www.elsevier.com / locate / bres
Research report
Prevention of 1-methyl-4-phenylpyridinium- and
6-hydroxydopamine-induced nitration of tyrosine hydroxylase and
neurotoxicity by EUK-134, a superoxide dismutase and catalase
mimetic, in cultured dopaminergic neurons
a b a ,
*
Kevin Pong , Susan R. Doctrow , Michel Baudry
a
Neuroscience Program, University of Southern California, Los Angeles, CA 90089-2520, USA b
Eukarion Inc., Bedford, MA, USA Accepted 8 August 2000
Abstract
Oxidative stress has been implicated in the selective degeneration of dopaminergic (DAergic) neurons in Parkinson’s disease (PD). In this study, we tested the efficacy of EUK-134, a superoxide dismutase (SOD) and catalase mimetic, on the nitration of tyrosine
1
hydroxylase (TH), a marker of oxidative stress, and neurotoxicity produced by 1-methyl-4-phenylpyridinium (MPP ) and
6-hydroxy-1
dopamine (6-OHDA) in primary DAergic neuron cultures. Exposure of cultures to 10mM MPP reduced dopamine (DA) uptake and the number of tyrosine hydroxylase immunoreactive (THir) neurons to 56 and 52% of control, while exposure to 30mM 6-OHDA reduced DA uptake and the number of THir neurons to 58 and 59% of control, respectively. Pretreatment of cultures with 0.5mM EUK-134
1
completely protected DAergic neurons against MPP - and 6-OHDA-induced neurotoxicity. Exposure of primary neuron cultures to either
1
MPP or 6-OHDA produced nitration of tyrosine residues in TH. Pretreatment of cultures with 0.5mM EUK-134 completely prevented
1
MPP - or 6-OHDA-induced nitration of tyrosine residues in TH. Taken together, these results support the idea that reactive oxygen
1
species (ROS) are critically involved in MPP - and 6-OHDA-induced neurotoxicity and suggest a potential therapeutic role for synthetic catalytic scavengers of ROS, such as EUK-134, in the treatment of PD. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Degenerative disease: Parkinson’s
Keywords: Catalase; Dopaminergic neurons; Oxidative stress; Parkinson’s disease; Reactive oxygen species; Superoxide dismutase
1. Introduction are indicative of oxidative stress in the substantia nigra.
Such changes include increased levels of lipid peroxida-Parkinson’s disease (PD) is a common neurodegenera- tion, protein oxidation, 3-nitrotyrosine formation, DNA tive disorder that is pathologically characterized by the oxidation and breaks, and decreased levels of ROS selective degeneration of dopaminergic (DAergic) neurons scavenging enzymes such as glutathione peroxidase (GPx) of the substantia nigra [1,24]. A growing body of evidence and catalase [33,34,40,45].
suggests that oxidative stress induced by reactive oxygen A number of neurotoxins that selectively damage DAer-species (ROS) is involved in this selective nigral cell gic neurons have been used to create animal models of PD.
1
degeneration [6,16,17,27,50]. Postmortem studies of PD Injections of either 1-methyl-4-phenylpyridinium (MPP ), patients have provided evidence for chemical changes that the active metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahy-dropyridine (MPTP), or 6-hydroxydopamine (6-OHDA) have been shown to produce some of the biochemical and
*Corresponding author. Tel.: 11-213-740-9188; fax: 11-213-740- 1
pathological changes that occur in PD [10,12]. MPP and
5687.
E-mail address: [email protected] (M. Baudry). 6-OHDA are selectively taken up by the plasma membrane
dopamine (DA) transporters and subsequently accumulate 2.2. DAergic neuron cultures within mitochondria. Both neurotoxins are presumed to
increase the formation of ROS, e.g. hydrogen peroxide Experimental protocols involving laboratory animals
?- ?
(H O ), superoxide (O ), and hydroxyl radicals ( OH).2 2 2 were approved by the University Animal Use Committee. However, there are still a number of unanswered questions Primary DAergic neuron cultures were prepared as de-regarding the similarities and differences between the scribed previously [41]. In brief, embryonic day 15 (E15) modes of actions of these two DAergic neuron toxins. rat fetuses (Sprague–Dawley, Charles River Laboratories,
?
The free radical nitric oxide (NO ) readily interacts with Wilmington, MA, USA) were collected, their brains were
?- 2
O2 to produce peroxynitrite (ONOO ), a potent oxidant removed, and a small piece of tissue comprising the ventral and nitrating agent [7,8]. One consequence of increased mesencephalic DAergic region was dissected out in
ice-2 21
ONOO formation is protein nitration on tyrosine res- cold phosphate buffered saline (PBS) without Ca and
21
idues, and analysis of protein nitration has thus been used Mg . Dissected pieces of tissue were pooled together and as a marker of oxidative stress under various experimental transferred to an enzymatic dissociation medium
contain-2
conditions [18,23,38,49]. ONOO has been proposed as a ing 0.2% trypsin (Life Technologies, Rockville, MD, USA) mediator of nigrostriatal damage in PD [28,30,44,47], an in Earle’s balanced salt solution, and incubated for 15 min idea supported by its effects on DA synthesis in PC 12 at 378C. After enzymatic dissociation, the trypsin solution cells [32] and on nitration and inactivation of tyrosine was aspirated and the tissue mechanically triturated with a hydroxylase (TH) in PC 12 cells and mouse striatum [2]. fire-polished Pasteur pipette in a complete medium [equal Based on the growing body of evidence implicating volumes of minimum essential medium and F-12 nutrient ROS in neurodegenerative diseases, several laboratories mixture (Life Technologies) supplemented with 0.1 mg / ml have developed small molecules that mimic antioxidant apotransferrin and 25 mg / ml insulin] containing 2,000 properties of endogenous enzymes. Salen–manganese IU / ml DNase. In these preparations, DAergic neurons complexes are synthetic compounds that exhibit both SOD represent approximately 5% of the total cell population. and catalase activities [4,19]. The prototype molecule, For DA uptake experiments and TH immunocytoch-EUK-8, has been shown to protect hippocampal slice emistry, single-cell suspensions were seeded on poly-L
-cultures from hypoxia-, acidosis-, and Ab-induced cell ornithine (0.1 mg / ml) and laminin (1 mg / ml; Life
Tech-5
death [11,39]. EUK-134, an analogue of EUK-8, sig- nologies) coated 96-well plates at a density of 0.3310 nificantly reduced brain infarction volume in a rat focal cells / well. For western blots, single-suspensions were ischemia model [5] and was neuroprotective against kainic seeded on similarly coated 100-mm dishes at a density of
7
acid neurotoxicity [46]. Taken together, these results 2.0310 cells / dish. suggest a potential role for synthetic SOD/ catalase
mimetics as therapeutic agents against various neurological 2.3. High-affinity DA uptake assay disorders.
In the present study, we tested the neuroprotective DA uptake experiments were performed on 10 days in effects of EUK-134 on both the nitration of tyrosine vitro (DIV) cultures using a method described by Proch-residues in TH and the neurotoxicity produced by the iantz [42] with modifications [41]. In brief, cultures were
1
neurotoxins MPP and 6-OHDA in primary cultures of washed with a modified Krebs–Ringer’s-phosphate buffer DAergic neurons. The results provide both convincing (120 mM NaCl, 1.3 mM Na EDTA, 5.6 mM glucose, 4.74
1
evidence that ROS are involved in both MPP - and 6- mM KCl, 1.8 mM CaCl , 1.2 mM MgSO , 32 mM sodium2 4
OHDA-induced neurotoxicity and strong support for the phosphate, 1 mM ascorbic acid, and 50mM pargyline) and
3
use of synthetic catalytic scavengers, such as EUK-134, in incubated for 60 min at 378C with 50 nM [ H]-DA (31 the treatment of PD. Ci / mmol; Du Pont / NEN, Wilmington, DE, USA). The cultures were washed twice with buffer, scintillation cocktail was added, and radioactivity was measured.
2. Materials and methods
2.4. TH immunocytochemistry 2.1. Materials
Sister cultures were fixed for 45 min at 378C with 4% EUK-134 was obtained from Eukarion, Inc. (Bedford, paraformaldehyde. Nonspecific binding was blocked by MA, USA) and stock solutions were made with water. incubating with PBS containing 1% Nonidet P-40, 1% Details of the structure and activity of EUK-134 have been BSA, and 5% goat serum for 90 min at 378C. Cultures
1
peroxidase ABC kit (Vector Laboratories, Burlingame, CA, USA) and diaminobenzidine substrate.
2.5. SDS–PAGE and western blot
SDS–PAGE and Western Blots were performed as described [41,46]. Cultures were grown in 100-mm dishes as described above. Forty-eight h after plating, medium was exchanged and cultures were returned to the incubator.
1
For MPP and 6-OHDA studies, cultures were treated with 0.5mM EUK-134 or control media on DIV 6. On DIV 8,
1
cultures were exposed to either 10 mM MPP , 30 mM 6-OHDA or control media for 3 h. Cultures were washed with ice-cold PBS and subsequently lysed with lysis buffer (137 mM NaCl, 20 mM Tris, 1 mM MgCl , 1 mM CaCl ,2 2
0.2 mM vanadate, 10% glycerol, 1% NP-40, and 1 mM phenylmethylsufoxyl fluoride). The lysates were mixed and centrifuged. Supernatants were transferred to microfuge tubes and incubated with the anti-TH antibody (6mg / ml, Chemicon, Temecula, CA, USA) overnight at 48C and Protein A-Sepharose (4 mg / ml, Pharmacia Biotech, Pis-cataway, NJ, USA) for 1 h at room temperature. The immunoprecipitates were pelleted and washed three times with lysis buffer. Immunoprecipitates were run on SDS– PAGE and transferred to nitrocellulose membranes. Mem-branes were blocked with 3% gelatin and probed with either the anti-TH antibody (1:200, Chemicon) or the anti-nitrotyrosine antibody (1:200, Upstate Biotechnology, Lake Placid, NY, USA) overnight, at room temperature. Secondary antibodies conjugated to alkaline-phosphatase (Bio-Rad, Hercules, CA, USA) were used to visualize corresponding levels of nitration and TH protein levels.
3. Results
1
3.1. MPP and 6-OHDA decrease DA uptake and
number of TH-immunoreactive neurons in mesencephalic
1
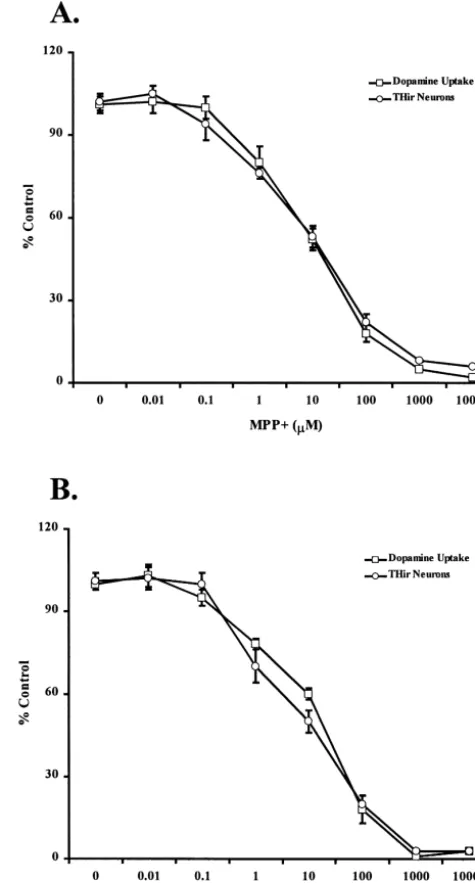
cultures Fig. 1. Effects of increasing concentrations of MPP and 6-OHDA in
DAergic neuron cultures. DAergic neuron cultures were prepared from E15 rat embryos. Cultures were exposed for 1 h to various concentrations
Embryonic mesencephalic neuronal cultures were pre- 1
of MPP and 6-OHDA, ranging from 0.01mM to 10mM. Cultures were
pared and maintained in 96-well tissue culture plates as then processed for high affinity DA uptake and TH immunocytoch-previously described [41]. A concentration-response analy- emistry. (A) DA uptake and number of THir neurons of cultures treated
1 1
sis was initially done with MPP and 6-OHDA to de- with various concentrations of MPP . Data are expressed as percent of values in untreated control cultures, and are means6S.D. of 3–5
termine relative levels of cytotoxicity in our cultures. On
experiments. (B) DA uptake activity and number of THir neurons of
DIV 9, cultures were exposed to various concentrations of
cultures treated with various concentrations of 6-OHDA. Data are
1
either MPP or 6-OHDA for 1 h. Culture media were expressed as percent of values in untreated control cultures, and are changed three times at the end of the exposure period. means6S.D. of 3–5 experiments.
Twenty four h later, high-affinity DA uptake was measured and TH immunocytochemistry was performed. Both
neuro-toxins decreased DA uptake activity and the number of 59% or control values, respectively (Fig. 1B). These THir neurons in a dose-dependent manner. Exposure to 10 concentrations of neurotoxins did not exhibit any
non-1
mM MPP reduced DA uptake activity and the number of specific neurotoxicity, as determined by visual inspection THir neurons to 56 and 52% of control values, respectively of overall culture viability (data not shown) and were used (Fig. 1A). Exposure to 30 mM 6-OHDA reduced DA for all subsequent experiments.
Table 1
done with EUK-134 to determine relative levels of toxicity 1
Effects of EUK-134 on MPP and 6-OHDA-induced alterations in the
or trophic activity in our cultures. EUK-134 did not exhibit a number of THir neurons
any toxic or trophic effects at concentrations ranging from
Treatment Number of THir
1 nM to 1 mM. Moderate toxicity was apparent at
neurons (% control)
concentrations .10 mM. EUK-134 was highly toxic at
Control 10063.4
concentrations.50mM. This toxicity was not specific for
0.5mM EUK-134 10063.0
DAergic neurons, but was a non-specific toxicity, effecting 1
10mM MPP 5263.2*
all cells in the cultures (data not shown). 1
0.5mM EUK-134110mM MPP 9264.4
30mM 6-OHDA 5964.5*
3.2. EUK-134 protects DAergic neurons against MPP1 0.5mM EUK-134130mM 6-OHDA 9463.7 a
and6-OHDA induced neurotoxicity Sister cultures were treated with 0.5mM EUK-134 or control media 48
h prior to exposure to neurotoxins. Cultures were exposed to either 10 mM MPP1or 30mM 6-OHDA for 1 h. Cultures were processed for TH
To determine whether EUK-134 treatment could protect
1 immunocytochemistry 24 h later. The number of THir neurons was
DAergic neurons against MPP - or 6-OHDA-induced
determined and expressed as a percent of control. Data are means6S.D.
neurotoxicity, we examined DA uptake and the number of of 3–5 separate experiments. *P,0.0001, two-tailed Student’s t-test. THir neurons. Cultures were treated on DIV 7 with various
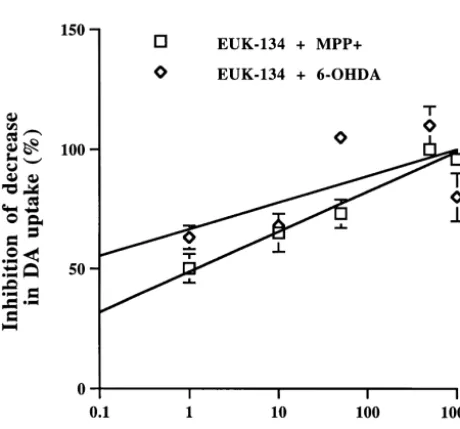
concentrations of EUK-134, ranging from 1 nM to 1mM.
1
On DIV 9, cultures were exposed to either 10mM MPP the number of THir neurons to 59% of control values (Fig. or 30 mM 6-OHDA for 1 h. High-affinity DA uptake was 1B). Similarly, pretreatment of cultures with increasing measured 24 h later. As previously determined, 10 mM EUK-134 concentrations resulted in a
concentration-depen-1
MPP reduced DA uptake and the number of THir dent protection against 6-OHDA-induced decrease in DA neurons to 56 and 52% of control levels, respectively (Fig. uptake (Fig. 2). 0.5mM EUK-134 also provided the best 1A). Pretreatment of cultures with increasing EUK-134 protection for DAergic neurons against 6-OHDA-induced concentrations resulted in a concentration-dependent decrease in DA uptake and number of THir neurons (Table
1
protection against MPP -induced decrease in DA uptake 1). Since pretreatment with 0.5mM EUK-134 provided the
1
(Fig. 2). Complete protection was provided at a con- best protection against MPP - and 6-OHDA-induced centration of 0.5 mM EUK-134. This concentration of neurotoxicity, this concentration of EUK-134 was used for
1
EUK-134 was also optimal in inhibiting MPP -induced all subsequent experiments.
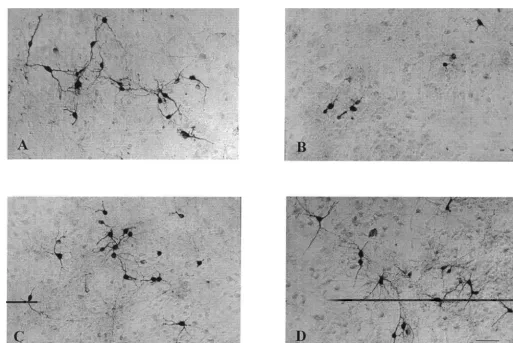
decrease in the number of THir neurons (Table 1). Thirty Representative micrographs were taken from sister
mM 6-OHDA reduced DA uptake to 58% of control and cultures fixed and processed for THir. Cultures exposed to
1
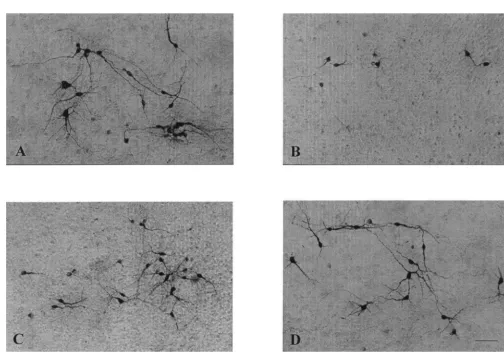
10 mM MPP showed a significant reduction in the number of cell bodies and neurites stained with anti-TH antibodies (Fig. 3B) when compared to control cultures (Fig. 3A). Pretreating cultures with 0.5 mM EUK-134 protected these qualitative and quantitative changes (Fig. 3C). Treatment of cultures with 0.5 mM EUK-134 alone had no toxic or trophic effects (Figs. 3D and 4D). Similarly, cultures exposed to 30 mM 6-OHDA showed significant reduction in the number of cell bodies and neurites stained with anti-TH antibodies (Fig. 4B) when compared to control cultures (Fig. 4A). Pretreatment with 0.5 mM EUK-134 also protected these morphological parameters against 6-OHDA-induced toxicity (Fig. 4C).
1
3.3. MPP - and 6-OHDA-induced tyrosine nitration of
TH is blocked by EUK-134
DAergic neuron cultures were prepared in 100-mm dishes as described. Cultures were treated with 0.5 mM EUK-134 or control medium on DIV 6. On DIV 8,
1 1
Fig. 2. Effects of EUK-134 on MPP - and 6-OHDA-induced decrease in cultures were exposed to either 10 mM MPP , 30 mM
DA uptake. Cultures were treated with various concentrations EUK-134, 6-OHDA or control medium for 3 h, then processed for ranging from 1 nM to 1mM, or control media 48 h prior to exposure 10 immunoprecipitation of tyrosine hydroxylase and
evalua-1
mM MPP or 30mM 6-OHDA for 1 h. Cultures were processed for DA
tion of its nitration by SDS–PAGE and western blot
uptake 24 h later. Data are expressed as percent of inhibition of the
1 analysis. Control cultures (Fig. 5A, lane 1) and cultures
decrease in DA uptake elicited by MPP or 6-OHDA at each EUK-134
1
Fig. 3. Effects of EUK-134 on MPP -induced alterations of THir neurons. Cultures were treated with 0.5mM EUK-134 or control medium 48 h prior to 1
1 1
h exposure to 10mM MPP . After exposure to 10 mM MPP , medium was changed three times, and cultures were processed for TH
immuno-1
cytochemistry 24 h later. Representative micrographs are shown here. (A) Untreated control cultures. (B) 10mM MPP . (C) 0.5mM EUK-134110mM
1
MPP . (D) 0.5mM EUK-134.
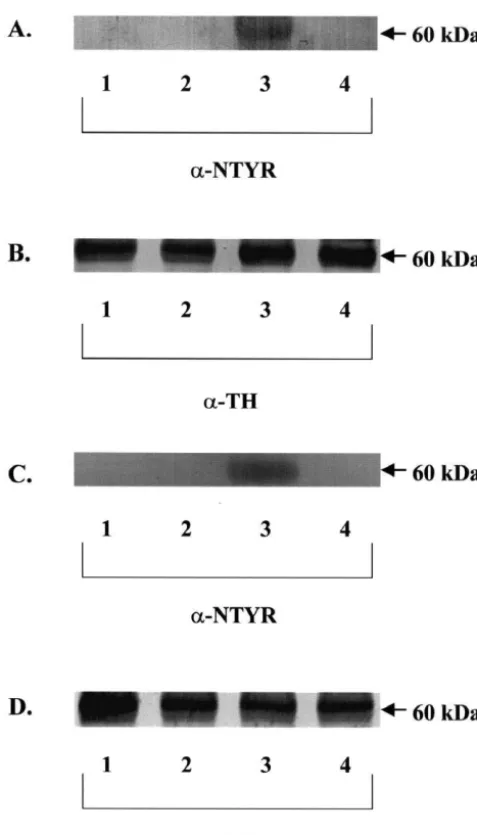
1
TH nitration. Exposure to 10 mM MPP resulted in TH THir neurons, and alteration of THir neuron morphology. nitration (Fig. 5A, lane 3). Pretreatment of cultures with In both cases, early oxidative stress in DAergic neurons
1
0.5 mM EUK-134 completely prevented MPP -induced was evidenced by TH nitration observed 3 h after treat-TH nitration (Fig. 5A, lane 4). Exposure to 30 mM 6- ment with either neurotoxin. It is generally assumed that
2
OHDA also resulted in TH nitration (Fig. 5C, lane 3), TH nitration reflects the generation of ONOO produced
?- ?
while pretreating cultures with 0.5 mM EUK-134 also from the interaction between O2 and constitutive NO
1
completely prevented 6-OHDA-induced TH nitration (Fig. [2,7,35]. Our results indicate that both MPP and 6-OHDA
?
-5C, lane 4). Control cultures (Fig. -5C, lane 1) and cultures treatment of DAergic neurons produce O2 that combine
? 2
treated with EUK-134 alone (Fig. 5C, lane 2) showed no with constitutive NO to form ONOO . This is consistent TH nitration. In these acute experiments, the levels of TH with the complete blockade of TH nitration by EUK-134, a
?
-protein remained unchanged (Fig. 5B and D) as described synthetic catalytic scavenger of O2 and H O . We do not2 2
by Ara et al. (1998). have evidence indicating that EUK-134 directly interacts
2 ?
with ONOO , NO or with NOS generation, but we cannot exclude the possibility that such an effect also could
4. Discussion contribute to its neuroprotective effect. However, it has
been shown that NOS inhibitors do not protect against Our study provides evidence for a central role of 6-OHDA toxicity [21,48], making this alternative not very
1
oxidative stress in MPP - and 6-OHDA-induced neuro- likely.
1
toxicity in cultured DAergic neurons. Specifically, we The neurotoxins MPP and 6-OHDA have been used demonstrated that pretreatment with EUK-134 completely extensively to generate both in vitro and in vivo models of
1 1
blocked MPP - and 6-OHDA-induced nitration of TH and PD. Hasegawa et al. [31] demonstrated that MPP
in-?
Fig. 4. Effects of EUK-134 on 6-OHDA-induced alterations of THir neurons. Cultures were treated with 0.5mM EUK-134 or control medium 48 h prior to 1 h exposure to 30 mM 6-OHDA. After exposure to 30 mM 6-OHDA, medium was changed three times, and cultures were processed for TH immunocytochemistry 24 h later. Representative micrographs are shown here. (A) Untreated control cultures. (B) 30 mM 6-OHDA. (C) 0.5 mM EUK-134130mM 6-OHDA. (D) 0.5mM EUK-134.
preparations while Cleeter et al. [14] demonstrated that that both neurotoxins exhibited an early, sustained rise in
1
MPP irreversibly inhibited complex I of the mitochon- ROS, although only 6-OHDA induced an ROS-related drial electron transport chain, an effect assumed to gener- collapse in mitochondrial membrane potential. Further-ate free radicals. Subsequent studies by various groups more, the C3 carboxyfullerene derivative, a potent anti-demonstrated that transgenic mice with increased copper / oxidant in several models of oxidative stress [20], was able zinc-SOD [43] or manganese-SOD [36] expression were to fully protect DAergic in the 6-OHDA paradigm, but
1
resistant to MPTP-induced neurotoxicity, further implicat- only partially protected against MPP -induced cell death. ing oxidative stress in the pathogenesis of MPTP neuro- In another study, Choi et al. [13] were able to protect the
toxicity. DAergic neuronal cell line, MN9D, against 6-OHDA- but
1
Various studies have shown that 6-OHDA is transported not MPP -induced cell death with the antioxidants N-into DAergic neurons via DA transporters where it is acetylcysteine or Mn(III)-tetrakis(4-benzoic acid)
por-?- ?
readily oxidized to produce H O , O , and OH [15,29].2 2 2 phyrin chloride (Mn-TBAP). The involvement of ROS in In addition, 6-OHDA has been shown in vitro to inhibit MPTP neurotoxicity is still a matter of debate, as both complex I and IV of the mitochondrial electron transport positive and negative results for ROS involvement have chain [25,26]. Furthermore, studies have demonstrated that been reported [13,22,30,32,37,44,47]. In vivo studies have transgenic mice which over-express GPx [9] or SOD [3] also provided some evidence for ROS involvement,
al-1
were resistant to 6-OHDA-induced neurotoxicity. These though the mechanisms involved in MPP -induced ROS results provide further support for a role of ROS in the formation and cell death remain unclear. Our results toxicity of 6-OHDA. provide conclusive evidence for a causal role of ROS in
1
More recent studies, however, have suggested different MPP toxicity, as we observed rapid TH nitration, an
1
mimetic EUK-134 completely blocked TH nitration and the subsequent decreases in DA uptake, number of THir neurons, and morphological alterations of these neurons. Furthermore, these findings suggest a potential therapeutic role for EUK-134 in the treatment of PD since EUK-134 appears to cross the blood–brain barrier, as intravenous or systemic administration provided significant protection in a focal cerebral ischemia model in rat [5] and against kainate-induced neuropathology in the rat [46].
Acknowledgements
We thank Dr. Wei Liu for technical assistance and helpful comments. This work was supported by a grant from Eukarion, Inc.
References
[1] Y. Agid, F. Javoy-Agid, M. Ruberg, Biochemistry of Neurotrans-mitters in Parkinson’s Disease, in: C.D. Marsden, S. Fahn (Eds.), Movement Disorders, Butterworth & Co, Stoneham, MA, 1987, pp. 166–230.
[2] J. Ara, S. Przedborski, A.B. Naini, V. Jackson-Lewis, R.R. Trifiletti, J. Horwitz, H. Ischiropoulos, Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), Proc. Natl. Acad. Sci. USA 95 (1998) 7659–7663.
[3] M. Asanuma, H. Hirata, J.L. Cadet, Attenuation of 6-hydroxy-dopamine-induced dopaminergic nigrostriatal lesions in superoxide dismutase transgenic mice, Neuroscience 85 (1998) 907–917. [4] M. Baudry, S. Etienne, A. Bruce, M. Palucki, E. Jacobsen, B.
Malfroy, Salen–manganese complexes are superoxide dismutase mimics, Biochem. Biophys. Res. Commun. 192 (1993) 964–968. [5] K. Baker, C.B. Marcus, K. Huffman, H. Kruk, B. Malfroy, S.R.
1 Doctrow, Synthetic combined superoxide dismutase / catalase
Fig. 5. Effects of EUK-134 on MPP - and 6-OHDA-induced TH
mimetics are protective as a delayed treatment in a rat stroke model: nitration. Cultures were treated with 0.5mM EUK-134 or control media
a key role for reactive oxygen species in ischemic brain injury, J. 48 h prior to exposure to neurotoxins. Cultures were exposed to either 10
1 Pharmacol. Exp. Therap. 284 (1998) 215–221.
mM MPP or 30mM 6-OHDA for 3 h. Cells were lysed with lysis buffer
[6] O. Bandmann, J. Vaughan, P. Holmans, C.D. Marsden, N.W. Wood, and the cell lysates were processed for immunoprecipitation of TH and
Association of slow acetylator genotype for N-acetyltransferase 2 analysis of nitration of tyrosine residues via SDS–PAGE and western blot
1 with familial Parkinson’s disease, Lancet 350 (1997) 1136–1139.
analysis. (A) TH nitration by MPP . Lane 1, control; lane 2, 0.5mM
[7] J.S. Beckman, W.H. Koppenol, Nitric oxide, superoxide, and perox-EUK-134; lane 3, 10mM MPP1; lane 4, 0.5mM EUK-134110 mM
1 ynitrite: the good, the bad, and ugly, Am. J. Physiol. 271 (1996)
MPP . (B) TH protein levels for (A). Lane 1, control; lane 2, 0.5mM
1 C1424–C1437.
EUK-134; lane 3, 10mM MPP ; lane 4, 0.5 mM EUK-134110 mM
1 [8] J.S. Beckman, Oxidative damage and tyrosine nitration from
perox-MPP . (C) TH nitration by 6-OHDA. Lane 1, control; lane 2, 0.5mM
ynitrite, Chem. Res. Toxicol. 9 (1996) 836–844. EUK-134; lane 3, 30mM 6-OHDA; lane 4, 0.5mM EUK-134130mM
[9] J.C. Bensadoun, O. Mirochnitchenko, M. Inouye, P. Aebischer, A.D. 6-OHDA. (D) TH protein levels for (C). Lane 1, control; lane 2, 0.5mM
Zurn, Attenuation of 6-OHDA-induced neurotoxicity in glutathione EUK-134; lane 3, 30mM 6-OHDA; lane 4, 0.5mM EUK-134130mM
peroxidase transgenic mice, Eur. J. Neurosci. 10 (1998) 3231–3236. 6-OHDA. Experiments were repeated 3–5 times, with a representative
[10] B.R. Bloem, I. Irwin, O.J.S. Buruma, J. Haan, R.A.C. Roos, J.W. blot shown here.
Tetrud, J.W. Langston, The MPTP model: versatile contributions to the treatment of idiopathic Parkinson’s disease, J. Neurol. Sci. 97 (1990) 273–293.
[11] A.J. Bruce, B. Malfroy, M. Baudry, Beta-amyloid toxicity in
addition, all these effects were prevented by EUK-134, a
organotypic hippocampal cultures: protection by EUK-8, a synthetic
synthetic catalytic scavenger of ROS.
catalytic free radical scavenger, Proc. Natl. Acad. Sci. USA 93
In conclusion, our study provides new evidence that
(1996) 2312–2316.
1
both MPP and 6-OHDA elicit oxidative stress in DAergic [12] R.S. Burns, C.C. Chiueh, S.P. Markey, M.H. Ebert, D.M. neurons, as measured by the nitration of tyrosine residues Jacobowitz, I.J. Kopin, A primate model of parkinsonism: selective
substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, superoxide formation and enhances NADH-dependent lipid peroxi-Proc. Natl. Acad. Sci. USA 80 (1983) 4546–4550. dation in bovine heart submitochondrial particles, Biochem. Bio-[13] W.S. Choi, S.Y. Yoon, T.H. Oh, E.J. Choi, K.L. O’Malley, Y.J. Oh, phys. Res. Commun. 170 (1990) 1049–1055.
Two distinct mechanisms are involved in 6-hydroxydopamine-and [32] H. Ischiropoulos, D. Duran, J. Horwitz, Peroxynitrite-mediated
1
MPP -induced dopaminergic neuronal cell death: role of caspases, inhibition of DOPA synthesis in PC12 cells, J. Neurochem. 65 ROS, and JNK, J. Neurosci. Res. 57 (1999) 86–94. (1995) 2366–2372.
[14] M.W.J. Cleeter, J.M. Cooper, A.H.V. Schapira, Irreversible inhibition [33] P. Jenner, Oxidative mechanisms in nigral cell death in Parkinson’s of mitochondrial complex I by 1-methyl-4-phenylpyridinium: evi- disease, Mov. Disord. 13 (Suppl 1) (1998) 24–34.
dence for free radical involvement, J. Neurochem. 58 (1992) 786– [34] J.N. Keller, M.P. Mattson, Roles of lipid peroxidation in modulation
789. of cellular signaling pathways, cell dysfunction, and death in the
[15] G. Cohen, R.E. Heikkila, The generation of hydrogen peroxide, nervous system, Rev. Neurosci. 9 (1998) 106–116.