Effect of atorvastatin and fluvastatin on the expression of
plasminogen activator inhibitor type-1 in cultured human
endothelial cells
Sophie Lopez, Franck Peiretti, Bernadette Bonardo, Ire`ne Juhan-Vague,
Gilles Nalbone *
INSERM EPI99-36and Uni6ersite´ de la Me´diterrane´e,Laboratoire d’He´matologie,Faculte´ de Me´decine,27Bd.Jean Moulin, 13385Marseille Cedex5,France
Received 21 September 1999; received in revised form 17 February 2000; accepted 1 March 2000
Abstract
Inhibitors of HMG-CoA reductase, namely statins, improve endothelial function independently of their cholesterol-lowering effects. Plasminogen activator inhibitor type-1 (PAI-1) plays a critical role in vascular pathophysiology both at the intra- and extravascular levels. We therefore investigated the effects of atorvastatin (ATOR) and fluvastatin (FLU) on PAI-1 and also tissue-type plasminogen activator (t-PA) synthesis in 20% fetal calf serum-cultured human umbilical vein endothelial cells (HUVEC) stimulated or not by recombinant human pro-inflammatory cytokines, i.e. tumor necrosis factor a (TNFa) and interleukin 1a (IL-1a). In non-stimulated HUVEC, ATOR and FLU significantly diminished (−50% at 2.0 mmol/l) the constitutive production of PAI-1 (mRNA level and protein secretion). This effect was prevented by addition of mevalonate (100
mmol/l). In HUVEC cultivated in 20% fetal calf serum, the t-PA antigen accumulation was not significantly altered, whereas in low serum concentration (1%) a significant stimulatory effect of ATOR (+30%) and FLU (+76%) was observed. In TNFa-stimulated cells, ATOR and FLU had a modest down-modulating effect (−17 and−20%, respectively) on TNFa-induced increase in PAI-1 synthesis. No effect of statins was observed in IL-1a-stimulated HUVEC, suggesting that statins do not interfere with the up-regulation of PAI-1 synthesis by pro-inflammatory cytokines. However, ATOR and FLU inhibited the TNFa-induced decrease in t-PA release. In conclusion, these results show that statins favorably modulate the expression of fibrinolytic factors produced by human endothelial cells. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Endothelial cells; Statins; PAI-1; Gene expression
www.elsevier.com/locate/atherosclerosis
1. Introduction
Statins were designed to specifically inhibit the en-zyme of the rate-limiting step of hepatic cholesterol synthesis (HMG-CoA reductase) and consequently, by enhancing LDL-cholesterol uptake, to lower circulating LDL-cholesterol [1]. Their efficiency was demonstrated by several clinical trials univocally showing a significant reduction in cardiovascular-related morbidity and mor-tality [2].
However, there is accumulating evidences that the
decrease in circulating LDL-cholesterol does not fully account for the beneficial therapeutic effect of statins on cardiovascular events, as cholesterol-independent mechanisms are also involved [3 – 8]. These cholesterol-independent mechanisms appear to be linked to the alteration of a large array of intracellular events regu-lated by metabolites downstream of mevalonate that is the immediate metabolite downstream of HMG-CoA [9,10]. For example, statins regulate the expression of proteins involved in vascular biology, such as MMP-9 in mouse macrophages [11], endothelin and NO syn-thase in vascular endothelial cells [12,13], tissue factor in monocytes [14,15] and adhesive molecules [16]. Also, statins reduce the platelet deposition on damaged vessel wall in a dyslipemic rabbit model [17].
* Corresponding author. Tel.: +33-4-91324507; fax: + 33-4-91254336.
E-mail address:[email protected] (G. Nalbone).
Coronary thrombosis is well recognized as the precip-itating event in acute ischemic heart diseases. In addi-tion to disrupaddi-tion of atheromatous plaque, systemic thrombogenic factors may contribute to the initiation and progression of coronary thrombosis and its clinical sequel [18]. Impaired fibrinolytic activity is often the consequence of an increase in the circulating level of a major inhibitor of fibrinolysis, the plasminogen activa-tor inhibiactiva-tor type-1 (PAI-1). Increased levels of this inhibitor are considered to be a risk factor of myocar-dial infarction, particularly in the case of the syndrome of insulin resistance in obese subjects with high levels of circulating triglycerides [19,20]. Clinical studies per-formed so far have not demonstrated univocal effects of statins on PAI-1 plasma levels [21 – 26], although the different clinical protocol designs and type of statin may account for the differences observed. In addition, as PAI-1 binds to the extracellular matrix [27], changes in circulating PAI-1 concentration probably poorly reflect local changes in the vascular wall [28]. In addi-tion to its funcaddi-tion as a regulator of fibrinolysis at the luminal face of endothelial cells, PAI-1 also regulates proteolysis-dependent migration and adhesion pro-cesses in the vascular wall [29]. This may have impor-tant pathophysiological consequences in terms of vascular remodeling as demonstrated for example by retroviral administration of PAI-1 gene in a model of aneurysm in the rat [30] or after vascular injury in PAI-1 knock-out mice [31].
Works devoted to in vitro studies of the effects of statins on the fibrinolytic potential of endothelial cells are scarce. In a recent study, Essig et al. [32] showed that lovastatin decreased the constitutive PAI-1 expres-sion in a transformed rat endothelial cell line whereas it increased the constitutive expression of t-PA in these cells and also in human umbilical vein endothelial cells (HUVEC). We therefore investigated the effect of statins on the synthesis of PAI-1 by HUVEC in non-stimulated conditions. TNF-a and IL-1a contribute to the inflammatory process in atherosclerosis and are major inducers of PAI-1 synthesis in cultured human endothelial cells [33,34]. We therefore studied if statins modulated the effects of pro-inflammatory cytokines on PAI-1 synthesis. To further investigate the modulation of the fibrinolytic balance of these cells by statins, we also included studies on t-PA synthesis. Two statins were studied: Atorvastatin (ATOR) and Fluvastatin (FLU).
Results showed that ATOR and FLU significantly decreased the synthesis of PAI-1 (mRNA and protein levels) in basal conditions, but modestly in HUVEC stimulated by pro-inflammatory cytokines. Both statins prevented the TNFa-induced decrease in t-PA production.
2. Materials and methods
2.1. Reagents
ATOR (calcium salt) and FLU (sodium salt) were provided as a powder by Parke – Davis and Novartis, respectively. Stock solutions (10 mmol/l) of ATOR and FLU were made in dimethylsulfoxide (DMSO) and in pure absolute ethanol, respectively, and aliquots stored at −20°C. The volume of DMSO or ethanol never exceeded 0.1% (v/v) and did not affect the PAI-1 and t-PA synthesis. Special precautions were taken to avoid direct exposure of statins to light, before and during incubation procedures. Statins were diluted in warm culture medium just before incubation with HUVEC. Recombinant human TNFa was from Amersham Bio-technology and recombinant human IL-1a from R&D Systems. The Moloney-Murine Leukaemia Virus Re-verse Transcriptase (M-MLV RT) and its appropriate buffer were purchased from Gibco BRL (Life Tech-nologies). Taq polymerase and its appropriate buffer were from Bioprobe. All molecular biological products (dNTP, random hexaprimers, RNasin®and appropriate buffers) were from Promega. Monoclonal antibodies against PAI-1 were kindly provided by P. Declerck and R. Lijnen (Leuven, Belgium). Cytochalasin D, meval-onate and geranylgeranylpyrophosphate (GGPP) were from Sigma – Aldrich.
2.2. Cell culture and experimental conditions
HUVEC were isolated and cultivated in 20% fetal calf serum (FCS), or 1% when specified, as previously described [35]. They were used for experiments at the third passage in six-well plates (2 ml/well) unless other-wise indicated. Preconfluent HUVEC were preincu-bated overnight with indicated doses of statin. The culture medium was then eliminated and replaced by fresh culture medium with the same dose of statin and TNFa (20 U/ml) or IL-1a (10 ng/ml) was or was not added. Total RNA was extracted 14 h later and condi-tioned culture medium was collected 24 h later. It was centrifuged at 14 000 rpm at 4°C for 10 min and the supernatant was stored at −20°C pending PAI-1 and t-PA assays. In the experiments in which mevalonate or GGPP was added, HUVEC were first treated as above with statin, then mevalonate (100mmol/l) or GGPP (15
mmol/l) was added and RNA was extracted at 14 h and culture medium collected at 24 h after addition of mevalonate.
2.3. RNA extraction and RT-PCR analysis
HUVEC were cultivated in 25 cm2
cDNA synthesis were performed as described [36]. The amplified fragment for human PAI-1 is of 284 bp, base position 10977 – 11260 (GenBank accession number X04744). Amplified fragment for eEF1a used as a house keeping gene [36] is of 289 bp, base position 382 – 670 (GenBank accession number X03558). PCR was performed on a Perkin-Elmer thermocycler (Ge-neAmp 2400). PCR conditions were calibrated in pre-liminary experiments, allowing determination of the cycle number for which there was a significant de-tectable spot of the amplified fragment. Twenty-five and 18 cycles were selected for PAI-1 and eEF1a, respectively. Specificity of the amplified fragment was assessed by demonstrating that appropriate restriction enzymes generated the expected clivage fragments. PCR started for 2 min at 95°C followed by cycles consisting of: 60 s at 58°C, 90 s at 72°C, and 45 s at 97°C. Amplification was terminated by 5 min at 72°C and products were visualized and photographed under UV radiation following gel agarose (2%) electrophoresis.
2.4. Protein assays
Total proteins of cell lysates were assayed according to specifications of the bicinchoninic acid protein assay kit of Sigma. PAI-1 antigen assay was performed on supernatants from conditioned culture medium by ELISA as described by Declerck et al. [37]. t-PA anti-gen assay was performed on the same supernatants using the ELISA kit of Biopool International, accord-ing to the specifications of the manufacturer. Concen-trations of PAI-1 and t-PA antigens in the culture medium were normalized with intracellular proteins. Lactate dehydrogenase (LDH) activity, released in the culture medium and used as an index of cell injury, was assayed using a clinical routine assay on an automatic analyzer (Hitashi).
2.5. Statistics
Because of the variability between the different HU-VEC preparations, each determination was performed in triplicate and at least five different preparations of HUVEC were tested. Results are expressed as mean9 S.D. Comparison was analyzed by ANOVA test and significance calculated atPB0.05 using the Fisher test.
3. Results
3.1. Effect of ATOR and FLU on PAI-1 synthesis in basal conditions
We first determined the effect of increasing doses of ATOR and FLU on HUVEC viability. We consistently observed that concentrations above 1.0mmol/l of either
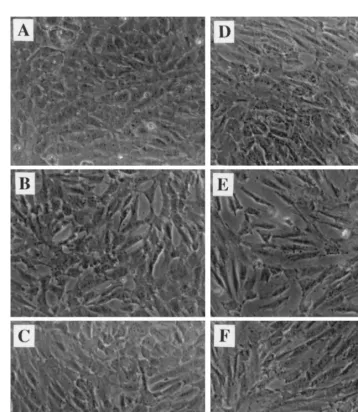
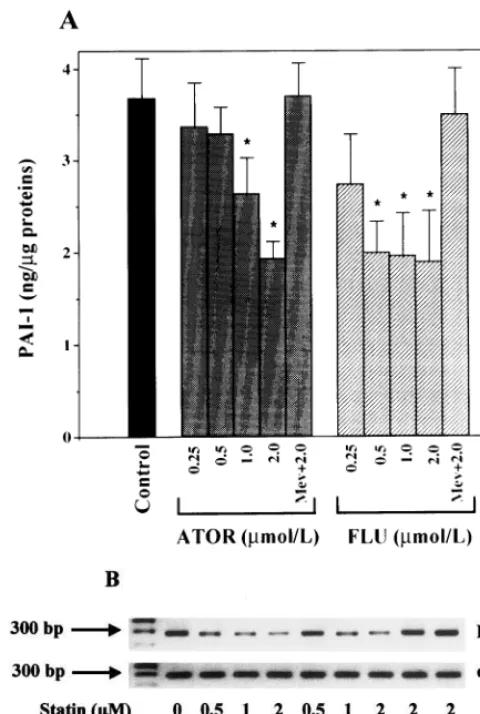
ATOR or FLU induced increasingly marked changes in the morphological appearance of HUVEC character-ized by some cell retraction and stretching (Fig. 1). For these reasons, we selected a range of concentration from 0.25 up to 2.0mmol/l between which no significant increase in LDH release could be observed (Table 1). As shown in Fig. 2(A), ATOR and FLU decreased the basal accumulation in culture medium of PAI-1 antigen measured at 24 h. The decrease became significant (PB0.05) at 0.5 mmol/l with FLU (−46%) and at 1.0
mmol/l with ATOR (−30%). At 2mmol/l the decrease was of 50% for the two statins. At the same concentra-tion, the effects of ATOR and FLU were not statisti-cally different (P\0.05). To investigate the molecular level at which statins exerted their down-regulating effects, we analyzed the PAI-1 mRNA levels by semi-quantitative RT-PCR. As shown in Fig. 2(B), a signifi-cant reduction in PAI-1 mRNA levels was observed with either ATOR (1 and 2 mmol/l) or FLU (0.5 – 2
mmol/l). To further investigate the mode of action of statin, HUVEC were pretreated overnight with the statins at 2.0 mmol/l and then with mevalonate (100
mmol/l). Mevalonate alone did not alter basal PAI-1 synthesis (not shown). However, as shown in Fig. 2, mevalonate significantly reversed the inhibitory effect of both ATOR and FLU tested at 2.0 mmol/l. The levels of PAI-1 antigen that accumulated in the culture medium, as well as those of PAI-1 mRNA, measured 24 and 14 h after mevalonate addition, respectively, were similar to those of control untreated HUVEC. GGPP, which is a down-stream metabolite of meval-onate, was shown to reverse lovastatin-induced de-crease in PAI-1 synthesis in cultured rat endothelial cells [32]. In HUVEC treated by ATOR or FLU (2
crease in t-PA antigen following lovastatin treatment. We therefore cultured HUVEC in 1% FCS and incu-bated them with ATOR and FLU at 2mmol/l. In these conditions, t-PA production was significantly increased by 30.093.5% and 76.498.8% (n=6,PB0.05), with ATOR and FLU, respectively (not shown).
3.2. Effect of ATOR and FLU on PAI-1 synthesis in TNFa-stimulated HUVEC
TNFa (20 U/ml) drastically enhanced (Fig. 3) PAI-1 accumulation (by a factor of 3.8) in the culture medium and mRNA levels, which is concordant with previous
Fig. 1. Phase contrast microphotography of HUVEC: (A) control untreated cells; (B) ATOR 2.0mmol/l; (C) FLU 2.0mmol/l; (D) TNFa(20 U/ml); (E) ATOR 2.0mmol/l+TNFa; (F) FLU 2.0mmol/l+TNFa.
Table 1
Effect of ATOR and FLU on LDH release from HUVECa
ATOR (mmol/l) FLU (mmol/l)
None
2.0 1.0
2.0 1.0
208.296.5
Control 197.697.4 208.792.5 205.799.7 196.096.5
201.791.1
TNFa 192.091.7 205.392.0 199.092.6 202.097.0
Fig. 2. Effect of ATOR and FLU on basal synthesis of PAI-1 in HUVEC. (A): PAI-1 accumulation in the culture medium 24 h after addition of statins. (B): PAI-1 and eEF1amRNA levels analyzed by RT-PCR 14 h after addition of statins. Values are means9S.D. (n=12) of four experiments each performed in triplicate. Differences are significant at *PB0.05 (ANOVA, Fisher test) over control cells without treatment.
Fig. 3. Effect of ATOR and FLU on PAI-1 synthesis in HUVEC stimulated by TNFa. HUVEC were pretreated overnight by statins, rinsed and then stimulated by TNFa, still in the presence of statins. (A): PAI-1 accumulation in the culture medium 24 h after addition of TNFa(20 U/ml). To facilitate comparison, PAI-1 accumulation in unstimulated conditions is repeated (black bar). (B): PAI-1 and eEF1a mRNA levels analyzed by RT-PCR 14 h after addition of TNFa. Values are means9S.D. (n=12) of four experiments each performed in triplicate. Differences are significant at *PB0.05 (ANOVA, Fisher test) over TNFa-stimulated cells.
data [33,34]. In the presence of ATOR or FLU, LDH release was not affected (Table 1). These statins mod-estly inhibited TNFa-induced PAI-1 accumulation in the culture medium. This inhibition did not exceed 17 and 25% for ATOR (at 1.0 mmol/l) and FLU (at 0.5
mmol/l), respectively, and was not reflected by
signifi-cant changes of PAI-1 mRNA levels. Il-1a is also a potent inducer of PAI-1 synthesis in HUVEC. In our conditions, IL-1aincreased PAI-1 synthesis from 3.19
Table 2
Effect of ATOR and FLU on t-PA release from HUVECa
ATOR (mmol/l) FLU (mmol/l)
None
2.0 1.0 2.0
1.0
0.2790.026 0.2790.01 0.3390.02 0.2990.014
Control 0.2890.02
0.1390.03** 0.2190.02
TNFa 0.1790.03* 0.2390.07 0.2090.06*
aHUVEC were treated as in described in Table 1. Conditioned media were collected and assayed for t-PA antigen. Values (ng t-PA/mg of total proteins) are means9S.D. (n=9) of three separate experiments each performed in triplicate.
0.5 up to 13.791.3 ng PAI-1/mg of total proteins (n=6). ATOR or FLU at 1.0 mmol/l did not signifi-cantly alter this IL-1a-induced increase in PAI-1 syn-thesis, as the decrease did not exceed 8% (data not shown).
We also investigated if ATOR or FLU altered the synthesis of t-PA. TNFais known to decrease the basal synthesis of t-PA in HUVEC [33], which was also observed herein by a significant (PB0.01) decrease in t-PA accumulation (−52%) when compared with un-stimulated cells (Table 2). Pretreatment of HUVEC (cultivated in 20% FCS) with either FLU or ATOR reduced the inhibitory effect of TNFa on t-PA release. However, because of some wide dispersion of t-PA levels among the different HUVEC preparations, the only significant reversal effect of statins (i.e. no statisti-cal significant decrease versus control; P\0.05) was observed with ATOR at 2.0 mmol/l (−22%) and with FLU at 1.0 mmol/l (−15%).
4. Discussion
The beneficial effect of statins on cardiovascular dis-eases appears over and above the well established and proven decrease in circulating LDL-cholesterol. By in-terfering with prenylation of transducing proteins, statins may alter the expression of proteins implicated in vascular function [9,10]. The dysregulation of the fibrinolytic potential of endothelial cells is critically involved in the development and progression of atherothrombosis [38]. In this study, we analyzed whether the two statins ATOR and FLU altered the levels of PAI-1 in HUVEC placed in basal and proinflammatory conditions. Clearly, both statins sig-nificantly decreased the synthesis of PAI-1 in HUVEC cultivated in the presence of 20% serum. The effects of ATOR and FLU were not statistically different one from the other, although FLU tended to act at lower concentrations than ATOR, which may reflect their different hydrophobic properties. These results are in line with those of Essig et al. [32] who showed, in a rat transformed endothelial cell line, that lovastatin down-regulated PAI-1 expression, as regards both activity and mRNA levels. The inhibitory effect of FLU and ATOR on PAI-1 antigen release is reflected by a de-crease in PAI-1 mRNA level and is prevented by addi-tion of mevalonate. This strongly suggests that statins exerted their effect on PAI-1 gene expression through inhibition of the mevalonate pathway. Intermediate metabolites of this pathway, such as farnesylpyrophos-phate or GGPP, are involved in the prenylation of transducing proteins, allowing proper interaction with the membrane and consequently an optimal transmis-sion of the information through the cytoskeleton. In this way, it was demonstrated that prenylation of Rho
GTPases by GGPP was inhibited by lovastatin [39]. Interestingly, in rat endothelial cells, GGPP was able to prevent the lovastatin-induced down-regulation of PAI-1 synthesis [32]. It was proposed that lovastatin induced a defect in the prenylation of Rho protein that in turn altered its interaction with the cytoskeleton [32]. Inter-estingly, we also observed with HUVEC that GGPP almost completely reversed the down-modulating effect of statins. However, in HUVEC, cytoskeletal reorgani-zation itself does not seem to be responsible for the decrease in PAI-1 synthesis, as we observed that the disrupter of actin filaments, cytochalasin D, did not significantly alter PAI-1 synthesis. This result can be analyzed in the light of the results of Zohar et al. [40], who showed in NIH3T3 cells that Rho-effector molecules regulating actin structure are distinct from those signaling to the nucleus. The effect of statins on the activation of PAI-1 synthesis by proinflammatory cytokines (TNFaand IL-1a) was modest as the optimal decrease we observed (−17 to −20%) was with TNFa. This suggests that FLU or ATOR does not interfere with the proinflammatory cytokines-triggered pathway leading to PAI-1 gene activation. One may thus hypothesize that, in vivo, statins would signifi-cantly regulate PAI-1 production in normal or mildly activated endothelial cells rather than those lining an advanced inflamed atherosclerotic lesion. This, how-ever, does not rule out the possibility that the signalling pathway triggered by other types of inducers of PAI-1 synthesis present in the atherosclerosic lesion can be down-regulated by statins. A different behavior was observed with t-PA synthesis in HUVEC. In basal conditions (i.e. 20% FCS), ATOR and FLU did not significantly alter t-PA release. However, in HUVEC and in rat endothelial cells incubated in low serum concentration and treated with lovastatin, Essig et al. [32] observed an increase in t-PA synthesis. In this study, we also observed that HUVEC incubated in 1% FCS increased the t-PA release in the presence of statin with a more marked effect of FLU. However, at this stage of investigation, the pathophysiological relevance of the relationships between statins, serum depletion and t-PA synthesis remains to be clarified. Interestingly, ATOR and FLU reduce the TNFa-induced decrease in t-PA antigen accumulation, suggesting that these statins interfere with some factors of the TNFa-triggered transduction pathway that down-regulate t-PA gene transcription. The differential regulation of PAI-1 and t-PA syntheses by ATOR and FLU in basal and TNFa-stimulated conditions underlines the complex regulation that statins exert on cell function and trafficking.
5. Note added in proof
While this paper was in press, Bourcier and Libby (Arterioscler Thromb Vasc Biol, 2000;20:556 – 62) re-ported that simvastatin down-regulated PDGF- and TGFb-induced PAI-1 synthesis in human endothelial cells.
Acknowledgements
This work was supported by funds from INSERM and Parke – Davis France. S. Lopez was a recipient of funds from GEHT-Sanofi and from Fondation pour la Recherche Me´dicale. The authors wish to thank M. Verdier for PAI-1 assays, V. Thome´ for preparing cell cultures and J. Ansaldi for t-PA assays.
References
[1] Dujovne CA. New lipid lowering drugs and new effects of old drugs. Curr Opin Lipidol 1997;8:362 – 8.
[2] Bucher HC, Griffith LE, Guyatt GH. Systematic review on the risk and benefit of different cholesterol-lowering interventions. Arterioscler Thromb Vasc Biol 1999;19:187 – 95.
[3] Williams JK, Sukhova GK, Herrington DM, Libby P. Pravas-tatin has cholesterol-lowering independent effects on the artery wall of atherosclerotic monkeys. J Am Coll Cardiol 1998;31:684 – 91.
[4] Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Gold-man S, Flaker GC, Braunwald E, for the Cholesterol and Recurrent Events (CARE) Investigators. Inflammation, pravas-tatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation 1998;98:839 – 844.
[5] Shiomi M, Ito T. Effect of cerivastatin sodium, a new inhibitor of HMG-CoA reductase, on plasma lipid levels, progression of atherosclerosis, and the lesional composition in the plaques of WHHL rabbits. Br J Pharmacol 1999;126:961 – 8.
[6] Bustos C, Hernandez-Presa MA, Ortego M, et al. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflam-mation in a rabbit model of atherosclerosis. J Am Coll Cardiol 1998;32:2057 – 64.
[7] Kano H, Hayashi T, Sumi D, et al. A HMG-CoA reductase inhibitor improved regression of atherosclerosis in the rabbit aorta without affecting serum lipid levels: possible relevance of up-regulation of endothelial NO synthase mRNA. Biochem Bio-phys Res Commun 1999;259:414 – 9.
[8] Mercuri M, Bond MG, Sirtori CR, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic mediterranean population. The Carotid Atherosclerosis Italian Ultrasound Study. Am J Med 1996;101:627 – 34.
[9] Vaughan CJ, Murphy MB, Buckley BM. Statins do more than just lower cholesterol. Lancet 1996;348:1079 – 82.
[10] Bellosta S, Bernini F, Ferri N, et al. Direct vascular effects of HMG-CoA reductase inhibitors. Atherosclerosis 1998;137:S101 – 9.
[11] Bellosta S, Via D, Canavesi M, Pfister P, Fumagalli R, Paoletti R, Bernini F. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thromb Vasc Biol 1998;18:1671 – 8.
[12] Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, Sanchez-Pascuala R, Hernandez G, Diaz C, Lamas S. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, Atorvas-tatin and SimvasAtorvas-tatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest 1998;101:2711 – 9.
[13] Laufs U, La Fata V, Plutzsky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG-CoA reductase in-hibitors. Circulation 1998;97:1129 – 35.
[14] Colli S, Eligni S, Lalli M, Camera M, Paoletti R, Tremoli E. Vastatins inhibit tissue factor in cultured human macrophages. A novel mechanism of protection against atherothrombosis. Arte-rioscler Thromb Vasc Biol 1997;17:265 – 72.
[15] Ferro D, Basili S, Alessandri C, Mantovani B, Cordova C, Violi F. Simvastatin reduces monocyte tissue-factor expression type IIa hypercholesterolaemia. Lancet 1997;350:1222.
[16] Kimura M, Kurose I, Russell J, Granger DN. Effects of fluvas-tatin on leucocyte-endothelial cell adhesion in hypercholes-terolemic rats. Arterioscler Thromb Vasc Biol 1997;17:1521 – 6. [17] Alfon J, Pueyo Palazon C, Royo T, Badimon L. Effects of
statins in thrombosis and aortic lesion development in a dys-lipemic rabbit model. Thromb Haemost 1999;81:822 – 7. [18] Fuster V, Badimon L, Badimon JJ, Chesebro JH. The
pathogen-esis of coronary artery disease and the acute coronary syn-dromes. New Engl J Med 1992;326:310 – 8.
[19] Juhan-Vague I, Pyke SDM, Alessi MC, Jespersen J, Haverkate F, Thompson SG. Fibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. Circulation 1996;94:2057 – 63.
[20] Sobel BE, Woodcock-Mitchell J, Schneider DJ, Holt RE, Marut-suka K, Gold H. Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients. Circulation 1998;97:2213 – 21.
[21] Wada H, Mori Y, Kaneko T, Wakita Y, Minamikawa K, Ohiwa M, Tamaki S, Yokoyama N, Kobayashi T, Deguchi K. Hyper-coagulable state in patients with hypercholesterolemia: effects of pravastatin. Clin Ther 1992;14:829 – 34.
[22] Zambrana JL, Velasco F, Castro P, et al. Comparison of bez-afibrate versus lovastatin for lowering plasma insulin, fibrinogen, and plasminogen activator inhibitor-1 concentrations in hyper-lipemic heart transplant patients. Am J Cardiol 1997;80:836 – 40. [23] Mitropoulos KA, Armitage JM, Collins R, et al. Randomized placebo-controlled study of the effects of simvastatin on haemo-static variables, lipoproteins and free fatty acids. The Oxford Cholesterol Study Group. Eur Heart J 1997;18:235 – 41. [24] Dangas G, Badimon JJ, Smith DA, et al. Pravastatin therapy in
hyperlipidemia: effects on thrombus formation and the systemic hemostatic profile. J Am Coll Cardiol 1999;33:1294 – 304. [25] Bevilacqua M, Bettica P, Milani M, et al. Effect of fluvastatin on
lipids and fibrinolysis in coronary artery disease. Am J Cardiol 1997;79:84 – 7.
[26] Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins. Implications for cardiovascular event reduction. J Am Med Assoc 1998;279:1643 – 50.
[27] Schleef RR, Loskutoff DJ, Podor TJ. Immunoelectron micro-scopic localization of type 1 plasminogen activator inhibitor on the surface of activated endothelial cells. J Cell Biol 1991;113:1413 – 23.
[28] Lang IM, Marsh JJ, Olman MA, Moser KM, Loskutoff DJ, Schleef RR. Expression of type 1 plasminogen activator inhibitor in chronic pulmonary thromboemboli. Circulation 1994;89:2715 – 21.
[30] Allaire E, Hasenstab D, Kenagy RD, Starcher B, Clowes MM, Clowes AW. Prevention of aneurysm development and rupture by local overexpression of plasminogen activator inhibitor-1. Circulation 1998;98:249 – 55.
[31] Carmeliet P, Moons L, Lijnen R, Janssens S, Lupu F, Collen D, Gerard RD. Inhibitory role of plasminogen activator inhihitor-1 in arterial wound healing and neointima formation. Circulation 1997;96:3180 – 91.
[32] Essig M, Nguyen G, Prie´ D, Escoubet B, Sraer J-D, Friedlander G. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells. Circ Res 1998;83:683 – 90.
[33] Schleef RR, Bevilacqua MP, Sawdey M, Gimbrone MA Jr, Loskutoff DJ. Cytokine activation of vascular endothelium. J Biol Chem 1988;263:5797 – 803.
[34] van den Berg EA, Sprengers ED, Jaye M, Burgess W, Maciag T, van Hinsbergh VWM. Regulation of plasminogen activator in-hibitor-1 mRNA in human endothelial cells. Thromb Haemost 1988;60:63 – 7.
[35] Chautan M, Latron Y, Anfosso F, Alessi MC, Lafont H, Juhan-Vague I, Nalbone G. Phosphatidylinositol turnover
dur-ing stimulation of plasminogen activator inhibitor-1 secretion induced by oxidized low density lipoproteins in human endothe-lial cells. J Lipid Res 1993;34:101 – 10.
[36] Lopez S, Peiretti F, Morange P, et al. Activation of plasminogen activator inhibitor-1 synthesis by phorbol esters in human promyelocyte HL-60. Thromb Haemost 1999;81:415 – 22. [37] Declerck PJ, Alessi MC, Vertrken M, Kruithof EKO,
Juhan-Vague I, Collen D. Measurement of plasminogen activator in-hibitor 1 (PAI-1) in biological fluids with a murine monoclonal antibody, based on enzyme-linked immunoadsorbant assay. Blood 1988;71:220 – 7.
[38] Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998;91:3527 – 61.
[39] Koch G, Benz C, Schmidt G, Olenik C, Aktories K. Role of Rho protein in lovastatin-induced breakdown of actin cytoskeleton. J Pharmacol Exp Ther 1997;283:901 – 9.
[40] Zohar M, Teramoto H, Katz B-Z, Yamada KM, Gutkind JS. Effector domain mutants of Rho dissociate cytoskeletal changes from nuclear signalling and cellular transformation. Oncogene 1998;17:991 – 8.