Summary We studied the effects of light quality and nutrient supply on growth and nitrogen accumulation in silver birch (Betula pendula Roth) seedlings to test three hypotheses: (1) growth of birch seedlings is sensitive to changes in light quality; (2) the response of birch seedling growth to light quality depends on nutrient supply; and (3) assimilation and allocation of nitrogen by birch seedlings are affected by light quality. The two light regimes simulated the spectral quality of sunlight and shadelight, but did not differ in photosynthetic photon flux density, and the two nutrient supply regimes dif-fered in the rate of supply, but not in the composition, of mineral nutrients.
Accumulation and allocation of dry weight and nitrogen were strongly affected by nutrient supply regime, but light quality had little effect. During the first 15 days of the experi-ment, the largest effect of light quality was on height growth, which was greater in seedlings in simulated shadelight than in seedlings in simulated sunlight. Light quality had little effect on dry weight and nitrogen allocation to the stem during this period. However, at the end of the experiment (Day 29), there was an increase in N concentration per unit dry weight in leaves and stems of seedlings in the simulated shadelight plus high nutrient supply treatment.
Keywords: allocation, assimilation, nitrogen, nutrient supply, photomorphogenesis, shadelight, sunlight.
Introduction
Because plants both modify their light environment and re-spond to it, a detailed understanding of plant responses to light is needed to elucidate the mechanisms underlying the morpho-logical and physiomorpho-logical processes in canopies and plant com-munities (e.g., Ballaré 1994, Aphalo and Ballaré 1995). Shade-avoiding species such as silver birch (Betula pendula Roth) (Atkinson 1992) tend to be more responsive to changes in light quality than shade-tolerant species (Smith 1994).
In many species, the perception of low R/FR ratios by phytochrome triggers several responses, including increased stem elongation, reduced leaf/stem dry weight ratio, increased shoot/root dry weight ratio, and reduced photosynthetic capac-ity, which are presumably adaptive for plants growing in dense
populations (e.g., Smith 1994, Barreiro et al. 1992). Blue light also triggers several photomorphogenic responses, including partial inhibition of hypocotyl elongation. Moreover, phyto-chrome-mediated responses and those dependent on blue light often interact (Mohr 1994).
Most research on plant photomorphogenesis has centered on the effects of light quality, e.g., R/FR, on carbon assimilation and partititoning. This has been a useful approach for studying agricultural crops and their weeds, which usually grow in rich fertilized soils. However, tree seedlings from boreal forests grow in nutrient-poor soils where nutrient supply is often a growth-limiting factor. Under these conditions, a study of plant photomorphogenesis that focuses on the effects of light quality on the acquisition and partitioning of mineral nutrients is likely to be rewarding. Depression of plant growth and a concurrent increase in allocation of dry matter to roots (Charles-Edwards et al. 1986, Levin et al. 1989), and inhibition of leaf area expansion (McDonald et al. 1992, Taylor et al. 1993) are well known responses to low nutrient supply. However, there are few reports on the interactions of light quality and mineral nutrition. Root growth was depressed by end-of-day FR in Glycine max (L.) (Kasperbauer et al. 1984). The activity of enzymes involved in N assimilation is enhanced by red light (R) (nitrate and nitrite reductase in several species (Johnson 1990, Mohr et al. 1992) and glutamine synthetase in Pinus sylvestris (L.) (Elmlinger and Mohr 1992)). These effects could alter the mineral nutrient economy of a plant in response to shading by its neighbors (see Aphalo and Ballaré 1995).
The objective of this study was to test three hypotheses: (1) growth of birch seedlings is sensitive to changes in light quality; (2) the response of birch seedling growth to light quality depends on nutrient supply; and (3) assimilation and allocation of nitrogen by birch seedlings are affected by light quality. We conducted a 2 × 2 factorial experiment, with light quality and mineral nutrient supply as the experimental fac-tors. The two light regimes simulated the spectral quality of sunlight and shadelight, but did not differ in photosynthetic photon flux density, and the two nutrient supply regimes dif-fered in the rate of supply, but not in the composition, of mineral nutrients. Data from sequential harvests were used for growth analysis.
Effects of light quality on growth and N accumulation in birch
seedlings
P. J. APHALO
1,2and T. LEHTO
1,21
Finnish Forest Research Institute, Suonenjoki Research Station, 77600 Suonenjoki, Finland 2 Current address: University of Joensuu, Faculty of Forestry, P.O. Box 111, 80101 Joensuu, Finland
Received July 10, 1995
Materials and methods
Plants
Betula pendula Roth (B. verrucosa Ehr.) seeds were germi-nated on sand in a greenhouse. The seeds were from a seed orchard established with material from different Finnish sites between 60°40′ N and 63°30′ N. When the seedlings had one or two true leaves visible, they were transplanted to Ray-Leach cells (165 cm3, SC-10 super cell, Stuewe and Sons, Corvallis, OR) containing a mix of sand and unfertilized peat (1/2, v/v). Fifteen days before the first harvest, all seedlings were watered once with a balanced nutrient solution containing 50 mg N dm−3. Nine days before the first harvest, the seedlings were placed in a growth chamber that provided a day/night air temperature of 20/15 °C, and a day/night water vapor satura-tion deficit of 5.8/3.5 mmol mol−1 (relative humidity 75/80%).
Treatments
The light quality treatments began on the day the seedlings were transferred to the growth chamber. The light source in the chamber consisted of 10 metal halide discharge lamps (HRI-T 250 W, Radium, Germany), supplemented with six incandes-cent lamps (25 W). The photoperiod was 20 h, with the lamps switched on in three incremental steps (4 metal halide + 2 incandescent lamps, 7 + 4, and 10 + 6) during the first 2 h of the photoperiod, and switched off in reverse order during the last 2 h. With all lamps on, the photosynthetic photon flux density (I) at the top of the plants was 225 µmol m−2 s−1 (measured with an LI-190SB quantum sensor, Li-Cor, Inc., Lincoln, NE). Taking into account the steps, the daily photon flux was 14.7 mol m−2 day−1, which is approximately 35% of the mean natural PAR in central Finland in May--July (Finnish Meteorological Institute 1993).
Light quality was modified with filters located approxi-mately 5 cm above the top of the seedlings (height adjusted weekly). The frame holding the filters fitted closely against the walls of the growth chamber, and although no vertical filters or barriers were used, border plants were used to prevent interfer-ence by stray light. Two kinds of filters were used: neutral (clear polyethylene film plus silver black scrim, Strand filter No. 270, Strand Lighting Ltd., Isleworth, UK) and green (moss green, Strand filter No. 422). The I under these filters differed by less than 1%, but the red (655--665 nm) to far-red (725--735 nm) photon flux ratio (R/FR) was 2.1 for the neutral filter (simulated sunlight, SU) and 0.5 for the green filter (simulated shadelight, SH). The photon flux density of blue light (450 nm) under the green filter was 20% of that under the neutral filter. Spectra are shown in Figure 1.
Nutrient treatments were started on the day of the first harvest (Day 0). Nutrients were supplied at two rates, but the proportions of the different elements were kept constant. A complete nutrient solution balanced for silver birch (Ingestad 1970) was provided at a rate of 0.86 mg N day−1 plant−1 for the high nutrient supply treatment (HN), and at a rate of 0.17 mg N day−1 plant−1 for the low nutrient supply treatment (LN). The solutions were prepared by dilution of equal amounts of two stock solutions. Solution A contained (per dm3): K
2SO4
24.48 g, K2HPO4 16.81 g, KH2PO4 15.44 g, KNO3 24.62 g, NH4NO3 110.0 g. Solution B contained (per dm3): Ca(NO3)2.4H20 20.68 g, Mg(NO3)2.6H2O 44.88 g, micronutri-ents stock solution 25 cm3, HNO
3 (70%) 1.8 g. The micronu-trient stock solution contained (per dm3): Fe(NO
3)3.9H2O 101.28 g, Mn(NO3)2.4H2O 36.56 g, Zn(NO3)2.4H2O 4.798 g, CuCl2.2H2O 1.610 g, Na2MoO4.2H2O 0.353 g, H3BO3 22.88 g, HNO3 (70%) 19.8 g.
Measurements and chemical analysis
Leaf area was measured with a leaf area meter (LI-3000, Li-Cor, Inc., Lincoln, NE) and stem height was measured to the nearest mm. Each seedling harvested was divided into leaf blades, stem plus petioles, and roots, and the plant parts were dried at 65 °C for 48 h before weighing. Each plant part was ground separately, but the four replicates were combined. For the larger plants, the samples were first coarsely ground in a mill, subsampled, and then reground in a mortar with a pestle. Nitrogen concentration was determined with an automatic analyzer (Model CHN-9000, Leco Co., St. Joseph, MI).
Chlorophyll concentration was estimated in vivo from trans-mittance spectra measured in the second youngest fully ex-panded leaf with a spectroradiometer equipped with an integrating sphere (Li-Cor LI-1800). The in vivo transmittance at 660 nm was calibrated against chlorophyll concentration per unit leaf area, measured in N,N-dimethylformamide extracts from leaf discs of birch seedlings using the equations of In-skeep and Bloom (1985). The R2 of the polynomial regression obtained was 0.98 (n = 32). The emission spectra of the light sources used was measured with the same spectroradiometer.
Experimental design, growth analysis calculations and statistical methods
number of remaining plants was small enough to allow a new spatially uniform arrangement, the seedlings were arranged at a lower density to reduce shading (the maximum leaf area index was 2.7 during the experiment).
The functional growth analysis method was used. Functions were fitted to the harvest means of the measured variables with time as the independent variable. The fitted functions were exponential polynomials. Relative growth rate (Rw) and rela-tive N accumulation rate (RN) were calculated as the deriva-tives of the regressions for dry weight (W) and nitrogen content (N) (see Table 2):
y
^′=b
1+ 2b2t, (1)
and confidence limits as:
y
^′±t
α/2× sd(y^′), (2) where:
sd(y^′)=√ Var(b1)+4t2Var(b2)+4tCov(b1,b2). (3)
Multivariate or univariate ANOVA was used to test the significance of differences between treatments. Height, leaf area, dry weight, and nitrogen content values and allometric ratios were ln-transformed before analyses or calculations, and are presented in the figures on a log scale.
Results Growth
Plant dry weight (W) increased throughout the experiment, but the relative rate of increase was smaller during the later stages than during the first 15 days of the experiment. At the final harvest on Day 29, W was between 12 and 18 times as much as on Day 0, depending on the treatments (Figure 2a). Shade-light (SH) had no effect on final W (P = 0.59), but the low nutrient supply rate (LN) decreased final W by 33% (P < 0.001), and there was a small but significant interaction (P = 0.029).
The relative rate of leaf area expansion decreased through-out the experimental period in all treatments (Figure 2b). Final leaf area (L) was 10 and 18 times as much as initial L for the LN- and HN-treated seedlings, respectively. Compared with final L of plants in the HN treatment, LN resulted in a 45% decrease in final L (P < 0.001), whereas shadelight had no effect on L (P > 0.20).
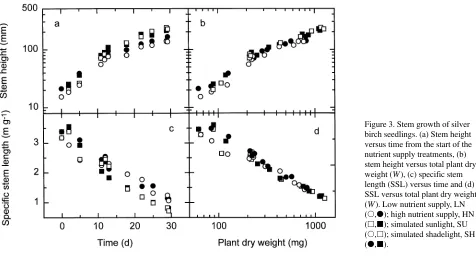
In the SH treatment, stem height (H) was initially increased by about 40%, but later in the study, the stem elongation rate was greater in SU-treated seedlings than in SH-treated seed-lings (Figure 3a). By the end of the experiment, H was similar in both light quality treatments (P > 0.20 for main effect and P = 0.086 for the interaction). In contrast, H was decreased by LN so that LN-treated plants were shorter than HN-treated plants at the final harvest (P < 0.001). A plot of H versus W (Figure 3b) shows that the effect of LN is mostly explained by
its effect on total plant size, whereas the effect of light quality is not.
Nitrogen content of the seedlings increased more slowly than W. On Day 2, the N content was 2.4 mg per seedling. Because the final N content was 4.2 and 9.5 times as much as the initial content in LN- and HN-treated seedlings, respec-tively, the final N content of LN-treated seedlings was only 44% of that of HN-treated seedlings (P < 0.001). Nitrogen content was only increased 3.4% by the SH treatment (P = 0.056). When plotted against W, differences in N content in response to LN remained large (data not shown).
Because N content increased at a slower rate than W accu-mulation, N concentration decreased during the course of the experiment. On Day 2, N concentrations in leaves per unit dry weight and per unit leaf area were 31 mg g−1 and 0.105 mg cm−2, respectively. Leaf N concentration per unit leaf area also decreased throughout the experiment, and this decrease was larger in LN-treated seedlings than in HN-treated seedlings (~50% in LN and ~25% in HN). At the time of the final harvest (Day 29), N concentrations in leaves and stems of LN-treated seedlings were only half those in HN-treated seedlings, whereas N concentration in the roots was less affected by nutrient supply (Table 1). There was no effect of light quality on N concentration in roots. However, in leaves and stems, the effect of light quality on N concentration depended on the nutrient supply. In the HN-treated seedlings, the SH treatment increased N concentration (P = 0.013 leaf, P= 0.052 stem), whereas in the LN-treated seedlings, SH treatment slightly decreased N concentration (P = 0.054 leaf, P = 0.100 stem). Figure 2. Dry weight and morphological attributes of silver birch seedlings. (a) Whole-plant dry weight (W) and (b) whole-plant leaf area (L) versus time. Low nutrient supply, LN (s,d); high nutrient
supply, HN (h,j); simulated sunlight, SU (s,h); simulated
Nitrogen concentration per unit leaf area was not affected by light quality (Table 1).
At the end of the experiment, chlorophyll concentration (expressed per unit leaf area) was reduced by LN (P < 0.001) but was not affected by the SH treatment (main effect P = 0.56, interaction P = 0.65). Estimated chlorophyll concentrations (means and standard error, in mg m−2) were: SU + HN: 196, SU + LN: 129, SH + HN: 209, SH + LN: 130, SE = 11.6.
Allocation
Because dry weight allocation is usually correlated with plant size (e.g., Evans and Hughes 1961), we plotted weight and N fractions against W (cf. Evans 1972, Peace and Grubb 1982). Otherwise, the effects of the treatments on allocation could
become confounded with their effects on plant size and onto-genic drift (Bourdôt et al. 1984). For example, leaf weight ratio (wleaf) at the final harvest was not significantly affected by any of the treatments (P = 0.191 for nutrient supply, P > 0.177 for light quality and P > 0.20 for the interaction). However, when
wleaf was plotted against W, a small but consistent decrease in
wleaf in response to LN was observed (Figure 4a, P < 0.001). A similar response pattern was observed for leaf N content ratio (Nleaf) (Figure 5a, P = 0.023). The SH treatment had no signifi-cant effect on wleaf or Nleaf when the whole data set was considered (P > 0.20). However, at Day 29, Nleaf was slightly higher in SU-treated seedlings than in SH-treated seedlings (P = 0.110) and slightly higher in HN-treated seedlings com-pared with LN-treated seedlings (P = 0.029). Neither wleaf nor
Nleaf changed appreciably with plant size.
Figure 3. Stem growth of silver birch seedlings. (a) Stem height versus time from the start of the nutrient supply treatments, (b) stem height versus total plant dry weight (W), (c) specific stem length (SSL) versus time and (d) SSL versus total plant dry weight (W). Low nutrient supply, LN (s,d); high nutrient supply, HN
(h,j); simulated sunlight, SU
(s,h); simulated shadelight, SH
(d,j).
Table 1. Nitrogen concentration in leaves, stems, and roots, and specific leaf area (SLA) of birch seedlings exposed to simulated sunlight (SU) or shadelight (SH), and high (HN) and low (LN) nutrient supply regimes. Values are the means of two harvests carried out on Day 29.
Treatment Nitrogen concentration SLA
Leaf (mg g−1) Stem (mg g−1) Root (mg g−1) Leaf (mg cm−2) cm2 g−1
SU + HN 22.0 13.0 15.5 0.076 289
SH + HN 25.0 14.2 17.5 0.079 317
SU + LN 14.5 8.1 12.0 0.054 270
SH + LN 12.6 7.1 12.3 0.053 237
Source of variation Probability from ANOVA
Nutrition < 0.001 < 0.001 0.003 0.001 0.014 Light quality 0.338 0.687 0.162 0.754 0.843
In contrast, stem weight ratio (wstem) increased with plant size, and the magnitude of the increase was mainly dependent on nutrient supply (Figure 4b, P < 0.001), but also slightly dependent on light quality (P = 0.028). In HN-treated seed-lings, the proportion of total W allocated to stem increased from 8% at the initial harvest to 25% at the final harvest, whereas in LN-treated seedlings wstem increased from 8 to 17%. Only a small part of this difference is explained by the difference in total W. Stem N content ratio (Nstem) followed a similar pattern (P = 0.006 for nutrient supply and P > 0.20 for light quality and interaction); however, N allocation to the stem in LN-treated seedlings decreased toward the end of the experi-ment (Figure 5b). The SH treatexperi-ment had no effect on wstem or
Nstem when the whole data set was considered (P > 0.20). As the size of the plants increased, root weight ratio (wroot) decreased, but it stabilized toward the end of the experiment. This decrease was smaller in LN-treated seedlings than in HN-treated seedlings (Figure 4c, P <0.001). A similar pattern was observed for root N content ratio (Nroot) (P = 0.005), except that Nroot increased toward the end of the experiment in LN-treated seedlings (Figure 5c). The SH treatment had no
effect on wroot or Nroot when the whole data set was considered (P > 0.20).
Leaf area ratio (L/W) first increased and later decreased as plant size increased (Figure 6). The small difference in re-sponse to the LN treatment observed at the final harvest, increased when leaf area ratio was plotted against W. Leaf area ratio followed the same temporal pattern in LN- and HN-treated seedlings, however, its value was lower in LN-HN-treated seedlings. By Day 29, specific leaf area (SLA) was decreased by LN (Table 1).
Specific stem length (SSL) decreased during the experi-ment, and when plotted against time, an effect of LN was apparent toward the end of the experiment (Figure 3c). On Day 29, SSL was increased by 50% by the LN treatment (P < 0.001). However, when SSL was plotted against W in-stead of time, the apparent effect of nutrition on SSL disap-peared (Figure 3d). The SH treatment increased SSL during the first part of the experiment when W was less than 120 mg per seedling; however, the effect disappeared as the seedlings grew (Figure 3d).
Figure 4. Dry weight allocation of silver birch seedlings. (a) Leaf weight ratio (wleaf), (b) stem weight ratio (wstem), and (c) root weight ratio (wroot) versus total plant dry weight (W). Low nutrient supply, LN (s,d); high nutrient supply HN (h,j); simulated sunlight, SU
(s,h); simulated shadelight, SH (d,j).
Figure 5. Nitrogen allocation of silver birch seedlings. (a) Leaf N ratio (Nleaf), (b) stem N ratio (Nstem), and (c) root N ratio (Nroot) versus total plant dry weight (W). Low nutrient supply, LN (s,d); high nutrient
supply, HN (h,j); simulated sunlight, SU (s,h); simulated
Functional growth analysis
We used functional growth analysis methods to calculate time courses of relative growth rate (Rw) and relative N accumula-tion rate (RN). Because there was no effect of SH on W or N content, the data from the two light quality treatments were pooled before fitting the growth curves (Table 2). Relative growth rate (the slope of the curves in Figure 2a) decreased during the experiment, and the LN treatment further decreased it. Although Rw and RN had similar values at Day 0, RN de-creased more quickly than Rw during the experiment and was also affected by the LN treatment. In the second half of the experiment, net assimilation rate per unit leaf area was lower in LN- than in HN-treated plants (data not shown). However, net assimilation rate per unit leaf N was similar in LN- and HN-treated seedlings throughout the experiment, although it varied with time (4.0 g day−1 g−1 N at Day 0, and 7.1 g day−1 g−1 N at Day 29).
Discussion
Nutrient supply
Silver birch is a pioneer species that prefers fertile forest sites and abandoned crop fields. Under conditions of optimal light and nutrient supply, seedlings can attain Rw values of up to 0.27 day−1 (Ingestad and Ågren 1992, Table 1).
Initial Rw was 0.12 day−1, which is lower than the value of aproximately 0.2 day−1 reported by Ingestad and McDonald (1989) under conditions of similar I but optimum nutrition. The N concentration of our plants was suboptimal from the beginning of the experiment (cf. seedlings under optimal nu-trition in Ingestad and Ågren 1992), and decreased even further during the second half of the study period. Although we took care to minimize shading, Rw decreased toward the end of the experiment; this was probably because we used a constant nutrient addition rate.
The response of the seedlings to altered nutrient supply was greater than the response to light quality. Similar increases in growth and decreases in allocation to roots with increased nutrient supply have been documented in silver birch both in the field (Viro 1974) and in controlled experiments (Ågren 1985, Ågren and Ingestad 1987), as well as in other broad-leaved tree species (e.g., Lehto and Grace 1994).
The nutrient supply treatments and plant size had little effect on leaf weight ratio. Most of the changes in allocation affected only roots and stems, a result qualitatively similar to that obtained in Eucalyptus by Sheriff (1992). Both in birch and in Eucalyptus, Nleaf was more sensitive than wleaf to nutrient supply. However, in birch, Nstem was decreased by LN, whereas in Eucalyptus, it was not affected by N supply (Sheriff 1992).
Light quality
We attempted to simulate only the spectral quality of sun- and shadelight. The results would have been different had we simulated both the spectral quality and photon flux density of these two kinds of light. Furthermore, although the spectrum of sunlight is fairly constant, that of shadelight varies both with the leaf area index of the canopy and with characteristics of the foliage forming the canopy. Values of R/FR photon flux ratio in the range of 0.1--0.75 are common in vegetation canopies (Smith 1994); the value of 0.5 in the present experiment corresponds to a relatively sparse canopy. The R/FR photon flux ratio in simulated sunlight was 2.1, which is higher than the ratio of 1.05--1.25 in real sunlight; however, this difference has only a small effect on the photoequilibrium of phyto-chrome.
In the early stages of the experiment, stem elongation was enhanced in SH-treated seedlings. However, there was no large effect of shadelight quality on assimilation and allocation of N or W. The explanation for the lack of effect of light quality on the growth rate of birch seedlings is that neither wleaf, leaf area Figure 6. Leaf area ratio versus total plant dry weight of silver birch
seedlings. Low nutrient supply, LN (s,d); high nutrient supply,
HN (h,j); simulated sunlight, SU (s,h); simulated shadelight,
SH (d,j).
Table 2. Parameters of growth equation. Time series data were fitted to y^ = b0+ b1t + b2t2, where y is the ln of the variable under consideration and t is time. Dry weight (W) and N content are in mg per plant and time is in days. Means of four replicates from each treatment and harvest were used, with the two light treatments bulked (n = 8 for N content, n = 22 for W).
Variable Treatment b0 SE b1 SE b2 SE R2
ratio nor net assimilation rate were affected. Rice and Bazzaz (1989) also found that light treatments have no effect on leaf area ratio when the results are expressed on a dry weight basis. The lack of an effect of light quality on net assimilation rate per unit leaf area can be explained by its lack of effect on both N and chlorophyll concentrations per unit leaf area.
When plants were compared at equal size, the main effect of light quality on birch seedlings was a change in SSL, which was smaller in SU-treated seedlings than in SH-treated seed-lings during the early stages of the experiment. However, this effect disappeared when the plants reached a certain size, possibly indicating the operation of a developmental switch. A similar decrease in responsiveness to natural canopy shade as seedlings grow has been reported for cucumber (Ballaré et al. 1991).
In herbaceous plants, an increase in stem extension is usu-ally accompanied by an increase in W of the stem, and these increases occur at the expense of both leaves and roots (McLaren and Smith 1978, Morgan and Smith 1979), resulting in a reduction in Rw. In our experiment, increased stem elon-gation was not accompanied by a decrease in Rw. Similar responses have been observed in other woody plants: in Fuch-sia magellanica Lam., increased stem elongation in response to end-of-day FR occurs through changes in SSL that do not affect Rw (Aphalo et al. 1991). In seedlings of the tropical tree Terminalia ivorensis A. Chev, a low R/FR increased stem elongation without decreasing Rw or increasing wstem, except at very low I (Kwesiga and Grace 1986).
If W accumulation is not depressed by low R/FR, the elon-gation response has no apparent cost in terms of carbon use. However, there are risks involved in a high elongation rate even if this can be achieved without carbon cost. For example, a seedling with a long thin stem is more exposed to mechanical damage by wind and rain, and to browsing by animals.
The increase in extension rate in response to shade depends on the habitat of the species (Kwesiga and Grace 1986, War-rington et al. 1989). For example, Kwesiga and Grace (1986) observed that, in Terminalia ivorensis, the main effect of in-creased R/FR was a substantial increase in leaf area ratio, whereas the leaf area ratio of Khaya senegalensis (Desr.) A. Juss. did not react to changes in R/FR. They attributed this difference to the different ecologies of the species. Terminalia is a pioneer species, whereas Khaya can survive extended periods shaded in the forest, and will start growing rapidly when a gap appears and I and R/FR increase. However, al-though birch is a pioneer species, we observed no effect of light quality on leaf area ratio.
We have discussed the response to light quality observed only in terms of R/FR; however, the light treatments also differed in their blue light content. Because phytochrome me-diated-responses are altered by blue light (Casal and Smith 1989, Mohr 1994), the effect of the simulated shadelight treat-ment is probably the result of a complex interaction involving two or more photoreceptors. In an outdoor experiment, Dale and Causton (1992) observed that shading by vegetation af-fects the assimilation of mineral nutrients and their allocation to different organs. In their experiment, in which the natural shade differed in both light quality and flux density from
sunlight, which was used as the control, the effects of shading on nutrient allocation differed from those on carbon allocation. In our experiment, in which light treatments differed only in light quality, allocation of dry matter was not affected and N allocation was only slightly different in seedlings from the two light treatments. These small differences caused significant changes in N concentration. Moreover, the magnitude and possibly also the direction of the changes in leaf N concentra-tion depended on the nutrient supply rate.
We conclude that: (1) stem elongation was affected by light quality at the early stages of the experiment, but only until plants reach a certain size; (2) there was no interaction between the effect of light quality on stem elongation and seedling nutritional status; (3) assimilation and allocation of N and W were only slightly affected by light quality; and (4) there was an effect of light quality on N concentration at the end of the experiment, evident in the shadelight-induced increase in N concentration per unit dry weight in leaves and stems of the seedlings receiving the higher nutrient supply.
Thus, although our results cannot be directly extrapolated to other species in which light quality has a more profound and long-lasting effect on dry weight allocation, we have demon-strated that light quality can alter the mineral nutrition of silver birch seedlings, and that this effect depends on the rate of mineral nutrient supply.
Caveat
A time series of growth data allows the use of two bases for comparison: size and age. Use of one rather than the other sometimes influences the interpretation of the results, but the appropriateness of one or the other is difficult to establish. It has been argued that plant size is preferable (e.g., Rice and Bazzaz 1989), because plant dry weight may reflect physi-ological age better than time (Bourdôt et al. 1984, and refer-ences therein). For example, dry weight allocation to stems is probably related to mechanical constraints that depend on plant size rather than on chronological age. It should be also noted that, in our data set, some effects and interactions present when data were plotted against time disappeared when the same data were plotted against W, but the opposite was not true. This is unlikely to happen by chance, and provides cir-cumstantial evidence for preferring W as a basis for compari-son.
Whichever basis was used, size or age, LN-treated seedlings always had a lower N concentration than HN-treated seedlings, demonstrating that, in both cases, the objective of testing for the effect of light quality in seedlings with different nutritional status was achieved.
Acknowledgments
References
Ågren, G.I. 1985. Theory for growth of plants derived from the nitrogen productivity concept. Physiol. Plant. 64:17--28.
Ågren, G.I. and T. Ingestad. 1987. Root:shoot ratio as a balance between nitrogen productivity and photosynthesis. Plant, Cell En-viron. 10:579--586.
Aphalo, P.J. and C.L. Ballaré. 1995. On the importance of informa-tion-acquiring systems in plant--plant interactions. Funct. Ecol. 9:5--14.
Aphalo, P.J., D. Gibson and A.H. Di Benedetto. 1991. Responses of growth, photosynthesis, and leaf conductance to white light irradi-ance and end-of-day red and far-red pulses in Fuchsia magellanica Lam. New Phytol. 117:461--471.
Atkinson, M.D. 1992. Betula pendula Roth (B. verrucosa Ehrh.) and B. pubescens Ehrh. Biological Flora of the British Isles, No. 175. J. Ecol. 80:837--870.
Ballaré, C.L. 1994. Light gaps: sensing the light opportunities in highly dynamic canopy environments. In Exploitation of Environ-mental Heterogeneity by Plants. Ecophysiological Processes Above and Below Ground. Eds. M.M. Caldwell and R.W. Pearcy. Aca-demic Press, San Diego, pp 73--110.
Ballaré, C.L., J.J. Casal and R.E. Kendrick. 1991. Responses of light-grown wild-type and long hypocotyl mutant cucumber seedlings to natural and simulated shade light. Photochem. Photobiol. 53:87--94.
Barreiro, R., J.J. Guiamet, J. Beltrano and E.R. Montaldi. 1992. Regulation of the photosynthetic capacity of primary bean leaves by the red:far-red ratio and photosynthetic photon flux density of incident light. Physiol. Plant. 85:97--101.
Bourdôt, G.W., D.J. Saville and R.J. Field. 1984. The response of Achillea millefolium L. (yarrow) to shading. New Phytol. 97:653--663.
Casal, J.J. and H. Smith. 1989. Effects of blue light pretreatments on internode extension growth in mustard seedlings after the transition to darkness: analysis of the interaction with phytochrome. J. Exp. Bot. 40:893--899.
Charles-Edwards, D.A., D. Doley and G.M. Rimmington. 1986. Mod-elling plant growth and development. Academic Press, Sydney, 235 p.
Dale, M.P. and D.R. Causton. 1992. The ecophysiology of Veronica chamaedrys, V. montana and V. officinalis. IV. Effects of shading on nutrient allocations----a field experiment. J. Ecol. 80:505--515. Elmlinger, M.W. and H. Mohr. 1992. Glutamine synthetase in Scots
pine seedlings and its control by blue light and light absorbed by phytochrome. Planta 188:396--402.
Evans, G.C. 1972. The quantitative analysis of plant growth. Black-well Scientific, Oxford, 734 p.
Evans, G.C. and A.P. Hughes. 1961. Plant growth and the aerial environment. I. Effect of artificial shading on Impatiens parviflora. New Phytol. 60:150--180.
Finnish Meteorological Institute. 1993. Measurements of solar radia-tion 1981--1990. Meteorol. Yearbook of Finland 81--90 (Part 4:1): 1--132.
Ingestad, T. 1970. A definition of optimum nutrient requirements in birch seedlings. I. Physiol. Plant. 23:1127--1138.
Ingestad, T., and A.J.S. McDonald. 1989. Interaction between nitrogen and photon flux density in birch seedlings at steady-state nutrition. Physiol. Plant. 77:1--11.
Ingestad, T. and G.I. Ågren. 1992. Theories and methods on plant nutrition and growth. Physiol. Plant. 84:177--184.
Inskeep, W.P. and P.R. Bloom. 1985. Extinction coefficients of chloro-phyll a and b in N,N-dimethylformamide and 89% acetone. Plant Physiol. 77:483--485.
Johnson, C.B. 1990. Signal transduction mechanisms in phytochrome action. In Signal Perception and Transduction in Higher Plants. Eds. R. Ranjeva and A.M. Boudet. Springer-Verlag, Berlin, Heidelberg, pp 229--247.
Kasperbauer, M.J., P.G. Hunt and R.E. Sojka. 1984. Photosynthate partitioning and nodule formation in soybean plants that received red or far-red light at the end of the photosynthetic period. Physiol. Plant. 61:549--554.
Kwesiga, F. and J. Grace. 1986. The role of the red/far-red ratio in the response of tropical tree seedlings to shade. Ann. Bot. 57:283--290. Lehto, T. and J. Grace. 1994. Carbon balance of tropical tree seedlings:
a comparison of two species. New Phytol. 127:455--463. Levin, S.A., H.A. Mooney and C. Field. 1989. The dependence of
plant root-shoot ratios on internal nitrogen concentration. Ann. Bot. 64:71--75.
McDonald, A.J.S., T. Lohammar and T. Ingestad. 1992. Net assimila-tion rate and shoot area development in birch (Betula pendula Roth) at different steady-state values of nutrition and photon flux density. Trees 6:1--6.
McLaren, J.S. and H. Smith. 1978. Phytochrome control of the growth and development of Rumex obtusifolius under simulated canopy light environments. Plant, Cell Environ.1:61--67.
Mohr, H. 1994. Coaction between pigment systems. In Photomorpho-genesis in Plants, 2nd Edn. Eds. R.E. Kendrick and G.H.M. Kronenberg. Kluwer Academic, Dordrecht, pp 353--373. Mohr, H., A. Neininger and B. Seith. 1992. Control of nitrate reductase
and nitrite reductase gene expression by light, nitrate and a plastidic factor. Bot. Acta 105:81--89.
Morgan, D.C. and H. Smith. 1979. A systematic relationship between phytochrome-controlled development and species habitat, for plants grown in simulated natural radiation. Planta 145:253--258. Peace, W.J.H. and P.J. Grubb. 1982. Interaction of light and mineral
nutrient supply on the growth of Impatients parviflora. New Phytol. 90:127--150.
Rice, S.A. and F.A. Bazzaz. 1989. Quantification of plasticity of plant traits in response to light intensity: comparing phenotypes at a common weight. Oecologia 78:502--507.
Sheriff, D.W. 1992. Roles of carbon gain and allocation in growth at different nitrogen nutrition in Eucalyptus camaldulensis and Euca-lyptus globulus seedlings. Austr. J. Plant Physiol. 19:637--652. Smith, H. 1994. Sensing the light environment: the functions of the
phytochrome family. In Photomorphogenesis in Plants, 2nd Edn. Eds. R.E. Kendrick and G.H.M. Kronenberg. Kluwer Academic, Dordrecht, pp 377--416.
Taylor, G., A.J.S. McDonald, I. Stadenberg and P.H. Freersmith. 1993. Nitrate supply and the biophysics of leaf growth in Salix viminalis. J. Exp. Bot. 44:155--164.
Viro, P.J. 1974. Fertilization of birch. Metsäntutkimuslaitoksen jul-kaisuja. Comm. Inst. For. Fenn. 81:1--38.