cereal technol-ogy, Adv. Cereal Sci. Technol. 10 (1990) 309 – 371.
[72] B.F. Harland, E.R. Morris, Phytate: A good or bad food component?, Nutrition Res. 15 (1995) 733 – 754. [73] S.E. Rickard, L.U. Thompson, Interactions and
biolog-ical effects of phytic acid, in: Antinutrients and Phyto-chemicals in Food, American Chemical Society Symposium Series No. 662, Washington D.C., 1997, pp. 294 – 312.
[74] L.F. Johnson, M.E. Tate, Structure of ‘phytic acids’, Can. J. Chem. 47 (1969) 63 – 73.
[75] D.S. Ertl, K.A. Young, V. Raboy, Plant genetic ap-proaches to phosphorous management in agricultural production, J. Environ. Qual. 27 (1998) 299 – 304. [76] D.T. Van der Molen, A. Breeuwsman, P.C.M. Boers,
Agricultural nutrient losses to surface water in the Netherlands: Impact, strategies, and perspectives, J. En-viron. Qual. 27 (1998) 4 – 11.
[77] V. Raboy, P. Gerbasi, Genetics of myo-inositol phos-phate synthesis and accumulation, in: B.B. Biswas, S. Biswas (Eds.), Inositol Phosphates, Phosphoinositides, and Signal Transduction, Subcellular Biochemistry se-ries, vol. 26, Plenum Press, New York, 1996, pp. 257 – 285.
[78] S.B. Shears, Inositol pentskis- and hexakisphosphate metabolism adds versatility to the actions of inositol polyphosphates novel effects on ion channels and protein traffic, in: B. Biswas, S. Biswas (Eds.), Inositol Phosphates, Phosphoinositides, and Signal Transduc-tion, Subcellular Biochemistry series, vol. 26, Plenum Press, New York, 1996, pp. 187 – 226.
[79] S.B. Shears, The versatility of inositol phosphates as cellular signals, Biochim. Biophys. Acta 1436 (1998) 49 – 67.
[80] L.R. Isbrandt, R.P. Oertel, Conformational states of
myo-inositol hexakis (phosphate) in aqueous solution. A 13C NMR, 31P NMR, and Raman spectroscopic investigation, J. Amer. Chem. Soc. 102 (1980) 3144 – 3148.
[81] L.G. Barrientos, P.P.N. Murthy, Conformational stud-ies of myo-inositol phosphates, Carbohydr. Res. 296 (1996) 39 – 54.
[82] A.T. Bauman, G.M. Chateauneuf, R.E. Brown, P.P.N. Murthy, Conformational inversion process in phytic acid: NMR spectroscopic and molecular modeling stud-ies, Tetrahedron Lett. 40 (1999) 4489 – 4492.
[83] J.J. Scott, F.A. Loewus, Phytate metabolism in plants, in: E. Graf (Ed.), Phytic acid: Chemistry and Applica-tions, Pilatus Press, Minneapolis, 1986, pp. 23 – 42. [84] J.N.A. Lott, J.S. Greenwood, G.D. Batten, Mechanisms
and regulation of mineral nutrient storage during seed development, in: J. Kigel, G. Galili (Eds.), Seed Devel-opment and Germination, Marcel Dekker, New York, 1995, pp. 215 – 235.
[85] L.R. Stephens, R.F. Irvine, Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphos-phate in Dictyostelium, Nature (London) 346 (1990) 580 – 583.
[86] P.J.M. van Haastert, P. van Dijken, Biochemistry and genetics of inositol phosphate metabolism in Dictyos
-telium, FEBS Lett. 410 (1997) 39 – 43.
ki-nase pathway required for efficient messenger RNA export, Science 285 (1999) 96 – 100.
[88] R.V. Tomlinson, C.E. Ballou, myo-Inositol polyphos-phate intermediates in the dephosphorylation of phytic acid by phytases, Biochemistry 1 (1962) 166 – 171. [89] R.V. Tomlinson, C.E. Ballou, Complete
characteriza-tion of myo-inositol polyphoshpates from beef brain phosphoinositides, J. Biol. Chem. 236 (1961) 1902 – 1906.
[90] D. Kostrewa, F. Leitch, A.G. D’Arcy, C. Broger, D. Mitchell, A.P.G.M. vanLoon, Crystal structure of phy-tase fromAspergillus ficuumat 2.5 A, resolution, Nature Struct. Biol. 4 (1997) 185 – 190.
[91] J.J. Scott, F.A. Loewus, A calcium-activated phytase from pollen of Lilium longiflorum, Plant Physiol. 82 (1986) 333 – 335.
[92] L. Barrietos, J.J. Scott, P.P.N. Murthy, Specificity of hydrolysis of phytic acid by alkaline phytase from lily pollen, Plant Physiol. 106 (1994) 1489 – 1495.
[93] P.T. Hawkins, D.R. Poyner, T.R. Jackson, A.J. Letcher, D.A. Lander, R.F. Irvine, Inhibition of iron-catalysed hydroxyl radical formation by inositol polyphosphates: a possible physiological function for
myo-inositol hexakisphosphate, Biochem. J. 294 (1993) 929 – 934.
[94] I.D. Spiers, S. Freeman, D.R. Poyner, C.H. Schwalbe, The first synthesis and iron binding studies of the natural product, myo-inositol 1,2,3-trisphosphate, Te-trahedron Lett. 36 (1995) 2125 – 2128.
[95] B.Q. Phillipy, E. Graf, Antioxidant functions of inositol 1,2,3-trisphosphate and inositol 1,2,3,6-tetrakisphos-phate, Free Radical Biol. Med. 22 (1997) 939 – 946. [96] C.-F. Huang, S.M. Voglmaier, M.E. Bembenek, A.
Saiardi, S.H. Snyder, Identification and purification of diphosphoinositol pentakisphosphate kinase, which syn-thesizes the inositol pyrophosphate bis(diphos-pho)inositol tetrakisphosphate, Biochemistry 17 (1998) 14998 – 15005.
[97] L.R. Stephens, T. Radenberg, U. Thiel, G. Vogel, K.-H. Khoo, A. Dell, T.R. Jackson, P.T. Kawkins, G.W. Mayr, The detection, purification, structural characteri-zation and metabolism of diphosphoinositol diphospho-inoistol pentakisphosphate(s) and bisdiphosphoinositol tetrakisphosphate(s), J. Biol. Chem. 268 (1993) 4009 – 4015.
[98] T. Munnik, R.F. Irvine, A. Musgrave, Phospholipid signalling in plants, Biochim. Biophys. Acta 1389 (1998) 222 – 272.
[99] G.G. Cote´, R.C. Crain, Why do plants have phospho-inositides?, BioEssays 16 (1994) 39 – 46.
[100] S. Carstensen, G. Pliska-Matyshak, N. Bhuvara-hamurthy, K.M. Robbins, P.P.N. Murthy, Biosynthesis and localization of phosphatidylinositol in barley aleurone cells, Lipids 34 (1999) 67 – 73.
[101] Y. Pak, Y. Hong, S. Kim, T. Piccariello, R.V. Farese, J. Larner, In vivochiro-inositol metabolism in the rat: A defect inchiro-inositol synthesis frommyo-inositol and an increased incorporation of chiro-[3H]inositol into phospholipid in the Goto-Kakizaki (G.K.) rat, Mol. Cells 8 (1998) 301 – 309.
[102] Y. Hong, Y. Pak, Identification of chiro -inositol-con-taining phospholipids and changes in their metabolism upon salt stress in soybean seedlings, Phytochemistry 51 (1999) 861 – 866.
[103] A. Lykidis, P.D. Jackson, C.O. Rock, S. Jackowski, The role of CDP-diacylglycerol synthase and phos-phatidyl synthase activity levels in the regulation of cellular phosphatidylinositol content, J. Biol. Chem. 272 (1997) 33402 – 33409.
[104] M.R. Low, A.R. Saltiel, Structural and functional roles of glycosylphosphatidylinositol in membranes, Science 239 (1988) 268 – 275.
[105] M.G. Low, The glycosyl-phosphatidylinositol anchor of membrane proteins, Biochim. Biophys. Acta 988 (1989) 427 – 454.
[106] A.R. Saltiel, Structural and functional roles of glyco-sylphosphoinositides, in: B.B. Biswas, S. Biswas (Eds.), Inositol Phosphates, Phosphoinositides, and Signal Transduction, Subcellular Biochemistry series, vol. 26, Plenum Press, New York, 1996, pp. 165 – 185.
[107] M. Kunze, J. Riedel, U. Lange, R. Hurwitz, R. Tis-chner, Evidence for the presence of GPI-anchored PM-NR in leaves of Beta 6ulgaris and from PM-NR in
barley leaves, Plant Physiol. Biochem. 35 (1997) 507 – 512.
[108] C. Stohr, F. Schuler, R. Tischner, Glycosyl-phos-phatidylinositol-anchored proteins exist in the plasma membranes of Chlorella Saccharophila (Kruger) Nad-son: Plasma-membrane-bound nitrate reductase as an example, Planta 196 (1995) 284 – 287.
[109] N. Morita, H. Nakazato, H. Okuyama, Y. Kim, G.A. Thompson, Evidence for a glycosylinositolphospho-lipid-anchored alkaline phosphatase in the aquatic plant
Spirodela oligorrhiza, Biochem. Biophys. Acta 1290 (1996) 3 – 62.
[110] D.K. Perry, Y.A. Hannun, The role of ceramide in cell signaling, Biochim. Biohys. Acta 1436 (1998) 233 – 243. [111] R.N. Trethewey, A.J. Krotzky, L. Willmitzer, Metabolic profiling: a Rosetta Stone for Genomics?, Curr. Opinion Plant Physiol. 2 (1999) 83 – 85.

![Fig. 10. Pyrophosphorylated inositol polyphosphates. (A) 5-PP-InsP5 and (B) 1,5-[PP]2-InsP4.](https://thumb-ap.123doks.com/thumbv2/123dok/1035558.929086/13.612.88.458.37.112/fig-pyrophosphorylated-inositol-polyphosphates-pp-insp-pp-insp.webp)