131
M
ICROSATELLITE VARIATION IN CASSAVA(M
ANIHOTESCULENTA
, E
UPHORBIACEAE)
AND ITS WILDRELATIVES
:
FURTHER EVIDENCE FOR A SOUTHERNA
MAZONIAN ORIGIN OF DOMESTICATION1K
ENNETHM. O
LSEN2 ANDB
ARBARAA. S
CHAALDepartment of Biology, Campus Box 1137, Washington University, St. Louis, Missouri 63130-4899 USA
Genetic variation at five microsatellite loci was used to investigate the evolutionary and geographical origins of cassava (Manihot
esculenta subsp. esculenta) and the population structure of cassava’s wild relatives. Two hundred and twelve individuals were sampled,
representing 20 crop accessions, 27 populations of cassava’s closest wild relative (M. esculenta subsp. flabellifolia), and six populations of a potentially hybridizing species (M. pruinosa). Seventy-three alleles were observed across all loci and populations. These data indicate the following on cassava’s origin: (1) genetic variation in the crop is a subset of that found in the wild M. esculenta subspecies, suggesting that cassava is derived solely from its conspecific wild relative. (2) Phenetic analyses group cassava with wild populations from the southern border of the Amazon basin, indicating this region as the likely site of domestication. (3) Manihot pruinosa, while closely related to M. esculenta (and possibly hybridizing with it where sympatric), is probably not a progenitor of the crop. Genetic differentiation among the wild populations is moderately high (FST 50.42, rST 50.54). This differentiation has probably arisen primarily through random genetic drift (rather than mutation) following recent population divergence.
Key words: cassava; Manihot esculenta; Manihot pruinosa; microsatellites, origin of domestication; population structure; wild relatives.
Cassava (Manihot esculenta subsp. esculenta Crantz) is a major crop of the tropics. Its starchy root (also known as man-ioc, tapioca, or yuca) is consumed daily by more than 500 million people (Best and Henry, 1992), making it the primary source of carbohydrates in sub-Saharan Africa (Cock, 1985), and the sixth most important crop worldwide (Mann, 1997). Cassava is grown primarily by subsistence farmers and, de-spite its global economic importance, has traditionally received less attention by researchers than have temperate crops (Cock, 1985). As a consequence, many fundamental questions about this ‘‘orphan crop’’ have only been addressed in recent years. One basic question concerns cassava’s origin of domesti-cation. The genus Manihot comprises 98 species spread throughout the Neotropics (Rogers and Appan, 1973), and in the past, various species from Mexico, Central America, and South America have all been proposed to be cassava’s closest wild relatives (reviews by Renvoize, 1972; Rogers and Appan, 1973; and Sauer, 1993; see also Olsen and Schaal, 1999). Tra-ditionally, no individual species was thought to be morpho-logically similar enough to cassava to be considered the crop’s sole progenitor. Many domestication hypotheses therefore speculated that cassava is a hybrid (‘‘compilospecies’’) derived
1Manuscript received 22 October 1999; revision accepted 25 May 2000. The authors thank Luiz Carvalho and Antonio Costa Allem for assistance with field collections; Tony Weisstein for assistance with tests of isolation by distance; Carolyn Ferguson, Jason Rauscher, and Alan Templeton for helpful comments on the manuscript; the Centro Nacional de Pesquisa de Recursos Gene´ticos e Biotecnologia (CENARGEN; Brası´lia, Brazil) for field support; and the Centro Internacional de Agricultura Tropical (CIAT; Cali, Colombia) for crop accessions. Funding for this work was provided by grants from the Explorer’s Club, the Jean Lowenhaupt Botany Fund, and National Science Foundation (Doctoral Dissertation Improvement Grant DEB-9801213) to K. Olsen, and by grants from the Rockefeller and Guggenheim foundations to B. Schaal.
2Author for reprint requests, current address: North Carolina State Univer-sity, Department of Genetics, Campus Box 7614, Raleigh, NC 27695-7614 USA (email: [email protected]).
from interbreeding Manihot species complexes in Mexico (Rogers, 1965; Rogers and Appan, 1973) and/or South Amer-ica (Rogers, 1963; Ugent, Pozorski, and Pozorski, 1986; Sauer, 1993).
These hybrid origin hypotheses were called into question by Allem (1987, 1994), who identified wild Manihot populations in Brazil that are morphologically close enough to cassava to be considered conspecific. Wild M. esculenta populations are referred to here as M. esculenta subsp. flabellifolia (Pohl) Ci-ferri (see Allem, 1994; Roa et al., 1997). Differences between cassava and M. esculenta subsp. flabellifolia are restricted al-most entirely to traits that would be selected upon during do-mestication (described below). Several recent studies have ex-amined the relationship between cassava and these wild M.
esculenta populations (Fregene et al., 1994; Roa et al., 1997;
Olsen and Schaal, 1999), and speculation has persisted that the crop’s origins may extend beyond this subspecies to other
Ma-nihot species (Fregene et al., 1994; Roa et al., 1997).
The most recent assessment of cassava’s origin (Olsen and Schaal, 1999) involved a phylogeographic study of M.
escu-lenta and a potentially hybridizing sympatric species, M. prui-nosa Pohl, based on DNA sequence variation in a low-copy
nuclear gene (Glyceraldehyde 3-phosphate dehydrogenase). The findings of that study suggested the following: genetic variation in M. esculenta subsp. flabellifolia alone can account for the crop’s genetic diversity, with no need to invoke other progenitor species; cassava is most closely related to popula-tions of M. esculenta subsp. flabellifolia occurring along the southern border of the Amazon basin; and the potentially hy-bridizing species M. pruinosa has apparently not contributed to the germplasm of the crop.
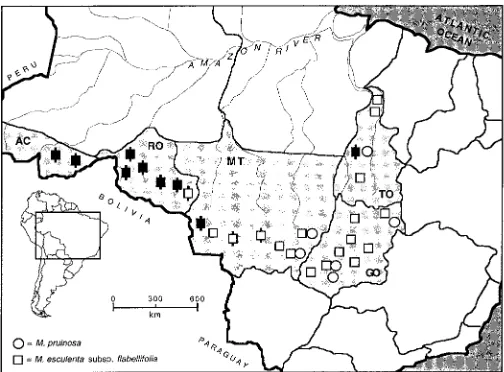
Fig. 1. Sampling locations for populations of M. esculenta subsp.
flabel-lifolia (squares) and M. pruinosa (circles) in Brazilian states adjoining the
Amazon basin. Black squares indicate populations grouped with cassava on the DC neighbor-joining distance tree; barred squares indicate populations grouped with cassava on the maximum likelihood distance tree (see Fig. 3). Brazilian states: AC, Acre; GO, Goia´s; MT, Mato Grosso; RO, Rondoˆnia; TO, Tocantins.
frequency variation at several unlinked loci. Although such data are not amenable to cladistic analyses (as are DNA se-quence data), they do provide multiple, independent assess-ments of genetic relationships. In the present study, we use this multilocus approach to reassess the question of cassava’s origin of domestication and the evolutionary relationships among cassava’s closest wild relatives. Using five variable mi-crosatellite loci, we analyze the same individuals sampled in the earlier phylogeographic study to test the findings of that study and to further elucidate the genetic structure of cassava’s wild relatives. First, do microsatellite allele frequency data confirm that cassava is derived solely from M. esculenta subsp.
flabellifolia—and specifically from those populations
occur-ring along the southern border of the Amazon basin? Second, what is the population structure of the crop’s wild relatives? In particular, what are the patterns of population differentiation within M. esculenta subsp. flabellifolia and M. pruinosa and is there any evidence of interspecific hybridization between them? This study is one of the first to compare microsatellite data with an intraspecific gene genealogy in an analysis of population structure in a plant species.
MATERIALS AND METHODS
Study system—The wild taxa of the study system occur primarily in central
and western Brazil and eastern Peru (Allem, 1992, 1994). Manihot esculenta subsp. flabellifolia is found in mesic forest patches (often in ravines) in the transition zone between the cerrado (savanna scrub) vegetation of the Bra-zilian Shield plateau and the lowland rainforest of the Amazon basin; it grows as a clambering understory shrub or treelet. In Brazil, this taxon occurs along the eastern and southern borders of the Amazon basin, in the states of To-cantins, Goia´s, Mato Grosso, Rondoˆnia and Acre. Manihot esculenta subsp.
flabellifolia differs from cultivated cassava in root and stem characteristics.
In addition to increased root tuberization, the crop also tends to have thick-ened, knobby stems with short internodes, and swollen leaf scars and stipule bases. This increased resource allocation to the stem probably reflects artificial selection for vegetative propagation in the crop; stem cuttings are the primary means by which cassava is propagated.
Although cassava is interfertile with M. esculenta subsp. flabellifolia (Roa et al., 1997) and is cultivated throughout the range of the wild subspecies, introgression from the crop is not thought to have significantly altered the genetic structure of the wild subspecies. Manihot esculenta subsp. flabellifolia is found in forest habitats where cassava is not grown, and cassava does not survive well in abandoned fields or as escapes from cultivation (Rogers, 1965; Allem, 1994; K. Olsen, personal observation). Moreover, because cassava is propagated almost exclusively by stem cuttings, unintentional spread of the crop by humans would be minimal.
The other species in the study system, M. pruinosa, is a small shrub found in dense cerrado vegetation in the Brazilian states of Tocantins, Goia´s, and eastern Mato Grosso. Although this species occurs in a different habitat from that of M. esculenta subsp. flabellifolia, the patchy nature of the cerrado-forest ecotone allows populations of the two species to grow in close prox-imity. Based on its morphological similarity to M. esculenta, M. pruinosa has been grouped into cassava’s ‘‘secondary gene pool’’ of potentially interfertile species (Allem, 1992); it is the only such species to occur in sympatry with
M. esculenta subsp. flabellifolia. Manihot pruinosa is included in the present
study to test the hypothesis that this species has contributed to genetic diver-sity in cassava via hybridization with M. esculenta subsp. flabellifolia.
Sampling and data collection—Populations of wild taxa were sampled
along two transects spanning most of the range of M. esculenta subsp.
fla-bellifolia: along the southern border of the Amazon basin (through the states
of Mato Grosso, Rondoˆnia, and Acre), and along the eastern border (through the states of Tocantins and Goia´s) (Fig. 1). This sampling area overlaps the range of M. pruinosa in the states of Tocantins, Goia´s, and southeastern Mato
Grosso. Collections were made during the months of November and Decem-ber in 1996 and 1997. Undisturbed populations of these species usually com-prise,15 individuals, and young leaf tissue from up to ten plants per pop-ulation was sampled and dried in silica gel. Voucher specimens from each population are housed at the Missouri Botanical Garden (MO), in St. Louis, and at the Centro Internacional de Pesquisa de Recursos Gene´ticos e Biotec-nologia (CEN), in Brası´lia, Brazil. From these wild taxa, 157 individuals of
M. esculenta subsp. flabellifolia, representing 27 populations, and 35
individ-uals of M. pruinosa, representing six populations, were included in analyses. Genetic diversity within cassava was assessed by sampling 20 landraces from the cassava ‘‘world core collection’’ maintained by the Centro Internacional de Agricultura Tropical, in Cali, Colombia.
DNA was extracted from dried leaves by a CTAB protocol (Hillis et al., 1996). Fourteen variable microsatellite loci have previously been identified in cassava (Chavarriaga-Aguirre et al., 1998), and these loci were surveyed for polymorphism in a sample of 12–36 individuals of M. esculenta subsp.
fla-bellifolia and M. pruinosa. Five loci with strong, unambiguous banding
pat-terns were selected for use in the study (GAGG5, GA134, GA16, GA12, and GA140); these loci are all composed of dinucleotide (GA) repeats.
PCR amplifications were carried out in 10-mL volumes, with each reaction containing the following: 10 mmol/L Tris-HCl (pH 9.0), 50 mmol/L KCl, 2.5 mmol/L MgCl2, 0.1% Triton X-100, 0.5 units taq polymerase (Promega), 200
mmol/L each dNTP, 0.2 mmol/L each primer (forward and reverse), 0.02
mmol/L [g32P]-ATP end-labeled forward primer, and;10 ng genomic DNA. Reaction conditions were the same for all five loci: one cycle of 948C (2 min); then 30 cycles of 948C (1 min), 558C (1 min), 728C (2 min); and finally one cycle of 728C (5 min). Following PCR, 2.0mL of formamide dye were added to each reaction, and reaction tubes were heated to 858C for 2 min, then snap-chilled in ice water. PCR products were electrophoresed on 6% polyacryl-amide gels and autoradiographed. A sequencing ladder of known length was included on gels to facilitate size determination of alleles.
Data analysis—Genetic diversity—Genetic polymorphism for each
(Guo and Thompson, 1992); a global test across loci and populations was constructed using Fisher’s method (Raymond and Rousset, 1995). In order to specifically test the hypothesis of heterozygote deficiency, the multiscore (U) test of Rousset and Raymond (1995) was employed. Tests for genotypic link-age disequilibrium among pairs of loci in each population were performed using Fisher’s exact tests (Raymond and Rousset, 1995), with unbiased P values again derived by a Markov chain method. For all tests, the global significance of multiple P values was assessed using a sequential Bonferroni adjustment (Rice, 1989), with an initialalevel of 0.05.
Population structure—Tests for the presence of population differentiation
were made using an unbiased estimated P value for a log-likelihood (G)-based exact test (Goudet et al., 1996). As a conservative approach, the test for genotypic differentiation was invoked. Genetic differentiation among popu-lations was then quantified for each species individually and for all wild pop-ulations together.
Methods for quantifying genetic differentiation from microsatellites are cur-rently debated (Goldstein et al., 1995a, b; Takezaki and Nei, 1996; Goldstein and Pollock, 1997). Some measures have been developed specifically for these markers (e.g., Slatkin, 1995; Goldstein et al., 1995a, b), which take into ac-count variation in allele sizes under a stepwise mutation model (SMM) (Ohta and Kimura, 1973). Under the SMM, alleles of similar size are assumed to be more closely related to each other than those of very different size. SMM-based measures of population differentiation are expected to be most accurate for populations that diverged long enough ago that current genetic differen-tiation reflects mutations accumulated since divergence (reviewed by Gold-stein and Pollock, 1997). In contrast, traditional measures, which do not as-sume an underlying mutational model, are thought to be more appropriate for recently diverged populations (Goldstein et al., 1995a, b; Takezaki and Nei, 1996; Goldstein and Pollock, 1997), since genetic differentiation among such populations will reflect the sorting of ancestral variation more than mutational divergence. For purposes of comparison, both types of measures were em-ployed in this study.
Genetic differentiation was quantified using F statistics (Weir and Cock-erham, 1984) and rstatistics (Michalakis and Excoffier, 1996) using GE-NEPOP. Therstatistics are analogous to F statistics but take into account differences in allele size under the SMM. The distribution of genetic variation in the study system was next quantified by an analysis of molecular variance (AMOVA; Excoffier, Smouse, and Quattro, 1992), using the program AR-LEQUIN (Schneider et al., 1997). Pairwise genetic distances among popula-tions were again calculated two ways, the first based solely on allele fre-quencies (Weir and Cockerham, 1984) and the second incorporating infor-mation on variances in allele sizes (Michalakis and Excoffier, 1996). Genetic variance was assessed at three hierarchical levels in the AMOVAs: within populations, among populations of the same species, and between the two species. Statistical significance of the variance measures was tested by non-parametric permutations (Schneider et al., 1997).
In order to test for isolation by distance among the wild populations, Man-tel’s (1967) permutation procedure in GENEPOP was used. A matrix of pair-wise great circle distances among populations was compared to a matrix of pairwise genetic distances (FSTandrST), with the Spearman rank correlation coefficient used as the test statistic. Isolation by distance was assessed for M.
esculenta subsp. flabellifolia and M. pruinosa populations independently, and
then for the study system as a whole. In addition, a multilocus estimate of the effective number of migrants per generation (Nm) was calculated by Slat-kin’s (1985) private alleles method, with corrections for sample size as given in Barton and Slatkin (1986).
Relationships among populations and taxa—In order to examine genetic
relationships among the wild populations, and cassava’s relationship to these populations, distance trees were inferred from the allele frequency data. Be-cause of the very close evolutionary relationship between cassava and its wild relatives, distance measures that do not assume an underlying mutational mod-el are probably most appropriate for these analyses (see also Tarr, Conant, and Fleischer, 1998; Wenburg, Bentzen, and Foote, 1998; Tessier and Ber-natchez, 1999). The chord distance, DC(Cavalli-Sforza and Edwards, 1967),
was chosen for the present study; DCassumes that differentiation among pop-ulations is due solely to random genetic drift. Using the program GENDIST in the PHYLIP computer package (version 3.5c; Felsenstein, 1995), a pairwise chord distance (DC) matrix was constructed, from which a neighbor-joining phenogram was generated with the program NEIGHBOR. For the sake of comparison, a neighbor-joining tree was also constructed using Slatkin’s (1995) RSTdistance, a SMM-based measure that estimates the fraction of the total variance of allele size occurring among populations. The RST distance matrix was generated in the MICROSAT program (Minch et al., 1996) and then imported into PHYLIP.
In addition to the two neighbor-joining trees, a maximum likelihood dis-tance tree was constructed in PHYLIP using the program CONTML. This algorithm operates under a Brownian motion model that, like DC, assumes evolution purely by random genetic drift (Felsenstein, 1995). The robustness of the DCand maximum likelihood distance trees was assessed by creating 100 bootstrap replicates of the data set with the SEQBOOT algorithm in PHYLIP, and then generating a majority-rule consensus tree in the CON-SENSE program. All distance trees were viewed in TREEVIEW (Page, 1996).
RESULTS
Genetic diversity—Variation at five microsatellite loci was
examined in 212 individuals from 27 populations of M.
es-culenta subsp. flabellifolia, six populations of M. pruinosa,
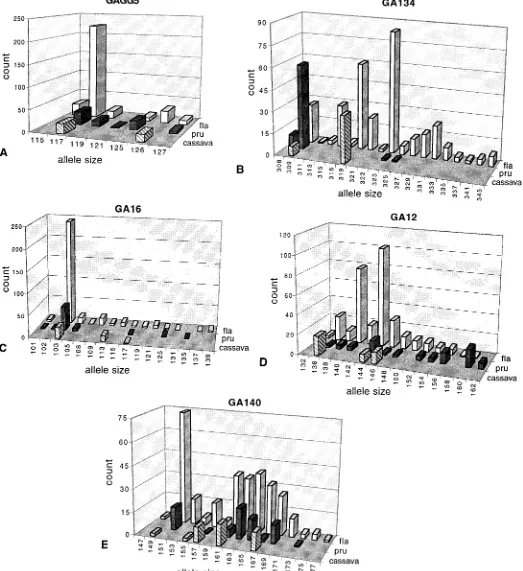
and 20 cassava accessions. The number of alleles observed per locus in this sample ranged from seven (GAGG5) to 19 (GA134) (mean5 14.606 2.01; Table 1, Fig. 2). Of the 73 alleles observed across all five loci, 15 occurred in cassava, and all of these cassava alleles were also observed in popu-lations of M. esculenta subsp. flabellifolia (Fig. 2). Thus, the cassava alleles are a subset of those found within the crop’s conspecific wild relative. At the interspecific level, all but five of the 35 alleles observed in M. pruinosa were shared with
M. esculenta subsp. flabellifolia (including ten of the 15
cas-sava alleles; Fig. 2); this pattern suggests a very close rela-tionship between the two species. Fifty-three alleles were unique to M. esculenta subsp. flabellifolia.
Observed heterozygosities averaged over all wild popula-tions ranged between 27 (GAGG5 and GA16) and 51% (GA140) (Table 1). In crop accessions alone, heterozygosity ranged from 35 (GA134) to 85% (GAGG5). Single-locus ex-act tests for Hardy-Weinberg equilibrium indicated significant deviations in nine out of 170 comparisons (P , 0.05; Table 1), a number comparable to what would be expected by Type I error alone. None of the individual probability tests was sig-nificant when the sequential Bonferroni correction was ap-plied. However, three populations showed significant hetero-zygosity deficits across loci (P,0.05; Table 1), and a global test across all populations and loci for both species (Fisher’s method) indicated a significant deviation from Hardy-Wein-berg expectations (x2
1405 232.7, P , 0.0001). Specifically
testing for heterozygote deficiency (U test) indicated statisti-cally significant deficits at two loci across all populations, both of which were significant following sequential Bonferroni cor-rections (GA134 and GA140; Table 1). Thus, there appears to be some evidence for nonrandom mating within populations.
Exact tests for genotypic linkage disequilibrium yielded sig-nificant deviations for three out of 222 comparisons (P ,
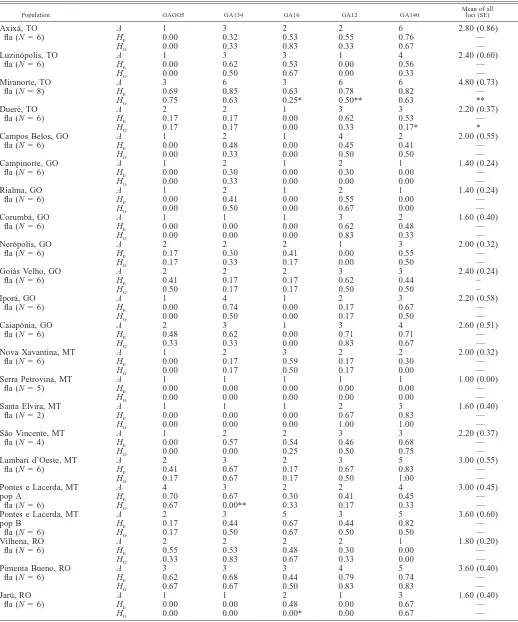
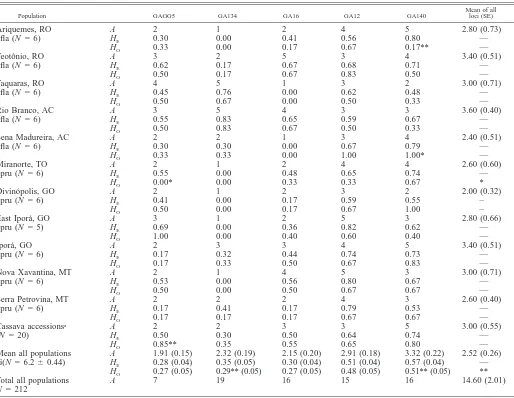
TABLE1. Allelic variability at five microsatellite loci in 27 populations of M. esculenta subsp. flabellifolia (fla), six populations of M. pruinosa (pru), and 20 accessions of cassava. Populations are listed in clockwise order around the eastern and southern border of the Amazon basin (see Fig. 1). Number of individuals examined (N), number of alleles per locus (A), heterozygosity expected under Hardy-Weinberg equilibrium (HE),
and observed heterozygosity (HO) are listed for each population and locus. Statistically significant deviations from Hardy-Weinberg expectations
are indicated by * (P,0.05) and ** (P,0.01).
Population GAGG5 GA134 GA16 GA12 GA140
Mean of all loci (SE) Luzino´ polis, TO
fla (N56) Campos Belos, GO
fla (N56) Goia´s Velho, GO
fla (N56) Nova Xavantina, MT
fla (N56) Serra Petrovina, MT
fla (N55) Santa Elvira, MT
fla (N52) Sa˜o Vincente, MT
fla (N54) Lambari d’Oeste, MT
fla (N56) Pontes e Lacerda, MT
pop A Pontes e Lacerda, MT
pop B Pimenta Bueno, RO
TABLE1. Continued.
Population GAGG5 GA134 GA16 GA12 GA140
Mean of all loci (SE) Rio Branco, AC
fla (N56) Sena Madureira, AC
fla (N56) Divino´ polis, GO
pru (N56) East Ipora´, GO
pru (N55) Nova Xavantina, MT
pru (N56) Serra Petrovina, MT
pru (N56) Cassava accessionsa
(N520) Mean all populations
G(N56.260.44) Total all populations
N5212
A 7 19 16 15 16 14.60 (2.01)
aAccessions from the cassava world core collection, Centro Internacional de Agricultura Tropical (CIAT): MARG11, MBOL3, MBRA12,
MBRA931, MCOL1505, MCOL1684, MCOL2215, MCR32, MCUB74, MECU82, MIND33, MMAL2, MMEX59, MNGA2, MPAN51, MPAR110, MPTR19, MTAI1, MVEN25, HMC1.
Population structure—Tests for genotypic heterogeneity
among the wild populations were highly significant for each of the five loci individually and for all loci combined (P ,
0.0005 following sequential Bonferroni adjustment). Quanti-tative estimates of this genetic differentiation differed between the two wild taxa, with M. esculenta subsp. flabellifolia show-ing greater interpopulation differentiation than M. pruinosa (multilocus FST5 0.42 and 0.28 for M. esculenta subsp.
fla-bellifolia and M. pruinosa, respectively; Table 2). These
mea-sures did not change dramatically when allele sizes were taken into account under the SMM (multilocusrST50.50 and 0.21
for M. esculenta subsp. flabellifolia and M. pruinosa, respec-tively; Table 2). Overall, these measures indicate a moderately high level of interpopulation differentiation. This pattern is also suggested by the corrected private alleles estimate of ef-fective interpopulation gene flow, which was less than 1.0 (Nm
50.32, 0.45 and 0.37 for M. esculenta subsp. flabellifolia, M.
pruinosa and all populations combined, respectively). At the
intrapopulation level, FISandrISvalues were generally positive
(FIS50.13,rIS50.23 across all populations of both species;
Table 2), a pattern consistent with the heterozygosity deficits observed in tests of Hardy-Weinberg equilibrium.
Fig. 2. Distributions of alleles observed at microsatellite loci (A) GAGG5, (B) GA134, (C) GA16, (D) GA12, and (E) GA140, in 157 individuals of M.
TABLE2. Genetic differentiation within and among populations of M. esculenta subsp. flabellifolia (fla), M. pruinosa (pru), and both taxa combined.
Locus
Fis
fla pru both
Fst
fla pru both
Fit
fla pru both
GAGG5
fla pru both
rst
fla pru both
rit
fla pru both
GAGG5
M. pruinosa alleles tend to be smaller than those of M. escu-lenta (Fig. 2b), and at locus GA12, where M. pruinosa alleles
tend to be larger (Fig. 2d).
Mantel tests for isolation by distance failed to reject the null hypothesis of no correlation between pairwise genetic differ-entiation (FSTandrST) and geographical distances among
pop-ulations (P. 0.20 for M. esculenta subsp. flabellifolia pop-ulations alone, M. pruinosa poppop-ulations alone, and all popu-lations combined). This result suggests that on the spatial scale examined, present population differentiation reflects gene flow processes other than limited, recurrent expansion from a re-stricted ancestral range. Alternatively, failure to detect isola-tion by distance may be an artifact of the small number of individuals examined per population, which limits the power of the test statistic.
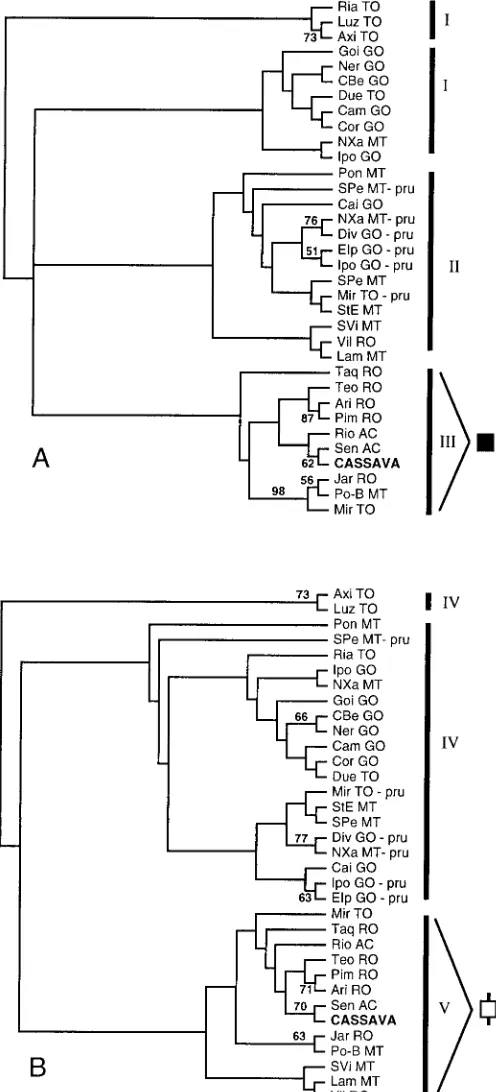
Relationships among populations and
taxa—Neighbor-joining and maximum likelihood distance methods were used to examine relationships among wild Manihot populations and cassava accessions. Bootstrap values were generally low on both types of distance trees, with most nodes associated with values of ,50% (Fig. 3a, b). Nonetheless, certain well-sup-ported features are shared between the neighbor-joining and maximum likelihood trees and can provide insight into genetic relationships in the study system. Most notable is cassava’s placement among southern Amazonian populations of M.
es-culenta subsp. flabellifolia. Both the DCneighbor-joining tree
and the maximum likelihood tree grouped cassava most close-ly with the population from Sena Madureira, Acre with rela-tively high bootstrap support (62 and 70%, repecrela-tively; Fig. 3a, b). Moreover, on both trees the crop falls within a cluster of populations from Acre, Rondoˆnia and western Mato Grosso (plus one aberrant population, Miranorte, in central Tocantins) (Figs. 1, 3a, b). This relationship was also observed on the
RST neighbor-joining tree (not shown), which placed cassava
in a cluster with four populations from Rondoˆnia and one from Acre.
While lacking in statistical support, the distance trees gen-erally grouped populations according to the geographical re-gion in which they occur. Thus, on the DC neighbor-joining
tree (Fig. 3a), the four main clusters of populations fell out in three regional categories: group I, which includes most M.
esculenta subsp. flabellifolia populations from Goia´s and
To-cantins; group II, consisting of most populations from Mato Grosso (plus westernmost Goia´s and easternmost Rondoˆnia), as well as the M. pruinosa populations; and group III, con-sisting of populations from Acre, Rondoˆnia, and westernmost Mato Grosso, plus the single population from Tocantins. On the maximum likelihood tree (Fig. 3b) two large clusters are defined: group IV, comprising most M. esculenta subsp.
fla-bellifolia populations from Tocantins, Goia´s, and eastern Mato
Grosso, plus all M. pruinosa populations; and group V, com-posed of M. esculenta subsp. flabellifolia populations from Acre, Rondoˆnia, and western Mato Grosso (plus the aberrant Tocantins population). Withdrawing the cassava accessions from the data set and reconstructing the distance trees did not alter the composition of these regional groupings.
Neither the neighbor-joining trees nor the maximum likeli-hood tree grouped the M. pruinosa populations into a discrete (‘‘monophyletic’’) cluster. Given the weak statistical support for these trees, this apparent lack of monophyly per se cannot be taken as evidence for reticulate evolution between M.
prui-nosa and M. esculenta subsp. flabellifolia. On the other hand,
the high proportion of alleles shared between the two species (Fig. 2) does suggest either introgression or shared ancestral polymorphisms between these species. It should be noted that the distance trees in Fig. 3 are unrooted and that relationships among the populations are all relative. Thus, the M. pruinosa populations should not be construed to be derived from M.
esculenta subsp. flabellifolia, as would be the implication on
a rooted tree.
DISCUSSION
Cassava’s origin of domestication—The microsatellite data
analyzed here corroborate the findings of our phylogeographic study (Olsen and Schaal, 1999). The crop’s genetic variation is a subset of that of its conspecific wild relative (Fig. 2), suggesting that M. esculenta subsp. flabellifolia is cassava’s sole wild progenitor. In addition, the microsatellite data con-firm that cassava is most closely related to populations of M.
esculenta subsp. flabellifolia occurring along the southern
bor-der of the Amazon basin (Figs. 1, 3). Thus, this southern eco-tone is independently identified as the crop’s likely geograph-ical origin of domestication.
hy-Fig. 3. (A) DCneighbor-joining distance tree and (B) maximum likelihood distance tree showing genetic relationships among 20 cassava accessions, 27 populations of M. esculenta subsp. flabellifolia, and six populations of M.
pruinosa (pru). Numbers on branches indicate bootstrap values of.50% (100 replications). Roman numerals indicate geographical regions (see text). Black and barred squares indicate wild populations grouped most closely with cas-sava (see Fig. 1).
potheses (e.g., Rogers and Appan, 1973; Sauer, 1993; Jen-nings, 1995) in two fundamental respects: (1) a single wild progenitor is specified, rather than a pool of unidentified hy-bridizing species, and (2) a single geographical area of do-mestication is proposed, rather than many independent sites throughout the Neotropics. Traditional domestication scenarios are strongly influenced by Rogers and Appan (1973), who, in their monograph of Manihot, emphasized the potential role of interspecific hybridization in the crop’s origin (see also Rogers, 1963, 1965). Many species of Manihot show very high levels of intraspecific morphological variation (Rogers and Appan, 1973; K. Olsen, personal observation). Rogers and Appan (1973) suggested that this variability is due to widespread in-terspecific hybridization in nature. Cultivars of cassava are particularly variable morphologically, and the monographers interpreted this variation as evidence that the crop is likely derived from a number of different species complexes in Me-soamerica and South America. They further argued that the wild populations resembling cassava most closely (i.e., M.
es-culenta subsp. flabellifolia) are actually feral crop derivatives.
Of the ‘‘truly’’ wild Manihot species, they contended that those occurring in Mesoamerica [particularly M. aesculifolia (H.B.K.) Pohl.] are cassava’s closest relatives, with many lo-cally occurring species also contributing to the crop wherever it is grown in the Neotropics.
This ‘‘compilospecies’’ hypothesis is clearly not supported by currently available data. Application of molecular genetic markers in Manihot has shown that M. esculenta (including both cassava and the wild subspecies) is more closely related to South American species than to those from Mesoamerica (Fregene et al., 1994; Roa, 1997; Roa et al., 1997; B. Schaal, unpublished data). In addition, observations that cassava’s ge-netic diversity is a subset of that found in M. esculenta subsp.
flabellifolia (see also Roa et al., 1997; Olsen and Schaal, 1999)
indicate that the former is derived from the latter, not the re-verse. Finally, in the absence of supporting evidence, we are skeptical that interspecific hybridization has played a major role in the origin of crop. Reports of natural hybridization in
Manihot remain largely anecdotal and are based almost
en-tirely on observations of morphological intermediacy in the field. This criterion is a poor indicator of hybridization (Riese-berg and Ellstrand, 1993; Riese(Riese-berg, 1995) and is likely to be particularly unreliable in Manihot, given the tremendous phe-notypic plasticity that occurs in cassava (Rogers and Fleming, 1973; Olson, 1997) and the morphological variability observed in its congeners (Rogers and Appan, 1973). In artificial cross-ing studies most species do not hybridize readily with cassava (e.g., Allem, 1992; Nassar et al., 1995; Nassar, Carvalho, and Vieira, 1996), and seeds reported from ‘‘successful’’ crosses are rarely tested for viability (Allem, 1992), let alone proof of hybrid parentage. Given current data on genetic variation in cassava and its relatives, it is not necessary to invoke multiple hybridization events to explain the crop’s origin. In this re-spect, the domestication of cassava parallels that of maize, which was believed to be of hybrid origin until molecular and other data proved otherwise (Iltis, 1983; Doebley, Goodman, and Stuber, 1984; Doebley, Renfroe, and Blanton, 1987).
The possibility remains that subsequent to domestication from M. esculenta subsp. flabellifolia, cassava’s genetic diver-sity has been influenced by occasional introgression from other
Manihot species. There is some evidence to suggest
sponta-neous hybridization between cultivated cassava and Manihot
former-ly cultivated as a source of rubber; hybridization between these species has been reported in Africa, where they are both in-troduced (Wanyera et al., 1992). Additional sampling of crop accessions and wild Manihot populations will be useful for assessing the potential influence of post-domestication inter-specific introgression on the crop’s current genetic diversity.
Site of domestication—The concordance of the multilocus
microsatellite data with our earlier assessment of cassava’s or-igin (Olsen and Schaal, 1999) strongly suggests that the crop arose from southern Amazonian populations of M. esculenta subsp. flabellifolia. If correct, this conclusion places cassava within an area of crop domestication that has also been pro-posed for the peanut (Arachis hypogaea), two species of chili pepper (Capsicum baccatum and C. pubescens) and jack bean (Canavalia plagiosperma) (reviewed by Piperno and Pearsall, 1998). Interestingly, archaeological evidence from central Rondoˆnia suggests the very early development of agricultural settlements in this region (e.g., preceramic ‘‘black soil’’ oc-cupation beginning about 4800 BP; Miller, 1992; B. Meggers, personal communication, Smithsonian Institution). Moreover, recent archaeological work in northern Bolivia has revealed evidence of an advanced, prehispanic aquacultural complex in this same area (Erickson, 2000). Taken together, these findings suggest that the southern Amazon basin may have been an important center for the development of lowland Neotropical agriculture. We are hopeful that the present study will en-courage further examination of early agriculture and crop do-mestication within this region.
Our sampling transects covered most of the eastern and southern borders of the Amazon basin. However, it is possible that there are additional wild M. esculenta populations occur-ring along the northern border of the Amazon basin, in the transition zone into the Guiana highlands of northern South America. Manihot populations from this area have been alter-natively classified either as M. esculenta subsp. flabellifolia (Allem, 1994), or as a distinct species, most notably M. tristis (Rogers and Appan, 1973; Allem, 1987; Roa et al., 1997). In an amplified fragment length polymorphism (AFLP) analysis of relationships among cassava and wild Manihot accessions, Roa et al. (1997) found that individuals classified as M. tristis were more distantly related to cassava than were wild M.
es-culenta individuals. This finding suggests that these more
northerly populations are probably not cassava’s closest wild relatives. More extensive population sampling from the north-ern Amazonian ecotone would be useful for clarifying cassa-va’s relationship to these populations.
Crop genetic diversity—As a species is brought into
do-mestication, founder events, population bottlenecks, and arti-ficial selection are all expected to reduce the genetic diversity of the crop in relation to its wild progenitor (Ladizinsky, 1985; Doebley, 1989; Eyre-Walker et al., 1998). In the present study, alleles observed in cassava constitute 22.1% of those found in
M. esculenta subsp. flabellifolia. This proportion is consistent
with previous comparisons of cassava and its wild relatives. In our phylogeographic analysis (Olsen and Schaal, 1999), cassava haplotypes represented 25% of those found in M.
es-culenta as a whole. Similarly, the AFLP survey of Roa et al.
(1997) showed 38 cassava cultivars to contain ;30% of the genetic variation found in 14 wild M. esculenta accessions. When our microsatellite variation is expressed in terms of total heterozygosity (Ht; Nei, 1973), the crop’s genetic diversity is
;23% less than that of M. esculenta subsp. flabellifolia (Ht5
0.68 and 0.52 for progenitor and crop, respectively). This amount of decrease falls within the range typically observed in other crop-relative comparisons (Doebley, 1989; Gepts, 1993).
Population structure of wild taxa—Genetic
diversity—Het-erozygote deficits (Table 1) and positive FIS and rIS values
(Table 2) suggest some degree of inbreeding in the wild pop-ulations. This observation is not surprising, given the life his-tory of these taxa. There is no known genetic self-incompat-ibility system in Manihot (although inflorescences are metan-drous), and the mechanism of seed dispersal (explosively de-hiscent pods) does not promote long-distance gene flow. Both of these traits would favor mating among relatives.
Another potential cause of heterozygosity deficits in these populations could be the occurrence of null (nonamplifying) alleles, which can cause heterozygotes to be misscored as ho-mozygous. Two lines of evidence suggest that null alleles are not the cause of the heterozygote deficits observed here. First, heterozygote deficits can be observed at all of the loci exam-ined (although statistical significance across populations is re-stricted to two loci; Table 1); the occurrence of null alleles at all five loci would be unlikely. Moreover, in an earlier survey of Manihot genetic diversity that included loci GAGG5 and GA12, no evidence for null alleles was found in M. esculenta or in four other Manihot species (Roa, 1997; see also Cha-varriaga-Aguirre et al., 1998).
Population differentiation—The distribution of alleles and
genotypes among the wild populations (Tables 1 and 2) indi-cates a moderate-to-high level of population differentiation. Estimates of differentiation among populations (FST andrST)
are greater for M. esculenta subsp. flabellifolia than for M.
pruinosa. This difference probably reflects the more restricted
sampling scheme used for the latter species. Manihot pruinosa was collected only in its region of sympatry with M. esculenta subsp. flabellifolia, and only six populations were examined (vs. 27 of M. esculenta subsp. flabellifolia). Another factor that could potentially affect measures of differentiation in the two species is ascertainment bias (e.g., Ellegren, Primmer, and Sheldon, 1995; Hutter, Schug, and Aquadro, 1998). When mi-crosatellites are isolated in a particular species, the selected loci tend to have higher levels of polymorphism in that focal species than in related species. The loci used in the present study were originally isolated in cassava (Chavarriaga-Aguirre et al., 1998), which could result in a bias towards polymor-phism in M. esculenta subsp. flabellifolia over M. pruinosa. Such a bias could lead to underestimates of genetic differen-tiation among M. pruinosa populations. However, observed levels of polymorphism do not suggest that this has occurred. The allelic diversity in M. pruinosa (35 alleles in 35 individ-uals) is proportionally more than twice that of M. esculenta subsp. flabellifolia (68 alleles in 157 individuals), the opposite of the pattern expected with ascertainment bias.
ran-dom genetic drift (Slatkin, 1995; Rousset, 1996). The latter situation most likely applies here. Morphological and molec-ular data suggest that M. esculenta and M. pruinosa are very recently diverged species (Allem, 1992; Olsen and Schaal, 1999; B. Schaal, unpublished data) within a recently radiated genus (Rogers and Appan, 1973; reviewed by Olsen and Schaal, 1999). At the same time, private alleles estimates of gene flow and estimates of interpopulation differentiation (FST
andrST) indicate that gene flow is low, not high. Thus,
con-temporary population structure has probably been shaped largely by random genetic drift. This conclusion is consistent with the large number of alleles shared between M. esculenta and M. pruinosa (Fig. 2), and it further suggests that the mea-sures chosen for distance tree construction (e.g., DC) are
ap-propriate, since they assume evolution solely by random ge-netic drift.
While the incidence of shared alleles between M. esculenta and M. pruinosa is very high, analyses of molecular variance (AMOVAs) indicate some divergence in overall allele sizes between the two species. In other interspecific comparisons using microsatellites, such divergence has been attributed to ascertainment bias (e.g., Ellegren, Primmer, and Sheldon, 1995), since allele length is positively correlated with poly-morphism in microsatellites (Ellegren, Primmer, and Sheldon, 1995; Goldstein and Pollock, 1997). In the present study, as-certainment bias would result in alleles of M. pruinosa being consistently shorter than those of M. esculenta subsp.
flabel-lifolia. This pattern is not observed (Fig. 2). Manihot pruinosa
alleles are significantly shorter only at one locus (GA134; t test, P , 0.0001) and were significantly longer at two loci (GAGG5 and GA12; t tests, P , 0.0001); there was no sig-nificant divergence between species at the other two loci (P
.0.06). Thus, the divergence in allele lengths between these two species is most likely the result of random genetic drift combined with some mutational divergence following speci-ation.
Relationships among the wild M. esculenta populations—
The DC and maximum likelihood trees generally group
pop-ulations according to the geographical regions in which they occur (Fig. 3). The sole exception is the population from Mir-anorte, Tocantins, which clusters with cassava’s closest wild relatives in the southern Amazon-border region (Figs. 1 and 3). One possible explanation for this grouping is that there has been introgression into this population from cultivated cassa-va. Cassava and M. esculenta subsp. flabellifolia are interfer-tile (Roa et al., 1997), and cassava is occasionally planted in close proximity to M. esculenta subsp. flabellifolia populations (K. Olsen, personal observation). On the other hand, the Mir-anorte population was sampled in primary forest, and we found no evidence for introgression into this population in our phylogeographic analysis (Olsen and Schaal, 1999). In that study, six haplotypes were observed in the Miranorte popu-lation, five of which were found only in the states of Tocantins and Goia´s, and none of which were observed in cassava. Con-sidering the weak statistical support for groupings on the mi-crosatellite distance trees, the placement of the Miranorte pop-ulation in the southern Amazonian cluster may not be of any real biological significance.
Interspecific relationships—The question of whether there
is interspecific hybridization between M. esculenta subsp.
fla-bellifolia and M. pruinosa remains unresolved. The high
pro-portion of alleles shared between these species (Fig. 2) and the intermingling of species on the distance trees (Fig. 3) are consistent with the occurrence of interspecific introgression. On the other hand, these features would be expected for any two recently diverged species, whether there is current hybrid-ization between them or not. Although M. pruinosa is consid-ered to fall within cassava’s ‘‘secondary gene pool’’ of poten-tially interfertile species (Allem, 1992), this designation is based on morphological similarity rather than evidence of hy-bridization; the interfertility of the two species has yet to be established in artificial crossing studies. Furthermore, these species are adapted to different habitats, and while populations of the two species can occur in close proximity, no putative hybrids were observed during field collections for this study. Definitive documentation of hybridization between these spe-cies would require more thorough sampling of M. pruinosa populations (including those plants outside the range of M.
esculenta subsp. flabellifolia), crossing experiments to
estab-lish interfertility, and possibly the use of more rapidly evolving genetic markers.
Regardless of whether or not interspecific hybridization oc-curs, we have little reason to suspect that M. pruinosa has contributed to cassava’s germplasm. Cassava is not grouped with M. pruinosa populations on distance trees (Fig. 3); more-over, the populations most closely related to cassava all occur west of the range of M. pruinosa (Figs. 1 and 3), suggesting little contact between M. pruinosa and cassava’s presumed progenitors. It is notable that these same patterns were ob-served in our phylogeographic study (Olsen and Schaal, 1999): the crop did not share haplotypes with M. pruinosa, and pop-ulations containing cassava haplotypes were all west of M.
pruinosa populations. Given current information on genetic
variation in M. esculenta, M. pruinosa, and other Manihot spe-cies (Fregene et al., 1994; Roa et al., 1997, Olsen and Schaal, 1999; B. Schaal, unpublished data), cassava appears to be de-rived solely from its conspecific wild relative, specifically from those populations occurring along the southern border of the Amazon. This information will be useful for crop improve-ment efforts (Tanksley and McCouch, 1997; M. Fregene, per-sonal commumication, Centro Internacional de Agricultura Tropical), for examining the genetics of domestication in this species (Gepts, 1993; Eyre-Walker et al., 1998), and possibly for setting priorities in preserving wild Manihot populations, many of which are threatened by deforestation.
LITERATURE CITED
ALLEM, A. C. 1987. Manihot esculenta is a native of the Neotropics. Plant
Genetic Resources Newsletter 71: 22–24.
———. 1992. Manihot germplasm collecting priorities. In W. M. Roca and A. M. Thro [eds.], Report of the First Meeting of the International Net-work for Cassava Genetic Resources, 87–110. Centro Internacional de Agricultura Tropical, Cali, Colombia.
———. 1994. The origin of Manihot esculenta Crantz (Euphorbiaceae).
Ge-netic Resources and Crop Evolution. 41: 133–150.
BARTON, N. H.,ANDM. SLATKIN. 1986. A quasi-equilibrium theory of the distribution of alleles in a subdivided population. Heredity 56: 409–415. BEST, R.,ANDG. HENRY. 1992. Cassava: towards the year 2000. In W. M. Roca and A. M. Thro [eds.], Report of the First Meeting of the national Network for Cassava Genetic Resources, 3–11. Centro Inter-nacional de Agricultura Tropical, Cali, Colombia.
CAVALLI-SFORZA, L. L.,ANDA. W. F. EDWARDS. 1967. Phylogenetic anal-ysis: models and estimation procedures. American Journal of Human
Genetics 19: 233- 257.
CHAVARRIAGA-AGUIRRE, P., M. M. MAYA, M. W. BONIERBALE, S. KRESOV
in cassava (Manihot esculenta Crantz): discovery, inheritance and vari-ability. Theoretical and Applied Genetics 97: 493–501.
COCK, J. H. 1985. Cassava: new potential for a neglected crop. Westfield, London, UK.
DOEBLEY, J. 1989. Isozymic evidence and the evolution of crop plants. In D. E. Soltis and P. S. Soltis [eds.], Isozymes in plant biology, 165–191. Dioscorides, Portland, Oregon, USA.
———, M. M. GOODMAN,ANDC. W. STUBER. 1984. Isoenzymatic variation in Zea (Gramineae). Systematic Botany 9: 203–218.
———, W. RENFROE,ANDA. BLANTON. 1987. Restriction site variation in
the Zea chloroplast genome. Genetics 117: 139–147.
ELLEGREN, H., C. R. PRIMMER,ANDB. C. SHELDON. 1995. Microsatellite ‘evolution’: directionality or bias? Nature Genetics 11: 360–362. ERICKSON, C. L. 2000. An artificial landscape-scale fishery in the Bolivian
Amazon. Nature 408: 190–193.
EXCOFFIER, L., P. E. SMOUSE,ANDJ. M. QUATTRO. 1992. Analysis of mo-lecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial-DNA restriction data. Genetics 131: 479–491.
EYRE-WALKER, A., R. L. GAUT, H. HILTON, D. L. FELDMAN, ANDB. S.
GAUT. 1998. Investigation of the bottleneck leading to the domestication of maize. Proceedings of the National Academy of Sciences, USA 95: 4441–4446.
FELSENSTEIN, J. 1995. Phylip (Phylogeny Inference Package) Version 3.57c. University of Washington, Seattle, Washington, USA.
FREGENE, M. A., J. VARGAS, J. IKEA, F. ANGEL, J. TOHME, R. A. ASIEDU, M. O. AKORODA,ANDW. M. ROCA. 1994. Variability of chloroplast DNA and nuclear ribosomal DNA in cassava (Manihot esculenta Crantz) and its wild relatives. Theoretical and Applied Genetics 89: 719–727. GEPTS, P. 1993. The use of molecular and biochemical markers in crop
evo-lution studies. In M. K. Hecht et al. [eds.], Evoevo-lutionary biology, 51–94. Plenum, New York, New York, USA.
GOLDSTEIN, A. R. LINARES, L. L. CAVALLI-SFORZA,ANDM. W. FELDMAN. 1995a. An evaluation of genetic distances for use with microsatellite loci. Genetics 139: 463–471.
———, ———, ———, and ———. 1995b. Genetic absolute dating based on microsatellites and the origin of modern humans. Proceedings of the
National Academy of Sciences, USA 92: 6723–6727.
———,ANDD. D. POLLOCK. 1997. Launching microsatellites: a review of mutation processes and methods of phylogenetic inference. Journal of
Heredity 88: 335–342.
GOUDET, J., M. RAYMOND, T.DEMEEU¨ S,ANDF. ROUSSET. 1996. Testing differentiation in diploid populations. Genetics 144: 1933–1940. GUO, S. W.,ANDE. A. THOMPSON. 1992. Performing the exact test of
Hardy-Weinberg proportion for multiple alleles. Biometrics 48: 361–372. HILLIS, D. M., B. K. MAPLE, A. LARSON, S. K. DAVIS,ANDE. A. ZIMMER.
1996. Nucleic acids IV: sequencing and cloning. In D. M. Hillis, C. Moritz, and B. K. Maple [eds.], Molecular systematics, 321–381. Sinauer, Sutherland, Massachusetts, USA.
HUTTER, C. M., M. D. SCHUG,ANDC. F. AQUADRO. 1998. Microsatellite
variation in Drosophila simulans: a reciprocal test of the ascertainment bias hypothesis. Molecular Biology and Evolution. 15: 1620–1636. ILTIS, H. H. 1983. From teosinte to maize: the catastrophic sexual
transmu-tation. Science 222: 886–894.
JENNINGS, D. L. 1995. Cassava: Manihot esculenta (Euphorbiaceae). In J.
Smartt and N. W. Simmonds [eds.], Evolution of crop plants, 128–132. Wiley, New York, New York, USA.
LADIZINSKY, G. 1985. Founder effect in crop-plant evolution. Economic
Bot-any 39: 191–198.
MANN, C. 1997. Reseeding the green revolution. Science 277: 1038–1043.
MANTEL, N. 1967. The detection of disease clustering and a generalized regression approach. Cancer Research 27: 209–220.
MICHALAKIS, Y.,ANDL. EXCOFFIER. 1996. A generic estimation of popu-lation subdivision using distances between alleles with special interest to microsatellite loci. Genetics 142: 1061–1064.
MILLER, E. T. 1992. Archaeology in the hydroelectric projects of Eletronorte: preliminary results. Smithsonian Institution, Washington, D.C., USA. MINCH, E., A. RUIZ-LINARES, D. GOLDSTEIN, M. FELDMAN,ANDL. L. CAV
-ALLI-SFORZA. 1996. Microsat: a computer program for calculating var-ious statistics on microsatellite allele data. Version 1.5. Stanford Uni-versity Medical Center, Stanford, California, USA.
NASSAR, N. M. A., C. G. CARVALHO,ANDC. VIEIRA. 1996. Overcoming
crossing barriers between cassava, Manihot esculenta Crantz and a wild relative, M. Pohlii Warwa. Brazilian Journal of Genetics 19: 617–620. ———, N. M. A. NASSAR, C. VIEIRA,ANDL. S. SARAIVA. 1995.
Cytoge-netic behaviour of the interspecific hybrid of Manihot neusana Nassar and cassava, M. esculenta Crantz, and its backcross progeny. Canadian
Journal of Plant Science 75: 675–678.
NEI, M. 1973. Analysis of gene diversity in subdivided populations.
Pro-ceedings of the National Academy of Sciences, USA 70: 3321–3323.
———, F. TAJIMA,ANDY. TATENO. 1983. Accuracy of estimated phyloge-netic trees from molecular data. Journal of Molecular Evolution 19: 153– 170.
OHTA, T., ANDM. KIMURA. 1973. The model of mutation appropriate to estimate the number of electrophoretically detectable alleles in a genetic population. Genetical Research 22: 201–204.
OLSEN, K. M.,ANDB. A. SCHAAL. 1999. Evidence on the origin of cassava: phylogeography of Manihot esculenta. Proceedings of the National
Academy of Sciences, USA 96: 5586–5591.
OLSON, P. D. 1997. Phenotypic plasticity: a quantitative genetic study using
Manihot esculenta Crantz. Ph.D. dissertation, Washington University, St.
Louis, Missouri, USA.
PAGE, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358.
PIPERNO, D. R.,ANDD. M. PEARSALL. 1998. The origins of agriculture in the lowland Neotropics. Academic, New York, New York, USA. RAYMOND, M.,ANDF. ROUSSET. 1995. GENEPOP: population genetics
soft-ware for exact tests and ecumenicism. Journal of Heredity 86: 248–249. RENVOIZE, B. S. 1972. The area of origin of Manihot esculenta as a crop
plant—a review of the evidence. Economic Botany 26: 352–360. RICE, W. R. 1989. Analyzing tables of statistical tests. Evolution 43: 223–
225.
RIESEBERG, L. H. 1995. The role of hybridization in evolution: old wine in new skins. American Journal of Botany 82: 944–953.
———,ANDN. C. ELLSTRAND. 1993. What can morphological and molec-ular markers tell us about plant hybridization. Critical Reviews in the
Plant Sciences 12: 213–241.
ROA, A. C. 1997. Estimacio´n de la diversidad gene´tica en Manihot spp. mediante morfologı´a y marcadores moleculares. Master’s thesis, Univ-ersidad del Valle, Cali, Colombia.
———, M. M. MAYA, M. C. DUQUE, J. TOHME, A. C. ALLEM,ANDM. W. BONIERBALE. 1997. AFLP analysis of relationships among cassava and other Manihot species. Theoretical and Applied Genetics 95: 741–750. ROGERS, D. J. 1963. Studies of Manihot esculenta Crantz and related species.
Bulletin of the Torrey Botanical Club 90: 43–54.
———. 1965. Some botanical and ethnological considerations of Manihot
esculenta. Economic Botany 19: 369–377.
———, ANDS. G. APPAN. 1973. Manihot and Manihotoides (Euphorbi-aceae): a computer assisted study. Hafner, New York, NY.
———,ANDH. S. FLEMING. 1973. A monograph of Manihot esculenta with
an explanation of the taximetrics methods used. Economic Botany 27: 1–113
ROUSSET, F. 1996. Equilibrium values of measure of population subdivision for stepwise mutation processes. Genetics 142: 1357–1362.
———,ANDM. RAYMOND. 1995. Testing heterozygote excess and deficien-cy. Genetics 140: 1413–1419.
SAUER, J. D. 1993. Historical geography of crop plants: a select roster. CRC Press, Boca Raton, Florida, USA.
SCHNEIDER, S., J.-M. KUEFFER, D. ROESSLI,ANDL. EXCOFFIER. 1997. Ar-lequin: a software for population genetic data analysis. Genetics and Biometry Laboratory, University of Geneva, Switzerland.
SLATKIN, M. 1985. Rare alleles as indicators of gene flow. Evolution 39: 53– 65.
———. 1995. A measure of population subdivision based on microsatellite allele frequencies. Genetics 139: 457–462.
TAKEZAKI, N.,ANDM. NEI. 1996. Genetic distances and reconstruction of phylogenetic trees from microsatellite dna. Genetics 144: 389–399. TANKSLEY, S. D., ANDS. R. MCCOUCH. 1997. Seed banks and molecular
maps: unlocking genetic potential from the wild. Science 277: 1063– 1066.
TARR, C. L., S. CONANT,ANDR. C. FLEISCHER. 1998. Founder events and variation at microsatellite loci in an insular passerine bird, the Laysan finch (Telespiza cantans). Molecular Ecology 7: 719–731.
and genetic diversity across generations assessed by microsatellites among sympatric populations of landlocked Atlantic salmon (Salmo salar L.). Molecular Ecology 8: 169–179.
UGENT, D., S. POZORSKI,ANDT. POZORSKI. 1986. Archaeological manioc (Manihot) from coastal Peru. Economic Botany 40: 78–102.
WANYERA, N. M. W., S. K. HAHN,ANDM. E. AKEN’OVA. 1992. Introgres-sion of ceara rubber (Manihot glaziovii Muell-Arg.) into cassava (M.
esculenta Crantz): a morphological and electrophoretic evidence. In M.
O. Akoroda [ed.], Root crops for food security in Africa, 125–130.
Pro-ceedings of the Fifth Triennial Symposium of the International Society for Tropical Root Crops-Africa Branch, Kampala, Uganda, 22–28 No-vember 1992.
WEIR, B. S.,ANDC. C. COCKERHAM. 1984. Estimating F-statistics for the analysis of population structure. Evolution 38: 1358–1370.