Electrophysiologic Studies in the Dorsal Raphe
Nucleus and Hippocampus
Jeffrey Sprouse, John Braselton, and Linda Reynolds
Background: The ability of pindolol to block 5-HT1A
autoreceptors on serotonin-containing neurons in the raphe nuclei is thought to underlie the clinical reports of enhanced efficacy and rate of improvement in depressed patients treated with pindolol/selective serotonin reuptake inhibitor (SSRI) combinations. Selectivity for somatoden-dritic 5-HT1A autoreceptors is a crucial requirement, as
blockade of postsynaptic 5-HT1Asites may jeopardize the
therapeutic response. Previous investigators have probed the effects of pindolol on serotonergic dorsal raphe cell firing in animal species; here we confirm their findings and extend them to include observations on postsynaptic 5-HT1A receptors in the hippocampus.
Methods: Extracellular single-unit recordings were made
in rats using standard electrophysiologic techniques. Fir-ing rates of serotonin-containFir-ing neurons in the dorsal raphe nucleus and CA3 hippocampal pyramidal neurons were monitored and the effects of pindolol given alone or in combination with an SSRI (fluoxetine) or a 5-HT1A
antagonist (WAY-100,635) were determined.
Results: Pindolol inhibited the firing rates of serotonergic
dorsal raphe neurons in a dose-dependent manner. Recov-ery to baseline firing rates was gradual, but this inhibition could be acutely reversed by WAY-100,635. A range of pindolol doses failed to block the inhibitory effects of fluoxetine on dorsal raphe cell firing. In the hippocampus, pindolol also inhibited cell firing as a function of dose, although these effects were insensitive to WAY-100,635 treatment.
Conclusions: The ability of pindolol to inhibit
serotoner-gic dorsal raphe cell firing is indicative of its agonist potential and is consistent with previous studies. The lack of observable antagonism of the SSRI-induced slowing of raphe unit activity casts doubt on the suitability of this mechanism of action to account for the positive findings in clinical studies utilizing pindolol/SSRI combinations. The 5-HT1A-independent inhibition of hippocampal CA3 cell
firing by pindolol suggests that this compound invokes
multiple pharmacologic actions, all of which need to be assimilated into any proposed mechanism of action. Biol
Psychiatry 2000;47:1050 –1055 © 2000 Society of
Bio-logical Psychiatry
Key Words: Pindolol, dorsal raphe nucleus, hippocam-pus, 5-HT1Aagonist, 5-HT1Aantagonist, SSRI
Introduction
T
he slow rate of improvement observed clinically with antidepressant treatment has long been attributed to the desensitization of serotonin 1A (5-HT1A) autoreceptors in the raphe nuclei (Blier and de Montigny 1994; Briley and Moret 1993). The activity of these neurons, once restored to normal firing rates, allows selective serotonin reuptake inhibitors (SSRIs) to enhance firing-dependent 5-HT release in raphe projection areas. From this scenario it has been predicted that 5-HT1Areceptor blockade would be useful in accelerating antidepressant activity (Artigas 1993; Blier and de Montigny 1994). As a test of this hypothesis, pindolol has been combined with SSRIs in small clinical studies due to its reported 5-HT1Aantagonist properties (Artigas et al 1996; McAskill et al 1998). Claims of more rapid onset or improved efficacy in clinical depression with the pindolol/SSRI combination rely on this mechanism, with the important added feature that pindolol is selective for cell body 5-HT1A autorecep-tors compared with postsynaptic 5-HT1A receptors (Ro-mero et al 1996). Such selectivity would appear to be crucial, as significant blockade of postsynaptic sites risks loss of the therapeutic response if activation of postsyn-aptic 5-HT1Areceptors is required for clinical activity, as is commonly thought (Blier et al 1987; de Vry 1995).A 5-HT1Aantagonist selective for cell body autorecep-tors would seem to be an unlikely entity given the large degree of receptor reserve reported in the dorsal raphe (Cox et al 1993; Meller et al 1990). Full agonists occupy only a small fraction of available receptors to yield their maximal effect; partial agonists achieve the same net
From Pfizer Central Research, Groton, Connecticut.
Address reprint requests to Jeffrey S. Sprouse, Ph.D., Pfizer Central Research, Dept. of Neuroscience, Groton CT 06340.
Received September 22, 1999; revised December 16, 1999; accepted December 17, 1999.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
1999c), although antagonist effects have also been claimed (Haddjeri et al 1999). The goal of our experiments was to independently characterize the agonist potential of acutely administered pindolol in the dorsal raphe and further to gauge its efficacy at postsynaptic 5-HT1A receptors. For these latter studies, pyramidal neurons of the hippocampal CA3 region were chosen for testing, as these cells are known to contain a dense concentration of 5-HT1A sites (Verge´ et al 1986) and to receive a moderate input from the dorsal raphe nucleus (Vertes 1991).
Methods and Materials
Electrophysiology
Extracellular single-unit recordings were made in choral hy-drate–anesthetized male Sprague–Dawley rats (250 –350 g) using standard electrophysiologic techniques (Sprouse and Aghajanian 1987, 1988). Serotonin-containing neurons in the dorsal raphe nucleus were identified online by their long-duration action potentials (1–2 msec) and slow rhythmic firing rate (0.5–3.0 Hz). Cells with these characteristics have been confirmed to be 5-HT neurons by intracellular double-labeling (Aghajanian and Van-dermaelen 1982). Unit activity was computed and stored in 10-sec bins triggered by individual neuronal spikes from an electronic discriminator and continuously plotted as a firing rate histogram (Spike 2, Cambridge Electronic Design, Cambridge, UK). Compounds were administered intravenously and the effects on firing rate noted.
Hippocampal CA3 pyramidal neurons were recorded and identified from their characteristic large amplitude (0.5–1.2 mV) and long duration (0.8 –1.2 msec) with single action potentials alternating with complex spike discharges. Because most of these units discharge infrequently in choral hydrate–anesthetized rats, a small iontophoretic current of acetylcholine (1– 6 nA) was used to activate silent or slowly discharging neurons to a physiologic firing rate (8 –12 Hz). Five-barreled micropipettes, used for this purpose, were arrayed as follows: the central barrel, for record-ing, was filled with a 2-mol/L NaCl solution and two side barrels, for microiontophoresis, contained 5-HT creatinine sulfate (0.5 mmol/L in 200 mmol/L NaCl, pH 4) or acetylcholine (20 mmol/L in 200 mmol/L NaCl, pH 4). A third side barrel containing a 2-mol/L NaCl solution was used for automatic current balancing. Pipettes were pulled and the tips broken back under microscopic control to a final width of 7–10mm. All drugs were retained with a28 nA current between ejections. A slowing of cell firing following brief ejections of 5-HT (10 nA, 1 min) confirmed the presence of serotonergic receptors on these neu-rons. As with the dorsal raphe recordings, unit activity was
DPAT, RBI, Natick, MA). Solutions for intravenous administra-tion were prepared daily using a saline vehicle (WAY-100,635, (6)-pindolol, (6)-8-OH-DPAT) or an acid/saline mixture (flu-oxetine). Doses were scaled to a final volume of 1 mL/kg and administered via lateral tail vein. Pilot studies indicated that the vehicles employed had no effect on cell firing.
Results
Inhibition of Serotonergic Dorsal Raphe Cell Firing by Pindolol
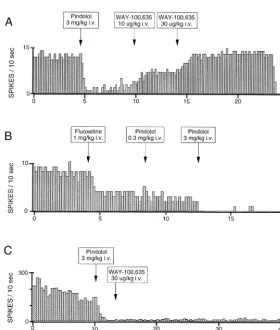
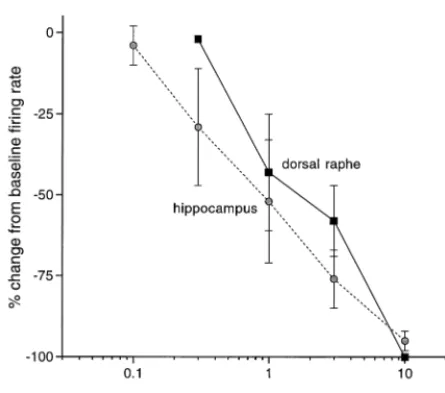
Following intravenous administration, pindolol inhibited the spontaneous firing rate of serotonergic dorsal raphe neurons in a dose-dependent manner. In general, the onset of the suppressant effect occurred within the first or second 10-sec sampling interval after drug injection (Fig-ure 1A); recovery to baseline rates was typically partial, proceeding gradually over the course of 20 –30 min, and occasionally absent. The mean inhibitory dose (ID50) value, calculated as the dose required to reduce firing rate to 50% of baseline, was 1.7 mg/kg (Figure 2). Complete inhibition of cell firing was consistently observed at the highest dose tested, approximately five to 10 times its ID50. The 5-HT1Aantagonist WAY-100,635 (Fornal et al 1996), given at doses that do not significantly affect baseline firing rate (10 and 30mg/kg IV, n56), abruptly reversed the inhibition produced by pindolol (Figure 1A). In pilot studies, a similar dose (17mg/kg IV) reduced the suppression of cell firing induced by 8-OH-DPAT (1
mg/kg IV) from 53%611% to 9%63% of the predrug baseline (n 5 5–7).
inhibition, also failed to show antagonist activity in the four cells tested.
Inhibition of CA3 Hippocampal Cell Firing by Pindolol
Intravenous administration of pindolol also inhibited hip-pocampal CA3 pyramidal neurons. As with the dorsal raphe recordings, onset was rapid and recovery was gradual and typically incomplete (Figure 1C). The ID50 values for pindolol in the hippocampus and dorsal raphe were similar (1.2 vs. 1.7 mg/kg IV), as were the slopes of the dose–response curves (Figure 2). Unlike the dorsal raphe recordings, however, WAY-100,635 (30 and 100
mg/kg IV) could not reverse pindolol-induced inhibition in the hippocampus, even though lower doses antagonized the inhibitory effects of pindolol in the dorsal raphe (Figure 1A).
Discussion
The results of the present study suggest that at high doses pindolol behaves as a 5-HT1Aagonist to completely inhibit
serotonergic dorsal raphe cell firing, confirming very similar findings published previously (Clifford et al 1998; Fornal et al 1999a, 1999b, 1999c). No evidence of 5-HT1A receptor antagonism was observed employing an SSRI to reduce unit firing rate, again consistent with earlier reports (Clifford et al 1998; Fornal et al 1999a) but inconsistent with the mechanism of action claimed in clinical studies with pindolol/SSRI combinations (McAskill et al 1998). An entirely new finding is the ability of pindolol to suppress CA3 hippocampal cell firing in a dose-dependent manner and, more importantly, in a WAY-100,635–insen-sitive manner. WAY-100,635 (30 and 100 mg/kg IV) could not reverse pindolol-induced inhibition in the hip-pocampus, even though lower doses antagonized the inhibitory effects of pindolol in the dorsal raphe. These data also discount the possibility that pindolol may alter hippocampal unit activity indirectly through terminal 5-HT1Bantagonism, another reported activity of pindolol (Assie and Koek 1996), as this mechanism would also be sensitive to 5-HT1Areceptor blockade. Blockade of cho-linergic input provided in the form of the acetylcholine iontophoretic currents would seem unlikely, given the Figure 1. Rate histograms showing (A) the inhibi-tion of a spontaneously firing serotonergic dorsal raphe neuron by intravenous administration of pin-dolol and its reversal by subsequent doses of the serotonin (5-HT1A) antagonist WAY-100,635, (B)
the inability of pindolol to block the suppressant effects of fluoxetine on a spontaneously firing serotonergic dorsal raphe neuron when given at a subthreshold dose (0.3 mg/kg IV) and one near the mean inhibitory dose value (3 mg/kg IV), and (C) the inhibitory effect of pindolol on the firing rate of a single unit in the CA3 hippocampal pyramidal cell layer and failed rescue by the 5-HT1A
receptor selectivity of pindolol analogs (van Koppen et al 1984), but blockade ofb-adrenoceptor excitation (Mueller et al 1981) is an obvious possibility. The observation that pindolol does not alter membrane potential in CA3 cells in hippocampal slices (Corradetti et al 1998) indeed suggests an effect on the neuronal input lost in tissue preparation. Regardless of the mechanism at work, these data demon-strate the potential for pindolol’s actions at receptor sites in addition to those scrupulously examined in recent studies and advocate a broader view of its net pharmaco-logic activities, particularly when one interprets clinical data.
Not diminished by the present findings are the earlier reports of mixed b-receptor/5-HT1Areceptor antagonists such as pindolol and propranolol as useful tools in dorsal raphe recordings. These studies were only demonstrations to confirm the 5-HT receptor subtype responsible for autoinhibition, as pindolol and propranolol were adminis-tered either by iontophoresis (Sprouse and Aghajanian 1986) or by bath application (Gelbach and Vandermaelen, 1987), and considerable effort in terms of experimental design was focused on utilizing iontophoretic currents or bath concentrations that did not suppress unit activity on their own. The use of these agents in vivo, prompted by the publication of the clinical studies, has been hampered by the inexact nature of systemic dosing relative to direct application in achieving extracellular levels that only block and do not activate 5-HT1A autoreceptors. As an example, low doses of pindolol (10 –200mg/kg IV) have
WAY-100,635) with the majority of agents falling into the partial agonist category (e.g., 8-OH-DPAT, buspirone, ipsapirone, gepirone). As noted in earlier work, com-pounds with partial agonist activity can block other partial agonists—for example, propranolol blocking the effects of 8-OH-DPAT or ipsapirone (Sprouse and Aghajanian 1986) or pindolol blocking the effects of gepirone (Gelbach and Vandermaelen 1987) or LSD (Haddjeri et al 1999). Antagonizing a full agonist such as 5-HT itself, however, appears to be an entirely different matter, with only limited blockade achieved by propranolol (Sprouse and Aghajanian 1986) or the lack of blockade with pindolol reported here and elsewhere. Within the class of mixed b/5-HT1A antagonists, only tertatolol has been shown to block the effects of endogenous 5-HT, and this conclusion is based only on the ability of this compound to modestly increase baseline firing rate in a subpopulation of dorsal raphe neurons (Jolas et al 1993; Prisco et al 1993).
Growing evidence of a long-loop feedback mechanism, in which activation of postsynaptic 5-HT1A receptors in turn suppresses cell firing in the raphe nuclei (Ceci et al 1994; Hajo´s et al 1999), suggests that this mechanism too should be considered in the action of pindolol. Overlaying such feedback on somatodendritic autoreceptor function, the effects on raphe firing rate become a function of the direct actions of agents on the autoreceptors and their indirect actions at some 5-HT1Asite distant from the raphe nuclei but nevertheless connected through a feedback loop. The inability of propranolol to block the inhibitory effects of 8-OH-DPAT on raphe cell firing when given systemically (Blier et al 1988), paired with its clear ability to do so when administered iontophoretically (Sprouse and Aghajanian 1986), may be evidence of the importance of the long-loop feedback mechanism relative to somatoden-dritic autoreceptor control (Blier et al 1988). In the case of pindolol, in vitro doses block the hyperpolarizing effects of 5-CT on dorsal raphe neurons (Corradetti et al 1998); its lack of antagonism in vivo again points to the potential importance of the feedback loop.
The profile of pindolol that emerges from the electro-physiologic data is one of 5-HT1Apartial agonism. Behav-ioral studies (Moore et al 1993; Sa´nchez et al 1996; Zhang and Barrett 1991) also show a mixed profile, as do clinical studies employing endocrine markers or temperature reg-ulation as surrogate endpoints (e.g., Meltzer and Maes Figure 2. Dose–response curves for the inhibition of CA3
1996). Still, the positive results of the pindolol/SSRI depression trials are not diminished by classifying pindo-lol as a 5-HT1A partial agonist rather than a selective 5-HT1A autoreceptor antagonist. Some receptor mecha-nism, likely involving 5-HT neurotransmission itself or regional targets of 5-HT neurotransmission, must be op-erative, although our results, particularly those in the hippocampus, suggest that pindolol has many pharmaco-logic actions, all of which need to be assimilated in any proposed mechanism of action.
Portions of this work were previously published in abstract form (Sprouse et al 1998).
References
Aghajanian GK, Vandermaelen CP (1982): Intracellular identi-fication of central norardrenergic and serotonergic neurons by a new double labeling procedure. J Neurosci 2:1786 –1792. Artigas F (1993): 5-HT and antidepressants: New views from
microdialysis studies. Trends Pharmacol Sci 14:262. Artigas F, Romero L, de Montigny C, Blier P (1996):
Acceler-ation of the effect of selected antidepressant drugs in major depression by 5-HT1Aantagonists. Trends Neurosci 19:378 –
383.
Assie M-B, Koek W (1996): (2)-Pindolol and (6)-tertatolol affect rat hippocampal 5-HT levels through mechanisms involving not only 5-HT1B, but also 5-HT1B receptors. Neuropharmacology 35:213–222.
Blier P, de Montigny C (1994): Current advances and trends in the treatment of depression. Trends Pharmacol 15:220 –226. Blier P, de Montigny C, Chaput Y (1987): Modifications of the serotonin system by antidepressant treatments: Implications for the therapeutic response in major depression. J Clin
Psychopharmacol 7(suppl 6):24S–35S.
Blier P, Stenberg S, Chaput Y, de Montigny C (1988): Electro-physiological assessment of putative antagonists of 5-hy-droxytryptamine receptors: A single-cell study in the rat dorsal raphe nucleus. Can J Physiol Pharmacol 67:98 –105. Briley M, Moret C (1993): Neurobiological mechanisms in-volved in antidepressant therapies. Clin Neuropharmacol 5:387– 400.
Ceci A, Baschirotto A, Borsini F (1994): The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotonin-ergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology 33:709 –713.
Clifford EM, Gartside SE, Umbers V, Cowen PJ, Hajo´s M, Sharp T (1998): Electrophysiological and neurochemical evidence that pindolol has agonist properties at the 5-HT1A
autorecep-tor in vivo. Br J Pharmacol 124:206 –212.
Corradetti R, Laaris N, Hanoun N, Laporte AM, Le Poul E, Hamon M, Lanfumey L (1998): Antagonist properties of (2)-pindolol and WAY 100635 at somatodendritic and postsynaptic 5-HT1Areceptors in the rat brain. Br J Pharma-col 123:449 – 462.
Cox RF, Meller E, Waszcak BL (1993): Electrophysiological evidence for a large reserve for inhibition of dorsal raphe cell firing by 5-HT1Aagonists. Synapse 14:297–304.
de Vry J (1995): 5-HT1Areceptor agonists: Recent developments
and controversial issues. Psychopharmacology 121:1–26. Fornal CA, Martin FJ, Mendlin A, Metzler CW, Bjorvatn B,
Jacobs BL (1999a): Pindolol increases extracellular 5-HT while inhibiting serotonergic neuronal activity. Eur J
Phar-macol 377:187–191.
Fornal CA, Martin FJ, Metzler CW, Jacobs BL (1999b): Pindo-lol, a putative 5-hydroxytryptamine1A antagonist, does not
reverse the inhibition of serotonergic neuronal activity in-duced by fluoxetine in awake cats: Comparison to WAY-100,635. J Pharmacol Exp Ther 291:220 –228.
Fornal CA, Martin FJ, Metzler CW, Jacobs BL (1999c): Pindolol suppresses neuronal activity and does not block the inhibition of serotonergic neurons produced by 8-hydroxy-2-(di-n-pro-pylamino)tetralin in awake cats. J Pharmacol Exp Ther 291:229 –238.
Fornal CA, Metzler CW, Gallegos RA, Veasey SC, McCreary AC, Jacobs BL (1996): WAY-100635, a potent and selective 5-hydroxytryptamine1A antagonist, increases serotonergic
neuronal activity in behaving cats: comparison with (S)-WAY-100135. J Pharmacol Exp Ther 278:752–762. Gartside SE, Umbers V, Hajo´s M, Sharp T (1995): Interaction
between a selective 5-HT1Areceptor antagonist and an SSRI
in vivo: Effects on 5-HT cell firing and extracellular 5-HT.
Br J Pharmacol 115:1064 –1070.
Gelbach G, Vandermaelen CP (1987): (2)-Pindolol blocks the inhibitory effect of gepirone, a 5-HT1Aagonist, on the firing
of serotonergic dorsal raphe neurons in the rat brain slice. Soc
Neurosci Abstr 13:1649.
Haddjeri N, de Montigny C, Blier P (1999): Modulation of the firing activity of rat serotonin and noradrenaline neurons by (6)pindolol. Biol Psychiatry 45:1163–1169.
Hajo´s M, Hajos-Korcsok E, Sharp T (1999): Role of the medial prefrontal cortex in 5-HT1A receptor-induced inhibition of
5-HT neuronal activity in the rat. Br J Pharmacol 126:1741– 1750.
Jolas T, Haj-Dahmane S, Lanfumey L, Fattaccini CM, Kidd EJ, Adrien J, et al (1993): (2)-Tertatolol is a potent antagonist at pre- and postsynaptic serotonin 5-HT1Areceptors in the rat
brain. Naunyn Schmiedebergs Arch Pharmacol 347:453– 463. McAskill R, Mir S, Taylor D (1998): Pindolol augmentation of
antidepressant therapy. Br J Psychiatry 173:203–208. Meller E, Goldstein M, Bohmaker K (1990): Receptor reserve
for 5-hydroxytryptamine1A-mediated inhibition of serotonin
synthesis: Possible relationship to anxiolytic properties of 5-hydroxytryptamine1A agonists. Mol Pharmacol 37:231–
237.
Meltzer HY, Maes M (1996): Effect of pindolol on hormone secretion and body temperature: Partial agonist effects. J
Neu-ral Transm 103:77– 88.
Moore NA, Rees G, Sanger G, Perrett L (1993): 5-HT1A
-mediated lower lip retraction: Effects of 5-HT1Aagonists and
antagonists. Pharmacol Biochem Behav 46:141–143. Mueller AL, Hoffer BJ, Dunwiddie TV (1981): Noradrenergic
Sa´nchez C, Arnt J, Moltzen E (1996): Assessment of relative efficacies of 5-HT1A receptor ligands by means of in vivo
animal models. Eur J Pharmacol 315:245–254.
Sheard MH, Zolovick A, Aghajanian GK (1972): Raphe neurons: Effect of tricyclic antidepressant drugs. Brain Res 43:690 – 693.
Sprouse J (1991): Inhibition of dorsal raphe cell firing by MDL73005EF, a novel 5-HT1Areceptor ligand. Eur J Phar-macol 201:163–169.
Sprouse JS, Braselton J, Reynolds L (1998): 5-HT1A agonist
activity of pindolol: Reversal of the inhibitory effects on cell firing in the dorsal raphe nucleus but not in the hippocampus by WAY-100,635. Ann N Y Acad Sci 861:274 –275. Sprouse JS, Aghajanian GK (1986): (2)-Propranolol blocks the
inhibition of serotonergic dorsal raphe cell firing by 5-HT1A
selective agonists. Eur J Pharmacol 128:295–298.
Psychiatry 7:5–15.
van Koppen CJ, Siero HL, Rodrigues de Miranda JF, Beld AJ, Ariens EJ (1984): Simultaneous assay of muscarinic and beta-adrenergic receptors using a double isotope technique.
Biochem Biophys Res Commun 120:665– 669.
Verge´ D, Daval G, Marcinkiewicz M, Patey A, El Mestikawy S, Gozlan H, Hamon M (1986): Quantitative autoradiography of multiple 5-HT1Areceptor subtypes in the brain of control or
5,7-dihydroxytryptamine-treated rats. J Neurosci 6:3474 – 3482.