www.elsevier.com / locate / bres
Research report
Dual modulation of excitatory synaptic transmission by agonists at
group I metabotropic glutamate receptors in the rat spinal dorsal horn
c a b c ,
*
´
´
Jie Zhong , Gabor Gerber , Ljubomir Kojic , Mirjana Randic
a
¨ ´
Department of Anatomy, Histology and Embryology, Semmelweis University of Medicine, Tuzolto utca 58., 1094 Budapest, Hungary
b
Department of Ophthalmology, Faculty of Medicine, University of British Columbia, 2550 Willow Street, Vancouver, BC, Canada V5Z 3N9
c
Department of Biomedical Sciences, Iowa State University, Ames, IA 50011, USA Accepted 3 October 2000
Abstract
The effects of group I metabotropic glutamate (mGlu) receptors on excitatory transmission in the rat dorsal horn, but mostly substantia gelatinosa, neurons were investigated using conventional intracellular recording in slices. The broad spectrum mGlu receptor agonist (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD), the group I mGlu receptor selective agonist (S )-3,5-dihydrox-yphenylglycine (DHPG), and the selective mGlu subtype 5 agonist (RS )-2-chloro-5-hydrox)-3,5-dihydrox-yphenylglycine (CHPG), all induce long-lasting depression of A primary afferent fibers-mediated monosynaptic excitatory postsynaptic potential (EPSP), and long-long-lasting potentiation of polysynaptic EPSP, and EPSP in cells receiving C-afferent fiber input. The DHPG potentiation of polysynaptic EPSP was partially or fully reversed by (S )-4-carboxyphenylglycine (S-4CPG), the mGlu subtype 1 preferring antagonist. 2-Methyl-6-(phenylethynyl)-pyridine, the potent and selective mGlu subtype 5 antagonist, partially reversed the CHPG potentiation of polysynaptic EPSP. The effects of DHPG on monosynaptic and polysynaptic EPSPs were reduced, or abolished, by the N-methyl-D-aspartate (NMDA)
receptor antagonistD(2)-2-amino-5-phosphonopentanoic acid (AP5). A clear and pronounced facilitation of the expression of DHPG- and
CHPG-induced enhancement of polysynaptic EPSP, and EPSP evoked at C-fiber strength, was seen in the absence of gamma-aminobutyric acid subtype A receptor- and glycine-mediated synaptic inhibition. Besides dual modulation of excitatory synaptic transmission, DHPG induces depression of inhibitory postsynaptic potentials evoked by primary afferent stimulation in dorsal horn neurons. In addition, group I mGlu receptor agonists produced a direct persistent excitatory postsynaptic effect consisting of a slow membrane depolarization, an increase in input resistance, and an intense neuronal discharge. Cyclothiazide and (S )-4-CPG, the mGlu receptor subtype 1 preferring antagonists, significantly attenuated the DHPG-induced depolarization. These results demonstrate that the pharmacological activation of group I metabotropic glutamate receptors induces long-term depression (LTD) and long-term potentiation (LTP) of synaptic transmission in the spinal dorsal horn. These types of long-term synaptic plasticity may play a functional role in the generation of post-injury hypersensitivity (LTP) or antinociception (LTD). 2000 Elsevier Science B.V. All rights reserved.
Theme: Excitable membranes and synaptic transmission
Topic: Long-term potentiation: physiology
Keywords: Excitatory postsynaptic potential; Group I metabotropic glutamate receptors; Spinal cord dorsal horn; Slice-intracellular recording technique
1. Introduction Glutamate acts through two broad classes of receptors, ion channel-linked (ionotropic) receptors, which include It is now well established that primary afferent fibers use a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate as a principal fast excitatory transmitter in the (AMPA), kainate, and N-methyl-D-aspartate (NMDA)
re-dorsal horn (DH) of the spinal cord, the first modulatory ceptors, and metabotropic receptors (mGluRs) which cou-site in the relay of sensory information to the brain [21,98]. ple via G-proteins to the intracellular second messenger cascades and ion channels. Eight mGlu receptor subtypes have been cloned to date and are classified into three
*Corresponding author. Tel.: 11-515-294-7793; fax: 1
1-515-294-groups based on structural homology, pharmacology and
2315.
´
E-mail address: [email protected] (M. Randic). signal transduction mechanisms: group I (mGlu receptors 1
and 5) are coupled to phospholipase C and stimulate mm thick) were cut with attached dorsal roots in an
21
phosphoinositide hydrolysis and intracellular Ca signal oxygenated (95% O , 5% CO ) Krebs-bicarbonate solu-2 2
transduction, whereas group II (mGlu receptors 2 and 3) tion (48C) on a vibratome and placed in a holding chamber and group III (mGlu receptors 4 and 6–8) are negatively (36618C) to recover for at least 1 h. A single slice was coupled to adenyl cyclase [1,16,79]. These receptors then transferred into an interface-type recording chamber modulate synaptic transmission and neuronal excitability. where it was submerged beneath an oxygenated superfus-Class I mGlu receptors have been postulated to play a role ing medium (flow-rate of about 3 ml / min, 34–358C) in synaptic plasticity such as long-term potentiation (LTP) containing (in mM): NaCl, 124; KCl, 1.9; KH PO , 1.2;2 4
[3,9,106] and long-term depression (LTD) of synaptic CaCl , 2.4; MgSO , 1.3; NaHCO , 26; glucose, 10; pH2 4 3
transmission [53,55,71] in the brain, as well as in the 7.4, 310–320 mOsm, and was equilibrated with 95% O ,2
spinal cord [32,82]. 5% CO .2
Although the presence of multiple mGlu receptors
(subtypes 1–5 and 7) in the spinal cord DH has been 2.2. Dorsal root stimulation and intracellular recording shown [10,46,54,70,89,92–94], their roles in physiology
and pathophysiology of synaptic transmission are not clear Intracellular recordings with sharp microelectrodes were and are complicated by the diversity of pre- and postsynap- made from DH neurons (laminae I–V), including sub-tic receptors. There is behavioral and electrophysiological stantia gelatinosa (SG, lamina II) cells. When viewed evidence that the activation of mGlu receptors, in par- under a dissecting microscope at a magnification of 10– ticular, mGlu subtypes 1 / 5, increases the excitability of the 403with transmitted illumination, the SG was distinguish-rat spinal DH neurons [51,64,65] and facilitates responses able as a translucent bend in the superficial DH, although it to NMDA and AMPA receptor activation was difficult to discern with certainty the border between [5,8,12,24,25,48,61,69,102–105]. Studies of the actions of laminae I and II. Under visual control, a single fiberglass mGlu receptor agonists and antagonists on responses of ([6010; O.D. and I.D., 1.0 and 0.58 mm, respectively; AM deep DH neurons [68,69,103–105] to noxious and non- Systems) microelectrode filled with 4 M potassium acetate noxious stimuli indicate that mGlu receptors are involved (pH 7.2) (DC resistance: 140–220 MV) was placed in the in mediating nociceptive inputs. In particular, group I SG or deep DH (DDH), and neurons were impaled by mGlu 1 / 5 receptors have been implicated in mediating oscillating the capacity compensation circuit of a high-nociception following a sustained noxious input [24– input impedance bridge amplifier (Axoclamp 2A, Axon 26,28,29,104,105]. However, the synaptic and cellular Instruments). A DC pen-recorder was used to record mechanisms underlying functional plasticity following membrane potentials continuously and a Digidata 1200 tissue or nerve injury are not known. Moreover, the data system with PCLAMP (version 6) software (Axon
Instru-described above are largely based on observations obtained ments) was used for data acquisition and analysis. Most from wide-dynamic-range neurons in deep DH laminae of recordings were obtained from cells with a stable resting rats and primates in vivo. membrane potential (more negative than 255 mV) and In the present study we examined the effects of group I with overshooting action potentials. The protocol for mGlu receptor activation by the group I and II mGlu assessing the effects of mGlu receptor agonists and antago-agonist (1S,3R)-1-aminocyclopentane-1,3-dicarboxylate nists on EPSPs was as follows. Monosynaptic and poly-(1S,3R-ACPD), (S )-3,5-dihydroxyphenylglycine (DHPG), synaptic EPSPs in DH neurons were evoked by ortho-a selective ortho-agonist for the group I mGlu receptors [88], ortho-and dromic electrical stimulation of primary afferent fibers in (RS )-2-chloro-5-hydroxyphenylglycine (CHPG), a selec- the lumbar dorsal root (L4 and / or L5) using a bipolar tive mGlu subtype 5 agonist [20] on the synaptic responses platinum wire electrode or glass suction electrode (with the mostly of rat superficial dorsal horn neurons to primary cathode internal). Single shocks (0.01–0.5 ms pulses, 2–35 afferent stimulation in vitro. Some of the results have been V), repeated at 2-min intervals, were given for at least 10 reported previously in abstract form [51,84,108]. min before, during (10 min), and for a 30–60 min period after bath administrations of chemicals. This frequency of stimulation was chosen for sampling data because it did
in relation to the primary afferents activated was done synaptic responses, EPSP area, was used. The area was solely on the basis of conduction velocity which was calculated under the curve of polysynaptic EPSPs in a calculated either by measuring the distance between the 180-ms time window starting at the onset of the EPSP. We stimulating electrode and the recording site on the dorsal first tested the stability of the synaptic and passive root and dividing by the conduction latencies of action membrane properties of DH neurons in slices over a period potentials recorded, or from the latency of evoked EPSPs of 10–20 min and next examined the changes in these and the distance from the stimulating electrode to the properties as a result of mGlu receptor-ligand treatment. recording site. Primary afferents conducting at velocity Over a recording period of 1–2 h, resting membrane above 15 m / s were classified as Ab [76], whereas those potential, input resistance, and the peak amplitude of the conducting between 1.5 and 15 m / s were classified as Ad, EPSP did not change significantly (#10% change in peak and those conducting below 1.5 m / s as C fibers. The amplitude) in DH neurons of untreated slices used in this minimum stimulus intensities and durations used to acti- study. All values are expressed as means6S.E.M. Statisti-vate Ad and C fibers were 3 V/ 0.1 ms and 5 V/ 0.5 ms, cal significance of data (P#0.05) has been assessed respectively. Stimulation of dorsal roots led to generation relative to control responses by use of either paired or of an EPSP. With small stimulus strength this EPSP was unpaired Student’s t-test, as appropriate.
graded in amplitude, had a fixed latency and monophasic
decay. As the stimulus strength was increased, however, a 2.4. Application of chemicals later slow polysynaptic component(s) was apparent. In
order to discriminate between monosynaptic and poly- Drugs were dissolved in oxygenated recording solution synaptic EPSPs, two criteria were used: (1) EPSPs latency immediately prior to use, and applied to the slices in should not change with increasing intensities of electrical known concentrations by addition to the superfusing stimulation; and (2) EPSPs should follow high frequency medium. All compounds were applied via the bath, and stimulation (50 Hz) with reduced amplitude but no change each neuron served as its own control. Drug-containing in latency. The polysynaptic EPSPs have variable latencies solution entered the recording chamber within 30–45 s of and show failures with high frequency stimulation. More- changing solutions, with complete exchange occurring over, the shapes and amplitudes of polysynaptic EPSPs are within 3 min. Drugs were administered for a sufficient variable in different trials when dorsal roots are stimulated period (5–10 min) to allow their full equilibration. Only at a constant intensity. Input resistance was measured at one cell in a slice was subject to one trial with mGlu 2-min intervals by passing a hyperpolarizing current pulse receptor agonist, the exception being the experiments using of 0.05 nA across the cell membrane and measuring the mGlu receptor antagonists where each cell was subjected voltage deflection produced. The current values were of to two or three trials.
sufficient duration (200–300 ms) to fully charge the Chemicals used and their sources were as follows: membrane capacitance. Bridge balance was monitored (2)-bicuculline methiodide and strychnine hydrochloride throughout experiments and corrected when necessary. To from Sigma (St. Louis), (1S,3R)-1-aminocyclopentane-1,3-reduce the increased spontaneous synaptic activity and dicarboxylate (1S,3R-ACPD),D
-2-amino-5-phosphonopen-subsequent action potential firing due to the removal of tanoate (D-AP5), (S )-4-carboxyphenylglycine (4-CPG),
21
synaptic inhibition, the Mg concentration in the super- (RS )-2-chloro-5-hydroxyphenylglycine (CHPG), 6-cyano-fusing solution was increased to 3 mM in the experiments 7-nitroquinoxaline-2,3-dione (CNQX), cyclothiazide where bicuculline and strychnine were applied to block the (CTZ), (S )-3,5-dihydroxyphenylglycine (DHPG), 6-nitro-GABA and glycine receptors.A 7-sulphanoylbenzo[ f ]quinoxaline-2,3-dione (NBQX), all obtained from Tocris Cookson Ltd. (Bristol, UK), 2-2.3. Data analysis methyl-6-(phenylethynyl)-pyridine (MPEP) kindly pro-vided by Novartis, Switzerland, tetrodotoxin (TTX; The mGlu receptor agonists were applied in the perfu- Alomone Labs, Jerusalem, Israel). All solutions were sate for 5–10 min in the absence or continuous presence of freshly prepared every day from stock solutions that were the antagonist. The magnitude of their effects in any stored at 2208C.
individual cell was determined by comparing the averaged peak amplitude of three consecutive EPSPs evoked
imme-diately prior to drug application (Vcontrol) to the averaged 3. Results
peak amplitude of three consecutive EPSPs measured at
the time of maximal change induced by mGlu receptor Stable intracellular recordings of up to 5 h were agonists (Vtreatment). Vtreatment was typically determined obtained from 119 DH (94 in LI and II; 25 in LIII–V) during application of mGlu receptor ligand, and also 20– neurons in the longitudinal (19) and transverse (86) spinal 22 min following the washout of mGlu receptor agonist, cord slices. The average resting membrane potential of and was expressed as percentage of control: Vtreatment/ these neurons was270.060.7 mV (mean6S.E.M), and the
previ-ous results [83,100]. Single shock electrical stimulation of applications of 10–100 mM (1S,3R)-ACPD for 10 min the primary afferent fibers in a L4 or L5 dorsal root caused a slow membrane depolarization (7.060.9 mV) in elicited monosynaptic and / or polysynaptic EPSPs in DH 20 (13 SDH and seven DDH cells) of 28 DH neurons as cells that were suppressed by 1–10 mM NBQX in a described previously [51,64]. This effect was associated reversible manner (to 360.8%, n526), suggesting that with nonsignificant changes in input resistance they were principally mediated by the AMPA subtype of (100.263.5% of control at 10 min of agonist application; glutamate receptors. A small component sensitive to n512). The depression of the EPSP amplitude was not application of the NMDA antagonistD-AP5 (100mM) was due to (1S,3R)-ACPD-induced depolarization since the
also observed, such that co-application of NBQX (10mM) membrane potential was always adjusted to its control andD-AP5 (100 mM) led to an almost complete block of level prior to dorsal root stimulation.
the EPSP [31,83,100].
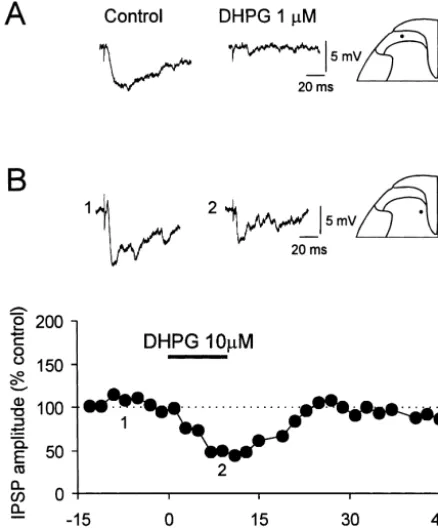
3.2. Potentiation of EPSPs in DH cells receiving inputs 3.1. Depression of monosynaptic EPSP and potentiation from C-afferent fibers
of polysynaptic EPSP by (1S,3R)-ACPD
Superfusion of spinal slices with (1S,3R)-ACPD (25– The activation of group I / II mGlu receptors by the 100 mM, 10 min) caused variable changes in peak am-prototypic broad-spectrum agonist (1S,3R)-ACPD resulted plitude of the EPSPs in DH cells (six SG and two DDH in a depression of A-fiber-mediated monosynaptic EPSP neurons) receiving input from C-fibers, that when averaged and potentiation of polysynaptic EPSP in DH neurons. showed an initial, statistically non-significant depression During the superfusion of slices with (1S,3R)-ACPD (25– during (1S,3R)-ACPD administration (to 62.2621.6% of 100mM, 10 min) the amplitude of the presumed A-fiber- control; n54) followed by a late reversible potentiation (to evoked monosynaptic EPSP was reduced in a dose-depen- 164.8613.8% of control; n54; P,0.05) upon washout dent manner in 14 (12 SG and two deep DH neurons) of (Fig. 2B). Because neurons that exhibited C-fiber strength-17 DH neurons studied both in longitudinal (n57) and evoked synaptic responses also received A-fiber-mediated transverse (n57) spinal slices (Fig. 1A, Table 1). How- input, and often polysynaptic EPSPs, it was usually ever, in three of 17 DH cells 25–100 mM (1S,3R)-ACPD possible to analyze C-fiber-evoked input only in the subset reversibly increased monosynaptic EPSPs both during and of neurons that did not receive a preceding A-fiber input. after washout of the agonist (Table 1). The peak depres- To investigate further the actions of (1S,3R)-ACPD on DH sion had a latency of 6–8 min and the effect persisted for cells receiving input only from C-afferents, our approach more than 25 min after the application of (1S,3R)-ACPD was to selectively block the action potential generation in was terminated (Fig. 1A). The depressant effect of large primary afferent fibers by using the sodium channel (1S,3R)-ACPD (100 mM) was reduced (from 55.4 to blocker TTX [97,99]. Fig. 2A illustrates a typical experi-79.1% of baseline) by the mGlu subtype 1 preferential ment in which TTX (0.5mM) when applied to DRG and antagonist 4-CPG (500 mM for 20 min), as illustrated in dorsal roots blocked the fast component of the synaptic Fig. 1B. response induced by low threshold DR stimulation, but did Besides depressing monosynaptic EPSP, (1S,3R)-ACPD not block the component induced by activation of high (10–50mM, 10 min) produced a reversible increase of the threshold, slowly (C) conducting afferents. The isolated peak amplitude of A-fiber-evoked polysynaptic EPSP, and C-fiber strength-evoked EPSP was almost completely the area under the curve of EPSPs (Fig. 1C) in eight cells blocked (Fig. 2B, trace 2 inset) during 6 min superfusion (four SG and four deep DH cells) examined (Table 2). The with 50 mM (1S,3R)-ACPD. Following removal of maximal effect had a latency of 8–10 min and it persisted (1S,3R)-ACPD from the bath, a sustained potentiation of for approximately 25 min (Fig. 1C). The potentiating the synaptic response (to about 200% of control) was effects of (1S,3R)-ACPD (100 mM) on a polysynaptic observed.
EPSP evoked by low-intensity stimuli (Fig. 1D, left
column), and a DR-evoked EPSP suprathreshold for action 3.3. Dual modulation of excitatory transmission and potential firing followed by longer-lasting polysynaptic synaptic plasticity following activation of the group I
activity evoked by high-intensity stimuli (Fig. 1D, right mGlu receptors
column), are illustrated in Fig. 1D. A significant increase in
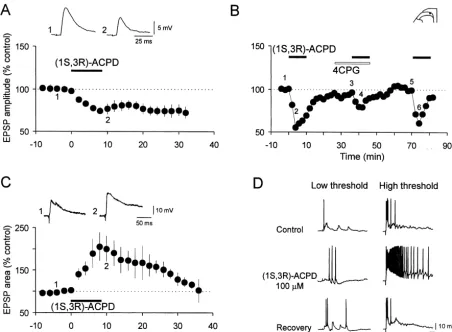
Fig. 1. Dual modulation of dorsal root-evoked EPSPs by the broad spectrum mGluR agonist (1S,3R)-ACPD in the spinal DH, and reduction of (1S,3R)-ACPD-induced depression of monosynaptic EPSP by the group I mGluR antagonist 4-CPG. (A) Bath application of (1S,3R)-ACPD (25–100mM, 10 min; n53 (25mM), n56 (50mM), n55 (100mM)) caused a depression of apparently monosynaptic Adfiber-evoked EPSPs (c.v. 1.6–5.8 m / s) in 14 of 17 neurons tested, which was sustained after the drug washout. Vm5 262 to286 mV, 18–23 day-old rats. (B) In a SG neuron receiving monosynaptic input from Adprimary afferent fibers (c.v. 2.5 m / s), superfusion of 100mM (1S,3R)-ACPD reduced the amplitude of the EPSPs evoked by orthodromic stimulation of a DR. 4-CPG (500 mM), the mGluR I preferential antagonist, produced no significant changes in the EPSPs by itself. However, the depressant effect of (1S,3R)-ACPD was reduced in the presence of 4-CPG in a reversible manner. Vm5 273 mV, 23 day-old rat. (C) Summarized data from eight DH neurons showing the time course of the potentiation of the DR-evoked polysynaptic EPSPs by (1S,3R)-ACPD (10–50mM, 6–10 min; n51 (10
mM), n52 (25mM), n55 (50mM)). The area under the curve of EPSPs showed a faster and greater increase following (1S,3R)-ACPD application in comparison with the EPSP amplitude (not shown). Vm5 270 to278 mV, 20–26 day-old rats. In this and in all subsequent figures the summary graphs (means6S.E.M.) show the time course of changes in the peak amplitude / area of EPSPs, whereas above the graph are displayed individual EPSPs taken at the time marked by the corresponding number, the solid bar above the graph indicates the time at which drug application occurred. (D) (1S,3R)-ACPD (100 mM, 5 min) increased the amplitude and duration of EPSP and action potential firing (right panel, middle trace) evoked by high (25 V, 0.5 ms) intensity stimulation of primary afferents. Upper row shows control responses, middle row responses obtained 5 min after the onset of (1S,3R)-ACPD application, lower row recovery following 12 min washout. Vm5 270 mV, 24 day-old rat.
MPEP, the potent and selective mGlu subtype 5 antagonist under the curve of polysynaptic EPSPs (Fig. 3D, Table 2),
[30]. in a dose-dependent manner (Fig. 3D inset-graph). The
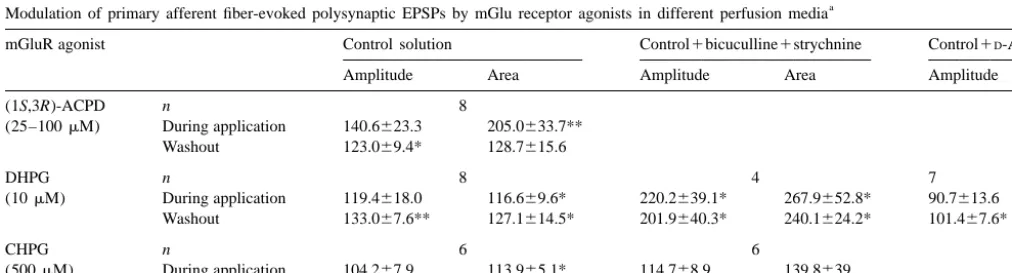
Table 1
a
Modulation of primary afferent fiber-evoked monosynaptic EPSPs by mGlu receptor agonists in different perfusion media
mGluR agonists Control solution Control1D-AP5
Depression Potentiation Depression
(1S,3R)-ACPD n 14 3
(25–100mM) During application 72.266.6** 166.2653.6
Washout 75.067.3* 126.463.9*
DHPG n 9 4 6
(10mM) During application 70.367.3** 129.9610.8* 92.763.7
Washout 79.569.7* 130.8613.9* 97.066.8*
CHPG n 7
(500mM) During application 85.764.0**
Washout 75.968.3*
a
Values, expressed as percentage of control (means6S.E.M), show the changes induced by (1S,3R)-ACPD, DHPG and CHPG in the peak amplitude of monosynaptic EPSPs evoked by stimulation of primary afferent fibers in lumbar dorsal roots. Values represent the peak change recorded during application of respective mGlu receptor agonist in control solution or in a solution containing 50mMD-AP5. Washout values are taken 16–22 min after removal of the agonist. Statistical significance of data is indicated by asterisks: * P,0.05; ** P,0.01.
Taken together, these data indicate that whereas mono- the normal medium. The mean value at 20 min following synaptic EPSPs are susceptible to both potentiation and washout was significantly different (P,0.01) from that in depression, polysynaptic EPSPs are potentiated by activa- the absence ofD-AP5 (Table 1). Furthermore, DHPG (10
tion of the group I mGlu receptor selective agonist DHPG. mM, 10 min) in the presence ofD-AP5 produced a small,
reversible decrease of the peak amplitude of DR-evoked 3.5. Depression and potentiation of synaptic responses polysynaptic EPSP during perfusion, followed by a
tran-by DHPG is dependent on NMDA receptor sient increase upon washout (Fig. 4B, Table 2). However, the mean value at 20 min after washout was significantly The sensitivity of DHPG-induced depression and poten- (P,0.05) lower than that from slices exposed to DHPG in tiation of EPSPs to the NMDA receptor antagonist D-AP5 the absence ofD-AP5.
was tested since some forms of activity-dependent,
long-term changes in synaptic strength have been shown to 3.6. Effects of bicuculline and strychnine on modulation require activation of this receptor system [1]. The effect of of synaptic responses by DHPG
the D-AP5 was first examined on the long-lasting
depres-sion of monosynaptic EPSP induced by DHPG. As shown Given the importance of inhibitory processes in the in Fig. 4A, DHPG (10 mM, 10 min) applied in the temporal and spatial control of sensory responses in the presence of D-AP5 (50 mM) induced a decrease in DH, and a recent evidence of co-localization of group I
amplitude of monosynaptic EPSP only in two of six SG mGlu5 with GABA in the superficial laminae of spinal DH cells during the application, but almost completely [46], we have investigated in the present study possible abolished a long-lasting depression induced by DHPG in interaction of DHPG with inhibitory processes within this
Table 2
a
Modulation of primary afferent fiber-evoked polysynaptic EPSPs by mGlu receptor agonists in different perfusion media
mGluR agonist Control solution Control1bicuculline1strychnine Control1D-AP5
Amplitude Area Amplitude Area Amplitude
(1S,3R)-ACPD n 8
(25–100mM) During application 140.6623.3 205.0633.7** Washout 123.069.4* 128.7615.6
Values, expressed as percentage of control (means6S.E.M.), show the changes induced by (1S,3R)-ACPD, DHPG and CHPG in the peak amplitude and the area of polysynaptic EPSPs evoked by stimulation of primary afferent fibers in lumbar dorsal roots. Values represent the peak change recorded during application of respective mGlu receptor agonist in control solution or in a solution containing bicuculline (5mM) and strychnine (2mM) orD-AP5 (50
dependent on the modulation of synaptic inhibition by the group I mGlu receptors. In support of this possibility, a marked reduction in the amplitude of DR-evoked IPSP in SG (Fig. 6A, n53) and DDH (Fig. 6B) was observed.
3.7. Effects of DHPG on membrane properties
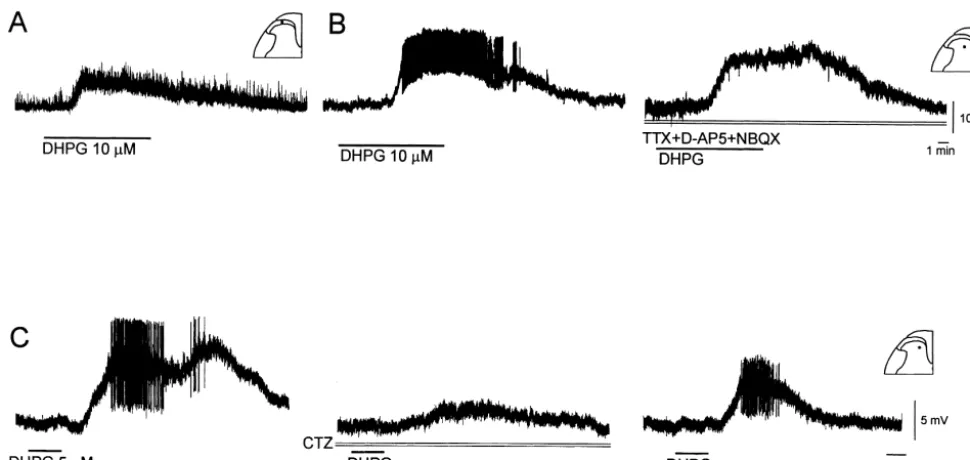
Bath application of 0.1–100 mM DHPG for 10 min caused a slow, dose-dependent and reversible membrane depolarization (6.660.6 mV, Fig. 7) associated with an increase in membrane input resistance (116.966.0% of control, measured at peak response; n517, P,0.05) in 28 of 38 DH neurons examined, or a hyperpolarization (23.860.9 mV) accompanied by a decrease in input resistance (68.6613.2% of control) in ten of 38 cells (31 SDH and seven DDH cells). The depolarizing and hy-perpolarizing responses outlasted the period of DHPG application for 11.062.2 min, n524 and 5.861.1 min,
n56, respectively. Two types of depolarizing responses to DHPG can be recorded from DH neurons. As illustrated in Fig. 7A, in most of SDH neurons the depolarization (6.060.5 mV, n521) was accompanied by an increase in baseline noise, whereas in the deep DH neurons, DHPG often produced a larger depolarization (8.661.8 mV, n57) and increase in excitability, as indicated by the generation of sustained firing of spontaneous action potentials (Fig. 7B). To determine whether the depolarization resulted
Fig. 2. Potentiation of EPSPs recorded from cells receiving C-fiber input
from a direct postsynaptic action on DH neurons, and to
by the broad spectrum mGluR agonist (1S,3R)-ACPD in the spinal DH.
eliminate the possibility that DHPG activated ionotropic
(A) The A-fiber component of the EPSP evoked by high intensity
glutamate receptors, the agonist was applied after blockade
stimulation (25 V, 0.5 ms) of a dorsal root was blocked by 0.5mM TTX
1
revealing the C-fiber component (c.v. 0.3 m / s). Vm5 278 mV, 26 day-old of voltage-dependent Na channels with TTX (0.5 mM) rat. (B) Summarized data from four DH neurons showing the time course and ionotropic glutamate receptors by NBQX (10mM) and of the initial depression and late potentiation of the EPSPs of cells
D-AP5 (30 mM). Under these conditions, DHPG produced receiving C-fiber inputs (c.v. 0.3–1.4 m / s) by (1S,3R)-ACPD (25–100
a depolarization (Fig. 7B, right trace) that was similar to mM, 10 min n51 (25mM), n52 (50mM), n5l (100mM)). Above the
that observed under control conditions (Fig. 7B, left trace).
graph are displayed individual EPSPs from an experiment where the
stimulated dorsal root was bathed in 0.5mM TTX to block A-fiber inputs. These data indicate that the depolarization is independent
Vm5 270 to278 mV, 22–26 day-old rats. of AMPA or NMDA receptor activation by increased
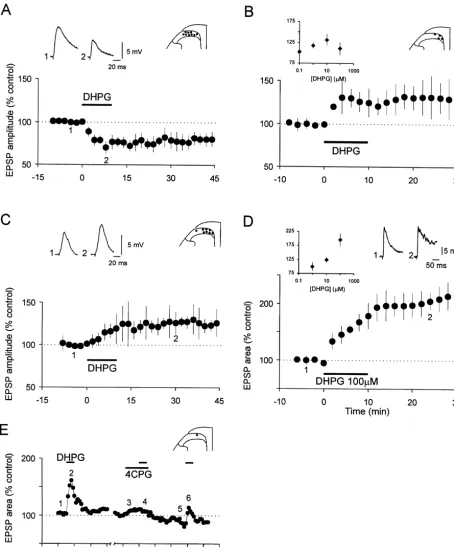
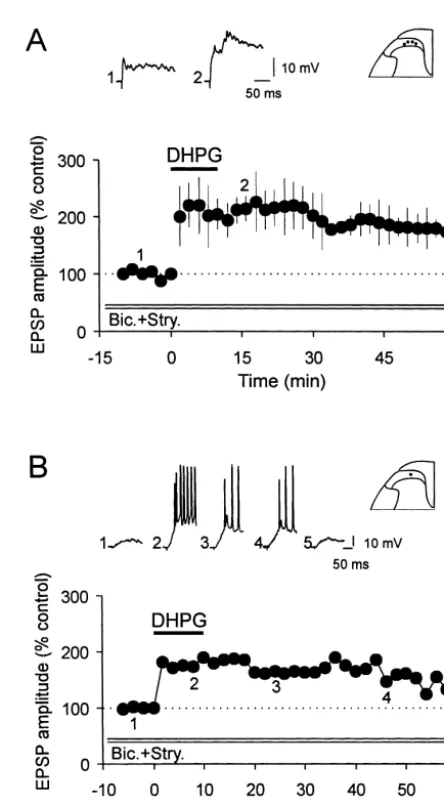
Fig. 4. NMDA receptor dependence of the S-DHPG-induced long-lasting depression and long-lasting potentiation of EPSPs. (A) The graph shows the pooled data from six SG cells in which the S-DHPG-inducedh10mM, 10 min) initial depression of monosynaptic EPSPs (Ad input: n56, 2.8–10.9 m / s) was reduced and later long-lasting depression abolished in
Fig. 5. Blockade of GABA - and glycine-mediated synaptic inhibition the presence of 50mMD-AP5, the NMDA receptor antagonist. Vm5 264 A
enhances the S-DHPG-induced potentiation of polysynaptic EPSPs. (A) to277 mV, 17–22 day-old rats. (B) Summary graph (n57) showing the
Summarized data showing the enhanced potentiation in the peak am-antagonism of the S-DHPG-induced (10mM, 10 min) potentiation of the
plitude of polysynaptic EPSPs induced by S-DHPG (10mM, 10 min) in polysynaptic EPSPs by DAP5 (50mM). Vm5 255 to 277 mV, 18–22
four SG neurons in the presence of bicuculline (5mM), strychnine (2 day-old rats.
21
mM) and a high concentration of Mg (3mM). Vm5 261 to289 mV, 17–19 day-old rats. B, The graph shows the time course of changes in the
was 5.161.1 mV and 0.0560.02 Hz (n511), respectively. peak amplitude of EPSPs caused by S-DHPG (10mM, 10 min) in a SG
The induction of oscillations was concentration-dependent neuron receiving input from C-afferent fibers (c.v. 0.7 m / s). Note also the long lasting potentiation of EPSPs accompanied by action potential firing
in that the number and frequency of oscillations increased
as shown on the individual EPSPs above the graph Vm5 261 mV, 17
with higher concentrations or time of exposure to DHPG.
day-old rat.
Experiments were also undertaken to determine if inhibi-tion plays a significant role in producing the
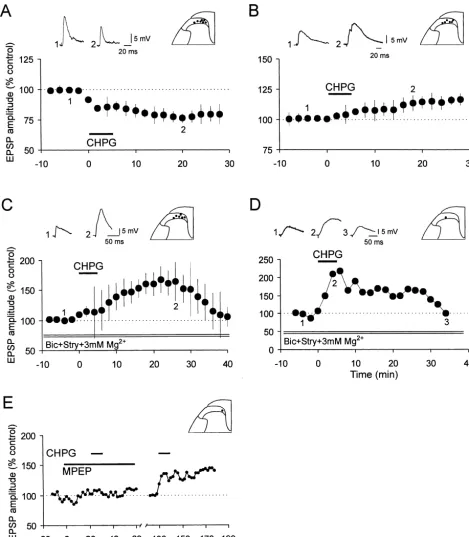
DHPG-induced oscillatory activity. An interesting finding was that produced a sustained depression (Fig. 9A, Table 1) or the oscillations induced by 10mM DHPG persisted in the potentiation (in 2 / 3 cells with 1 mM CHPG) of Ad -fiber-presence of bicuculline (5mM) and strychnine (2mM), the evoked monosynaptic EPSP and prolonged potentiation of GABAA and glycine receptor antagonists, in a cell ex- the polysynaptic EPSP (Fig. 9B, Table 2). Both effects hibiting excitatory synaptic noise (Fig. 8C), but were outlasted the period of CHPG application for more than 25 abolished (Fig. 8D, right trace) in the cell in which DHPG min. a-Methyl-6-(phenylethynyl)-pyridine (MPEP), the applied in a normal medium produced a marked increase in potent metabotropic glutamate receptor subtype 5 antago-inhibitory synaptic noise (Fig. 8D, left trace). nist [30], almost completely reversed the polysynaptic EPSP potentiation (Fig. 9E) caused by CHPG (n53). In 3.8. Dual modulation of DR-evoked EPSPs by CHPG the presence of bicuculline (5 mM), strychnine (2 mM),
21
elevation of intracellular calcium [83,87]. In addition, recent studies suggest the potential role of neurokinin 1 (NK1) and opioid receptors in the generation of LTP and LTD [56,57,82,84,108]. The results of this study provide the first demonstration of de novo long-lasting synaptic plasticity (LTP and LTD) in the spinal cord dorsal horn that is induced by activation of group I metabotropic glutamate receptors in the absence of repetitive presynaptic activity.
The present study addressed electrophysiologically and pharmacologically how group I mGlu receptors influence primary afferent-mediated synaptic transmission and plas-ticity in the rat spinal DH. This is a particularly important question in view of an emerging evidence that implicates group I mGlu receptors in nociception and hyperalgesia. The principal findings are that the activation of group I mGlu receptors by (1S,3R)-ACPD, DHPG and CHPG induced: 1) a long-lasting depression of Ad-fiber-mediated monosynaptic EPSP in neurons of both substantia gelatin-osa and deep dorsal horn, and 2) a long-lasting potentiation of polysynaptic EPSP. The effects of DHPG on mono-synaptic and polymono-synaptic EPSPs were inhibited byD-AP5.
The increases in polysynaptic EPSPs produced by DHPG and CHPG are greatly augmented after synaptic inhibition
Fig. 6. S-DHPG decreased the dorsal root-evoked inhibitory postsynaptic
is blocked. In addition, we confirmed previous
demonstra-potentials (IPSPs) in DH neurons. (A) S-DHPG (1mM, 10 min) blocked
the DR-evoked IPSP in a SG neuron. Vm5 264 mV, 19 day-old rat. (B) tion of a direct increase in excitability of DH neurons by The graph shows the time course of the reversible depression of DR- the mGlu receptor agonist (1S,3R)-ACPD [51,64] and evoked IPSPs following bath application of S-DHPG (10mM, 10 min) in
demonstrated that the effect is exerted via the activation of
a deep DH neuron. The individual IPSPs from the same experiment are
group I mGlu receptors.
shown above the graph. Vm5 254 mV, 18 day-old rat.
4.1. Long-lasting depression of Ad-fiber-mediated
monosynaptic EPSPs by activation of group I mGlu
control solution (Fig. 9C, Table 2). Moreover, under receptors
conditions of blockade of GABA - and glycine-mediatedA
synaptic inhibition, the EPSP-evoked at C-fiber strength (1S,3R)-ACPD, the group I and II mGlu receptor was substantially increased (Fig. 9D). agonist, primarily caused a sustained decrease in the Application of 0.5 mM CHPG for 5 min had no amplitude of the DR-evoked monosynaptic EPSP. This significant effect on the resting membrane potential (n5 effect appears to be specific for group I mGlu receptors, as 13), or membrane input resistance (n515). However, 1 it could be mimicked by DHPG and CHPG, the selective mM CHPG produced a prolonged depolarization (7.862.7 group I agonists, and was attenuated by 4-CPG, the group I mV, n55, lasting 9–32 min). In the presence of TTX (0.5 antagonist. Depression of the monosynaptic and, to a lesser mM), NBQX (10 mM) and D-AP5 (50 mM), the CHPG- extent, polysynaptic components of the dorsal root-evoked
induced depolarization was not modified, the finding ventral root potential (VRP) of the neonatal rat spinal cord suggesting a postsynaptic site of action. by (1S,3R)-ACPD [23,43–45,80, but see 11] and also EPSPs evoked by low or high intensity dorsal root stimulation in immature ventral horn neurons in vitro was
4. Discussion reported [50]. Class I mGlu receptors have also been shown to play a role in the long-term depression in the We have previously reported long-term potentiation brain [7,53,55,60,71–73,75].
Fig. 7. S-DHPG-induced depolarization of DH neurons. The group I mGluR agonist, S-DHPG (10mM, 10 min) induced a depolarization and increase in the baseline noise in both superficial (A) and deep (B) DH neurons. In the deep DH neuron the larger depolarization was associated with spontaneous action potential firing (B, left trace). In the presence of TTX (0.5mM), and ionotropic glutamate receptor antagonistsD-AP5 (30mM) and NBQX (10mM), the depolarization was not modified, the finding suggesting a postsynaptic site of action (B, right trace). Vm5 267 mV (A),273 mV (B), 19 day-old rats. (C) In the presence of ionotropic glutamate receptor antagonist CNQX (20mM), bath-applied S-DHPG (5mM for 90 s) to a deep DH neuron caused a large membrane depolarization associated with increased baseline noise and action potential firing (left trace). This effect is reversibly blocked by cyclothiazide (CTZ, 25mM), an antagonist at mGluR1 (middle trace). The inhibitory response was recorded 30 min after exposure of the slice to CTZ. Partial recovery of the S-DHPG-induced depolarization occurred 30 min after removal of CTZ (right trace). Vm5 262 mV, 21 day-old rat.
which belong to each of the three major groups, can serve 4.2. Activation of group I mGlu receptors induces long-as autoreceptors. Although the pharmacological profiles of lasting potentiation of DR-evoked polysynaptic EPSPs
mGlu autoreceptors at synapses between primary afferent
Fig. 8. S-DHPG-induced oscillatory activity recorded from DH neurons. (A) In a deep DH neuron S-DHPG (100 mM, 90 s) produced stereotypic oscillatory activity lasting over an hour in the presence of competitive ionotropic glutamate receptor antagonists CNQX (20mM) andD-AP5 (50mM). The
oscillations consisted of trains of depolarizations with overriding action potentials. Vm5 263 mV, 18 day-old rat. (B) In a SG neuron addition of bicuculline (5mM) and strychnine (2mM), the GABA and glycine receptor antagonists, to the perfusion medium augmented the amplitude of the oscillatory activityA
induced by S-DHPG (10mM, 10 min). To reduce the increased spontaneous synaptic activity and subsequent action potential firing due to the removal of
21
synaptic inhibition, the Mg concentration in the perfusing solution was increased to 3 mM. Vm5 279 mV, 18 day-old rat. (C,D) The increase in the baseline noise and the oscillatory activity induced by 10mM S-DHPG persisted, and oscillations were even enhanced (C, right trace) in the presence of bicuculline (5mM) and strychnine (2mM) in a cell exhibiting excitatory synaptic noise (C), but were abolished (D, right trace) in the cell in which
S-DHPG applied in a normal medium produced a marked increase in inhibitory synaptic noise (D, left trace, inset). The insets in C, D illustrate the
spontaneous synaptic activity taken at the time indicated by the arrow on a slower time scale. (C) Vm5 274 mV, 19 day-old rat. (D) Vm5 253 mV, 18 day-old rat.
21
responses [5,12,18,34,35]. In a few brain regions, however, Ca concentration, in the induction of LTP and LTD in the (1S,3R)-ACPD-induced potentiation seems to be pre- the brain and spinal cord [1,6,53,55,82]. The present study synaptically controlled [15,40,41]. There is also evidence demonstrates that the induction of long-lasting depression for a role of presynaptic group I mGlu receptors in the of monosynaptic EPSP and the magnitude of long-lasting positive modulatory control of neuronal glutamate release, potentiation of polysynaptic EPSP produced by DHPG in probably from primary afferent C-fibers, in the rat spinal the spinal cord DH, appears to be dependent on co-cord [27]. activation of group I mGlu and NMDA receptors, as the effects were reduced, or abolished, by blockade of either 4.3. DHPG-induced long-lasting depression /potentiation receptor class. Our finding of the NMDA receptor
Fig. 9. Effects of RS-CHPG on the synaptic responses of DH neurons. (A) Summarized data showing the time course of the depression of the monosynaptic EPSPs (Adinput: n57, 2–10 m / s) by bath-applied selective mGluR5 agonist RS-CHPG (0.5 mM, 5 min). Vm5 264 to280 mV, 17–23 day-old rats. (B) Bath application of RS-CHPG (0.5 mM, 5 min) in the control solution produced a small potentiation in the peak amplitude of the Ad-primary afferent-evoked polysynaptic EPSPs of six SG neurons (inset) recorded in response to electrical stimulation of a lumbar dorsal root. Vm5 265
21
to277 mV, 17–22 day-old rats. (C) In a medium containing bicuculline (5mM), strychnine (2mM) and a high concentration of Mg (3 mM), 0.5 mM
RS-CHPG-induced long-lasting potentiation in the peak amplitude of polysynaptic EPSPs recorded from SG neurons (inset) was increased (n56).
Vm5 258 to277 mV, 20–22 day-old rats. (D) In a SG neuron (inset) receiving input from C-fibers (c.v. 1.3 m / s), superfusion of RS-CHPG (0.5 mM, 5
21 min) potentiated the EPSPs during and after drug application in the presence of bicuculline (5mM), strychnine (2mM) and high concentration of Mg (3 mM). Vm5 269 mV, 21 day-old rat. (E) In the presence of MPEP (10mM), a potent mGluR antagonist, CHPG (1 mM, 5 min) had no significant effect on5
regions [71,72]. The relevant question to ask here is why the superficial laminae of spinal DH [46]. In spinal DH should NMDA receptor antagonists inhibit the LTD/ LTP neurons, we have shown that the blockade of GABA andA
of monosynaptic and polysynaptic transmission induced by glycine receptors by their selective antagonists had two DHPG? One possibility is that the requisite NMDA effects on the synaptic actions of DHPG. First, this receptor component is contributed by background NMDA treatment enhanced the size of the initial DHPG-induced receptor-mediated currents elicited by synaptically released potentiation of polysynaptic EPSPs and EPSPs evoked by glutamate and that NMDA receptor antagonists block these stimuli at C-fiber strength, and second, it greatly facilitated currents. Alternatively, tonic activation of synaptic and the likelihood and magnitude of the DHPG-induced long-extrasynaptic NMDA receptors might arise from the lasting potentiation of EPSPs. Although the mechanism(s) background levels of glutamate released from adjacent glia by which the absence of synaptic inhibition facilitates the cells [77]. Although the site(s) of interaction between DHPG-induced increase of synaptic responses has not been NMDA and group I mGlu receptors has not been examined explored in the present study, we propose that the facilita-in the present study, anatomical and other evidence tion is likely a result of a combination of direct excitatory suggests that the interactions can occur either presynapti- effects of DHPG on DH cells, and a decrease in inhibition. cally and / or postsynaptically. In agreement with post- This suggestion is supported by two findings: (1) In the synaptic presence of both group I mGlu and NMDA presence of bicuculline and strychnine DHPG increases receptors in the DH [46,58,89,94], a positive postsynaptic cell excitability and magnitude of EPSPs in DH neurons, interaction between NMDA and group I mGlu receptors and (2) DHPG decreases primary afferent-evoked IPSPs has been described in DH neurons [5,8,12,32,48,96]. recorded in the absence (Fig. 6) and the presence of the Besides the mutual amplification of receptor function, the ionotropic glutamate receptor antagonists (20mM CNQX, co-activation of NMDA and mGlu receptors may also 50mM D-AP-5). These observations are in general
agree-result in the enhancement of the effects mediated by ment with a recent report that disinhibition of spinal dorsal intracellular messengers, including calcium [13,42,63]. We horn GABA-ergic and glycinergic systems may facilitate have recently shown that DHPG increases NMDA-me- recruitment of NMDA-sensitive polysynaptic circuits [2].
21
diated Ca transients in the substantia gelatinosa neurons At present the mechanism(s) by which DHPG elicits [32]. Thus, the DHPG-induced depression / potentiation of depressant action on IPSPs is unknown. In the hippocam-synaptic transmission could be due to increase in cytosolic pus, the mGlu receptor-induced disinhibition is mediated
21 21
[Ca ] that may have been achieved either via Ca influx by reduced transmission at excitatory synapses onto
inhib-21
through NMDA receptor channels, or Ca release from itory interneurons and inhibitory synapses onto pyramidal the internal stores following activation of PLC–IP path-3 cells involved in polysynaptic pathways [18,19]. However,
21
way. Furthermore, a Ca dependence in mGlu receptor- in the striatum, Stefani et al. [90] have shown that the stimulated phosphoinositide breakdown in synaptosomes activation of mGlu receptors inhibits GABA-mediated has also been demonstrated [95], so it is possible that synaptic potentials in the presence of ionotropic glutamate NMDA receptor and mGlu receptor interactions can occur receptor antagonists via inhibition of the GABA release. presynaptically, consistent with the location of both group In summary, it is clear from both immunohistochemical I mGlu and NMDA receptors in DRG neurons [92,93], and [46] and present data that the net facilitatory effect of on primary afferent terminals [46,58]. group I mGlu receptor agonists reported in our pharmaco-In summary, the present work has revealed a further logical experiments must emerge from complex interaction degree of complexity in the interactions between glutamate involving a variety of mediators with different and oppos-receptors at a central glutamatergic synapse. The finding ing effects. Our results suggest a potential role of the that activation of NMDA receptors can facilitate the dorsal horn GABA-ergic and glycinergic interneurons in activation of group I mGlu receptors may have implica- providing spatial and temporal conditions for modifications tions for synaptic plasticity [6,59,79,82] and central sen- of synaptic strength during plasticity in information pro-sitization of DH neurons, a phenomenon that contributes to cessing in the somatosensory system as illustrated by pain [62]. nociception-related phenomena such as sensitization,
hy-peralgesia, and allodynia [78]. 4.4. Involvement of synaptic inhibition in group I mGlu
receptor-induced potentiation of polysynaptic EPSP
4.5. Pharmacological identification of mGlu receptors One of the prevalent mechanisms for the inhibition of regulating glutamatergic neurone excitability: group I primary afferent transmission is through polysynaptic mGlu receptors
inhibitory pathways mediated by GABAA and glycine
in-crease in membrane noise and a burst firing, in a majority synaptic transmission through neuronal circuits and pro-of DH neurons, which effects in the case pro-of DHPG were moting of synaptic plasticity.
accompanied by an increase in membrane input resistance. In addition, both 1S,3R-ACPD and DHPG induced a
spontaneous oscillatory activity in about one-third of tested 4.6. Synaptic plasticity induced by group I mGlu superficial DH and a greater proportion of DDH neurons. receptor activation: potential role in the superficial A direct excitatory effect of 1S,3R-ACPD with induction spinal dorsal horn
of intense neuronal discharges [51] and oscillations was
previously reported for deep DH neurons in the rat spinal Superficial spinal dorsal horn (SDH, laminae I and II) is slice preparation [64–66], and for DH neurons in the slices involved in modulation of nociceptive information, but of the turtle spinal cord [85]. Our results obtained in the synaptic and cellular mechanisms underlying the changes presence of TTX, CNQX, and D-APV suggest that the responsible for this function are not well understood.
depolarization is independent of AMPA / KA or NMDA Neuromodulation by substances released by primary affer-receptor activation, and that the effect of DHPG is likely to ents is thought to play a critical role in the development of result from activation of the group I mGlu subtype(s) on plasticity induced by nociceptive inputs [22,82]. The spinal the DH neurons themselves, rather than on afferent fibers mechanisms contributing to nociception-related phenom-presynaptic to these cells. ena (central sensitization, hyperalgesia, allodynia, analge-The agonist and antagonist pharmacology of the mGlu sia) are modifications in synaptic efficacy produced by receptor(s) responsible for the excitatory effects in DH activity-dependent changes [22] and by modulatory effects neurons is similar to that of the mGlu receptors that of neurotransmitters [82]. In addition, the modulation of mediate depolarization of spinal cord motoneurons intrinsic properties of neurons provides a potential post-[4,23,43–45,50,80]. In both sets of neurons, the effective- synaptic site of plasticity [64,66,85].
ness of the agonists tested correlates well with their Long-term synaptic plasticity (LTP and LTD) following capacity to generate the production of IP in brain slices3 stimulation of primary afferents, peripheral nerve, or [88] and to activate group I mGlu receptors in transfected induced by noxious stimulation or nerve injury, has been cells [91]. Taken together, the agonist and antagonist reported both in vitro and in vivo in the rat spinal cord. pharmacology strongly implicates both subtypes 1 and 5 of There is indication that LTP of excitatory synaptic trans-group I mGlu receptors, in the mediation of postsynaptic mission may play a role in the generation of post-injury depolarization in DH neurons. Group I mGluRs are also hypersensitivity, and LTD in antinociception [32,82,86]. the predominant mGlu receptors involved in increasing The cellular mechanisms underlying LTP and LTD are still excitability of cells in various brain regions [1,16,79]. not well understood. Metabotropic glutamate receptors are Although the ionic mechanism of depolarization has not thought to play a role in modulation of neuronal excitabili-been investigated in the present study, there is a large body ty and synaptic transmission in the brain, and contribute to of evidence indicating that activation of mGlu receptors by regulation of function in neural networks [79] including stimulation of glutamatergic afferents or exogenous appli- nociceptive circuits in the spinal cord DH [11,61,102]. The cation of mGlu receptor agonists increases neuronal ex- present in vitro intracellular data, taken together with citability by modulation of a variety of ion channels [1,16]. available in vivo evidence from extracellular studies mGlu receptors can exert direct excitatory effects on [11,68,69,74,102–105] suggest the presence of functional neurons by activation of non-selective cation currents, group I mGlu receptors on young rat spinal DH neurons,
21
including activation of a Ca -activated nonspecific cation that may play a role in induction of long-term changes of current [1,17,38,66,67,81,107]. In the hippocampus, reduc- responses to innocuous and noxious stimuli resulting in
1
tion of three K conductances have been suggested to hyperalgesia, allodynia, and analgesia. Our present study mediate the 1S,3R-ACPD-induced depolarization, an M- shows that both the mono- and polysynaptic responses in like current, a calcium-dependent slow after-hyperpolariz- the superficial DH are modulated by group I mGlu
1
molecular switch activated by metabotropic glutamate receptors
5. Conclusion
regulates induction of long-term potentiation, Nature 368 (1994) 740–743.
Superficial spinal dorsal horn is involved in the modula- [10] S.J. Boxall, S.W.N. Thompson, A. Dray, A.H. Dickenson, L. Urban, tion of nociceptive information but little is known regard- Metabotropic glutamate receptor activation contributes to
nocicep-ing the mechanisms underlynocicep-ing the changes responsible for tive reflex activity in the rat spinal cord in vitro, Neuroscience 74 (1996) 13–20.
this function. The precise role(s) of the synaptic activation
[11] S.J. Boxall, A. Berthele, D.J. Laurie, B. Sommer, W.
of metabotropic glutamate receptors in the regulation of
¨
Zieglgansberger, L. Urban, T.R. Tolle, Enhanced expression of
synaptic transmission and activity-dependent plasticity in metabotropic glutamate receptor 3 messenger RNA in the rat spinal this spinal cord region remains to be determined. However, cord during ultraviolet irradiation induced peripheral inflammation, the present demonstration that activation of metabotropic Neuroscience 82 (1998) 591–602.
´
[12] R. Cerne, M. Randic, Modulation of AMPA and NMDA responses
glutamate receptors can induce a long-lasting potentiation
in rat spinal dorsal horn neurons by
trans-1-aminocyclopentane-1,3-and a long-lasting depression raises the intriguing
possi-dicarboxylic acid, Neurosci. Lett. 144 (1992) 180–184.
bility that metabotropic glutamate receptors may be in- [13] R.A.J. Challiss, R. Mistry, D.W. Gray, S.R. Nahorski, Modulatory volved in the mechanisms underlying potentiation and effects of NMDA on phosphoinositide responses evoked by the depression of neuronal responses that are associated with metabotropic glutamate receptor agonist 1S,3R-ACPD in neonatal
rat cerebral cortex, Br. J. Pharmacol. 112 (1994) 231–239.
nociception-related phenomena such as hyperalgesia and
¨ ¨
[14] S. Charpak, B.H. Gahwiler, K. Do, T. Knopfel, Potassium
conduct-analgesia.
ances in hippocampal neurons blocked by excitatory amino acid transmitters, Nature 347 (1990) 765–767.
[15] D.R. Collins, Actions of agonists of metabotropic glutamate
re-Acknowledgements ceptors on synaptic transmission and transmitter release in the olfactory cortex, Br. J. Pharmacol. 108 (1993) 422–430.
[16] P.J. Conn, J.P. Pin, Pharmacology and functions of metabotropic
We thank Don-ho Youn for valuable help with
prepara-glutamate receptors A, Rev. Pharmacol. Toxicol. 37 (1997) 205–
tion of the manuscript. This effort was supported by grants
237.
from the National Science Foundation (IBN 9604654) and [17] V. Crepel, L. Aniksztejn, Y. Ben-Ari, C. Hammond, Glutamate
21
the Paralyzed Veterans of America Spinal Cord Research metabotropic receptors increase a Ca -activated non-specific
cat-Foundation (SCRF 1738). ionic current in CA1 hippocampal neurons, J. Neurophysiol. 72 (1994) 1561–1569.
[18] M.A. Desai, P.J. Conn, Excitatory effects of ACPD receptor activation in the hippocampus are mediated by direct effects on
References pyramidal cells and blockade of synaptic inhibition, J. Neurophysiol.
66 (1991) 40–52.
[1] R. Anwyl, Metabotropic glutamate receptors: electrophysiological [19] M.A. Desai, C.J. McBain, J.A. Kauer, P.J. Conn, Metabotropic properties and role in plasticity, Brain Res. Rev. 29 (1999) 83–120. glutamate receptor induced disinhibition is mediated by reduced [2] R. Bardoni, P.C. Magherin, A.B. MacDermott, Synaptic activation transmission at excitatory synapses onto interneurons and inhibitory of NMDA receptors drives action potential firing in the superficial synapses onto pyramidal cells, Neurosci. Lett. 181 (1994) 78–82. dorsal horn of the rat during early postnatal development, Eur. J. [20] A.J. Doherty, M.J. Palmer, J.M. Henley, G.L. Collingridge, D.E. Neurosci. (in press). Jane, (RS )-2-chloro-5-hydroxyphenylglycine (CHPG) activates [3] Z.I. Bashir, Z.A. Bortolotto, C.H. Davies, N. Berretta, A.J. Irving, mGlu5 but not mGlu1 receptors expressed in CHO cells and A.J. Seal, J.M. Henley, D.E. Jane, J.C. Watkins, G.L. Collingridge, potentiates NMDA responses in the hippocampus, Neuropharmacol-Induction of LTP in the hippocampus needs synaptic activation of ogy 36 (1997) 265–267.
glutamate metabotropic receptors, Nature 363 (1993) 347–350. [21] T.P. Doubell, R.J. Mannion, C.J. Woolf, The dorsal horn: state-[4] E.F. Birse, S.A. Eaton, D.E. Jane, P.L.St.J. Jones, R.H.P. Porter, dependent sensory processing, plasticity and the generation of pain, P.C.-K. Pook, D.C. Sunter, P.M. Udvarhelyi, B. Wharton, P.J. in: P.D. Wall, R. Melzack (Eds.), Textbook of Pain, 4th Edition, Roberts, T.E. Salt, J.C. Watkins, Phenylglycine derivatives as new Churchill-Livingstone, Edinburgh, 1999, pp. 165–181.
pharmacological tools for investigating the role of metabotropic [22] R. Dubner, M.A. Ruda, Activity-dependent neuronal plasticity glutamate receptors in the central nervous system, Neuroscience 52 following tissue injury and inflammation, Trends Neurosci. 15
(1993) 481–488. (1992) 96–103.
[5] D. Bleakman, K.I. Rusin, P.S. Chard, S.R. Glaum, R.J. Miller, [23] S.A. Eaton, D.E. Jane, P.L.St.J. Jones, R.H.P. Porter, P.C. Pook, D.C. Metabotropic glutamate receptors potentiate ionotropic glutamate Sunter, P.M. Udvarhelyi, P.J. Roberts, T.E. Salt, J.C. Watkins, responses in the rat dorsal horn, Mol. Pharmacol. 42 (1992) 192– Competitive antagonism at metabotropic glutamate receptors by
196. (S )-4-carboxyphenylglycine and (RS )-a
-methyl-4-carbox-[6] T.V.P. Bliss, G.L. Collingridge, A synaptic model of memory: yphenylglycine, Eur. J. Pharmacol. 244 (1993) 195–197.
long-term potentiation in the hippocampus, Nature 361 (1993) [24] K. Fisher, T.J. Coderre, The contribution of metabotropic glutamate 31–39. receptors (mGluRs) to formalin-induced nociception, Pain 68 (1996) [7] V.Y. Bolshakov, S.A. Siegelbaum, Postsynaptic induction and pre- 255–263.
synaptic expression of hippocampal long-term depression, Science [25] K. Fisher, T.J. Coderre, Comparison of nociceptive effects produced 264 (1994) 1148–1152. by intrathecal administration of mGluR agonists, NeuroReport 7 [8] A. Bond, D. Lodge, Pharmacology of metabotropic glutamate (1996) 2743–2747.
receptor-mediated enhancement of responses to excitatory and [26] K. Fisher, T.J. Coderre, Hyperalgesia and allodynia induced by inhibitory amino acids on rat spinal neurones in vivo, Neuro- intrathecal (RS )-dihydroxyphenylglycine in rats, NeuroReport 9 pharmacology 34 (1995) 1015–1023. (1998) 1169–1172.
)-S-DHPG-induced nociception depends on the release of glutamate from neonatal rat motoneurones and rat thalamic neurones, Neurophar-primary afferent C-fibers, Soc. Neurosci. Abstr. 24 (1998) 1869. macology 32 (1993) 725–727.
[28] M.E. Fundytus, K. Fisher, A. Dray, J.L. Henry, T.J. Coderre, In vivo [45] D.E. Jane, P.L.St.J. Jones, P.C.-K. Pook, H.-W. Tse, J.C. Watkins, antinociceptive activity of anti-rat mGluR1 and mGluR5 antibodies Actions of two new antagonists showing selectivity for different in rats, NeuroReport 9 (1998) 731–735. sub-types of metabotropic glutamate receptor in the neonatal rat
spinal cord, Br. J. Pharmacol. 112 (1994) 809–816. [29] M.E. Fundytus, M. Osborne, A. Dray, J.L. Henry, T.J. Coderre, An
antisense oligonucleotide targeting mGluR1 attenuates allodynia / [46] H. Jia, A. Rustioni, J.G. Valtschanoff, Metabotropic glutamate hyperalgesia in a model of chronic inflammation in rats, Soc. receptors in superficial laminae of the rat dorsal horn, J. Comp. Neurosci. Abstr. 24 (1998) 1870. Neurol. 410 (1999) 627–642.
¨ ¨
[30] F. Gasparini, K. Lingenhohl, N. Stoehr, P.J. Flor, M. Heinrich, I. [47] P. Jonas, J. Bischofberger, J. Sandkuhler, Corelease of two fast Vranesic, M. Biollaz, H. Allgeier, R. Heckendorn, S. Urwyler, M.A. neurotransmitters at a central synapse, Science 281 (1998) 419–424. Varney, E.C. Johnson, S.D. Hess, S.P. Rao, A.I. Sacaan, E.M. [48] M.W. Jones, P.M. Headley, Interactions between metabotropic and Santory, G. Velic¸elebi, R. Kuhn, 2-Methyl-6-(phenylethynyl)- ionotropic glutamate receptor agonists in the rat spinal cord in vivo, pyridine (MPEP): a potent, selective and systemically active mGlu5 Neuropharmacology 34 (1995) 1025–1031.
receptor antagonist, Neuropharmacology 38 (1999) 1493–1503. [49] I. Kangrga, M. Randic, Outflow of endogenous aspartate and´ ´
[31] G. Gerber, M. Randic, Excitatory amino acid-mediated components glutamate from the rat spinal dorsal horn in vitro by activation of of synaptically-evoked input from dorsal roots to deep dorsal horn low- and high-threshold primary afferent fibers. Modulation by neurons in the rat spinal cord slice, Neurosci. Lett. 106 (1989) m-opioids, Brain Res. 553 (1991) 347–352.
211–219. [50] A.E. King, X.H. Liu, Dual action of metabotropic glutamate
´
[32] G. Gerber, D.-H. Youn, C.H. Hsu, M. Randic, Spinal dorsal horn receptor agonists on neuronal excitability and synaptic transmission synaptic plasticity: involvement of group I metabotropic glutamate in spinal ventral horn neurons in vitro, Neuropharmacology 35
¨
receptors, in: J. Sandkuhler, B. Bromm, G.F. Gebhart (Eds.), Prog. (1996) 1673–1680.
Brain Res. 129 (2000) 115–133. [51] Lj. Kojic, M. Randic, Modulation of excitatory synaptic responses in´ ´ ´
[33] G. Gerber, J. Zhong, D.-H. Youn, M. Randic, Group II and group III rat spinal dorsal horn neurons by (1S,3R)-1-aminocyclopentane-1,3-metabotropic glutamate receptor agonists depress synaptic transmis- dicarboxylic acid, Soc. Neurosci. Abstr. 19 (1993) 1526.
sion in the rat spinal cord dorsal horn, Neuroscience 100 (2000) [52] P. Krieger, A. el Manira, S. Grillner, Activation of pharmacological-393–406. ly distinct metabotropic glutamate receptors depresses reticulos-[34] S.R. Glaum, R.J. Miller, Metabotropic glutamate receptors mediate pinal-evoked monosynaptic EPSPs in the lamprey spinal cord, J.
excitatory transmission in the nucleus of the solitary tract, J. Neurophysiol. 76 (1996) 3834–3841.
Neurosci. 12 (1992) 2251–2258. [53] C. Levenes, H. Daniel, F. Crepel, Long-term depression of synaptic´ [35] S.R. Glaum, R.J. Miller, Activation of metabotropic glutamate transmission in the cerebellum: cellular and molecular mechanisms
receptors produces reciprocal regulation of ionotropic glutamate and revisited, Prog. Neurobiol. 55 (1998) 79–91.
GABA responses in the nucleus of the tractus solitarius of the rat, J. [54] H. Li, H. Ohishi, A. Kinoshita, R. Shigemoto, S. Nomura, N. Neurosci. 13 (1993) 1636–1641. Mizuno, Localization of a metabotropic glutamate receptor, [36] H. Gotani, M. Kuno, F. Nakamura, S. Matsuura, Potentiation of mGluR7, in axon terminals of presumed nociceptive, primary excitatory postsynaptic potentials by a metabotropic glutamate afferent fibers in the superficial layers of the spinal dorsal horn: an receptor agonist (1S,3R-ACPD) in frog spinal motoneurons, Brain electron microscopic study in the rat, Neurosci. Lett. 223 (1997)
Res. 689 (1995) 281–288. 153–156.
1 ¨
[37] N.C. Guerineau, B.H. Gahwiler, U. Gerber, Reduction of resting K [55] D.J. Linden, Long-term synaptic depression in the mammalian brain, current by metabotropic glutamate and muscarinic receptors in rat Neuron 12 (1994) 457–472.
CA3 cells: mediation by G-proteins, J. Physiol. 474 (1994) 27–33. [56] X.-G. Liu, J. Sandkuhler, Characterization of long-term potentiation ¨
[38] N.C. Guerineau, J.-L. Bossu, B.H. Gahwiler, U. Gerber, Activation of C-fiber-evoked potentials in spinal dorsal horn of adult rat: of a nonselective cationic conductance by metabotropic gluta- essential role of NK1 and NK2 receptors, J. Neurophysiol. 78 matergic and muscarinic agonists in CA3 pyramidal neurons of the (1997) 1973–1982.
rat hippocampus, J. Neurosci. 15 (1995) 4395–4407. [57] X.-G. Liu, J. Sandkuhler, Activation of spinal N-methyl-D-aspartate [39] J. Harvey, M.J. Palmer, A.J. Irving, V.R.J. Clarke, G.L. Collingridge, or neurokinin receptors induces long-term potentiation of spinal
NMDA receptor dependence of mGlu-mediated depression of C-fibre-evoked potentials, Neuroscience 86 (1998) 1209–1216. synaptic transmission in the CA1 region of the rat hippocampus, Br. [58] H. Liu, H. Wang, M. Sheng, L.Y. Jan, Y.N. Jan, I. Basbaum, J. Pharmacol. 119 (1996) 1239–1247. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the [40] I. Herrero, M.T. Miras-Portugal, J. Sanchez-Prieto, Positive feed- spinal cord dorsal horn, Proc. Natl. Acad. Sci. USA 91 (1994)
back of glutamate exocytosis by metabotropic presynaptic receptor 8383–8387.
stimulation, Nature 360 (1992) 163–166. [59] R.C. Malenka, Synaptic plasticity in the hippocampus: LTP and [41] I. Herrero, M.T. Miras-Portugal, J. Sanchez-Prieto, Rapid desensiti- LTD, Cell 78 (1994) 535–538.
zation of the metabotropic glutamate receptor that facilitates gluta- [60] B. McCaffery, K. Cho, Z.A. Bortolotto, J.P. Aggleton, M.W. Brown, mate release in rat cerebrocortical nerve terminals, Eur. J. Neurosci. F. Conquet, G.L. Collingridge, Z.I. Bashir, Synaptic depression 6 (1994) 115–120. induced by pharmacological activation of metabotropic glutamate [42] A.J. Irving, G.L. Collingridge, J.G. Schofield, L-glutamate and receptors in the perirhinal cortex, Neuroscience 93 (1999) 977–984.
21
acetylcholine mobilise Ca from the same intracellular pool in [61] S.T. Meller, C.L. Dykstra, G.F. Gebhart, Acute mechanical hy-cerebellar granule cells using transduction mechanisms with differ- peralgesia is produced by coactivation of AMPA and metabotropic
21
ent Ca sensitivities, Cell Calcium 13 (1992) 293–301. glutamate receptors, NeuroReport 4 (1993) 879–882.
[43] M. Ishida, T. Saitoh, K. Shimamoto, Y. Ohfune, H. Shinozaki, A [62] M.J. Millan, The induction of pain: an integrative review, Prog. novel metabotropic glutamate receptor agonist: marked depression Neurobiol. 57 (1999) 1–164.
´
[64] V. Morisset, F. Nagy, Modulation of regenerative membrane prop- [83] M. Randic, M.C. Jiang, R. Cerne, Long-term potentiation and long-term depression of primary afferent neurotransmission in the erties by stimulation of metabotropic glutamate receptors in rat deep
rat spinal cord, J. Neurosci. 13 (1993) 5228–5241. dorsal horn neurons, J. Neurophysiol. 76 (1996) 2794–2798.
´
[84] M. Randic, J. Zhong, D.-H. Youn, G. Gerber, S.K. Park, J.M. [65] V. Morisset, F. Nagy, Nociceptive integration in the rat spinal cord:
Chung, Involvement of group I and II mGluRs in spinal dorsal horn role of non-linear membrane properties of deep dorsal horn neurons,
synaptic function following tissue or nerve injury, in: Proc. IASP Eur. J. Neurosci. 10 (1998) 3642–3652.
9th World Cong. on Pain, 1999, Abstr. 38. [66] V. Morisset, F. Nagy, Ionic basis for plateau potentials in deep
[85] R.E. Russo, F. Nagy, J. Hounsgaard, Modulation of plateau prop-dorsal horn neurons of the rat spinal cord, J. Neurosci. 19 (1999)
erties in dorsal horn neurones in a slice preparation of the turtle 7309–7316.
spinal cord, J. Physiol. Lond. 499 (1997) 459–474. ´
[67] K. Murase, M. Randic, P.D. Ryu, Tachykinins modulate multiple
¨
[86] J. Sandkuhler, Synaptic mechanisms of hyperalgesia, Prog. Brain ionic conductances in voltage-clamped rat spinal dorsal horn
neu-Res. 129 (2000) 81. rons, J. Neurophysiol. 61 (1989) 854–865.
¨ ´
[87] J. Sandkuhler, J.G. Chen, G. Cheng, M. Randic, Low-frequency [68] V. Neugebauer, P.-S. Chen, W.D. Willis, Role of metabotropic
stimulation of afferent Ad fibers induces long-term depression at glutamate receptor subtype mGluR1 in brief nociception and central
primary afferent synapses with substantia gelatinosa neurons in the sensitization of primate STT cells, J. Neurophysiol. 82 (1999)
rat, J. Neurosci. 17 (1997) 6483–6491. 272–282.
[88] D.D. Schoepp, J. Goldsworthy, B.G. Johnson, C.R. Salhoff, R.S. ¨
[69] V. Neugebauer, T. Lucke, H.-G. Schaible, Requirement of metabot- Baker, 3,5-Dihydroxyphenylglycine is a highly selective agonist for ropic glutamate receptors for the generation of inflammation-evoked phosphoinositide-linked metabotropic glutamate receptors in the rat hyperexcitability in rat spinal cord neurons, Eur. J. Neurosci. 6 hippocampus, J. Neurochem. 63 (1994) 769–772.
(1994) 1179–1186. [89] R. Shigemoto, S. Nakanishi, N. Mizuno, Distribution of the mRNA [70] H. Ohishi, S. Nomura, Y.Q. Ding, R. Shigemoto, E. Wada, A. for a metabotropic glutamate receptor (mGluR1) in the central Kinoshita, J.L. Li, A. Neki, S. Nakanishi, N. Mizuno, Presynaptic nervous system: an in situ hybridization study in adult and develop-localization of a metabotropic glutamate receptor, mGluR7, in the ing rat, J. Comp. Neurol. 322 (1992) 121–135.
primary afferent neurons: an immunohistochemical study in the rat, [90] A. Stefani, A. Pisani, N.B. Mercuri, G. Bernardi, P. Calabresi, Neurosci. Lett. 202 (1995) 85–88. Activation of metabotropic glutamate receptors inhibits calcium [71] S.H.R. Oliet, R.C. Malenka, R.A. Nicoll, Two distinct forms of currents and GABA-mediated synaptic potentials in striatal neurons,
long-term depression coexist in CA1 hippocampal pyramidal cells, J. Neurosci. 14 (1994) 6734–6743.
Neuron 18 (1997) 969–982. [91] Y. Tanabe, A. Nomura, M. Masu, R. Shigemoto, N. Mizuno, S. [72] S.M. O’Mara, M.J. Rowan, R. Anwyl, Metabotropic glutamate Nakanishi, Signal transduction, pharmacological properties, and receptor-induced homosynaptic long-term depression and depoten- expression patterns of two rat metabotropic glutamate receptors, tiation in the dentate gyrus of the rat hippocampus in vitro, mGluR3 and mGluR4, J Neurosci. 13 (1993) 1372–1378. Neuropharmacology 34 (1995) 983–989. [92] A. Valerio, M. Paterlini, M. Boifava, M. Memo, P.F. Spano, [73] L.S. Overstreet, J.F. Pasternak, P.A. Colley, N.T. Slater, B.L. Metabotropic glutamate receptor mRNA expression in rat spinal
Trommer, Metabotropic glutamate receptor mediated long-term cord, NeuroReport 8 (1997) 2695–2699.
¨ depression in developing hippocampus, Neurophysiology 36 (1997) [93] A. Valerio, P. Rizzonelli, M. Paterlini, G. Moretto, T. Knopfel, R.
831–844. Kuhn, M. Uemo, P.F. Spano, mGluR5 metabotropic glutamate
[74] J. Palecek, V. Paleckova, P.M. Dougherty, W.D. Willis, The effect of receptor distribution in rat and human spinal cord: a developmental
trans-ACPD, a metabotropic excitatory amino acid receptor agonist, study, Neurosci. Res. 28 (1997) 49–57.
´ ´ ´ ¨ ¨
on the responses of primate spinothalamic tract neurons, Pain 56 [94] Z. Vidnyanszky, J. Hamori, L. Negyessy, D. Ruegg, T. Knopfel, R. ¨
(1994) 261–269. Kuhn, T.J. Gorcs, Cellular and subcellular localization of the [75] M.J. Palmer, A.J. Irving, G.R. Seabrook, D.E. Jane, G.L. Collin- mGluR5a metabotropic glutamate receptor in the rat spinal cord,
gridge, The group I mGlu receptor agonist S-DHPG induces a novel NeuroReport 6 (1994) 209–213.
21 form of LTD in the CA1 region of the hippocampus, Neuro- [95] M. Vignes, E. Blanc, M. Recasens, Stimulation of Ca -activated pharmacology 36 (1997) 1517–1532. non-specific cationic channels by phospholipase C-linked glutamate [76] J.-S. Park, T. Nakatsuka, K. Nagata, H. Higashi, M. Yoshimura, receptors in synaptoneurosomes? Eur. J. Neurosci. 7 (1995) 1791–
Reorganization of the primary afferent termination in the rat spinal 1802.
dorsal horn during post-natal development, Dev. Brain Res. 113 [96] N. Voitenko, S.D. Hocherman, G. Gerber, J. Zhong, I. Sonea, M.
21 ´
(1999) 29–36. Randic, Increase in intracellular free Ca concentration elicited by [77] V. Parpura, T.A. Basarsky, P.G. Haydon, Astrocyte-neuron sig- group I metabotropic glutamate receptors activation in the rat spinal
naling, in: F. Conti, T.P. Hicks (Eds.), Excitatory Amino Acids and dorsal horn, Soc. Neurosci. Abstr. 24 (1998) 586.
the Cerebral Cortex, MIT Press, Cambridge, MA, 1996, pp. 167– [97] P.J. Waddell, S.N. Lawson, Electrophysiological properties of
sub-174. populations of rat dorsal root ganglion neurons in vitro,
Neuro-[78] O. Paulson, E.J. Moser, A model of hippocampal memory encoding science 36 (1990) 811–822.
and retrieval: GABA-ergic control of synaptic plasticity, Trends [98] W.D. Willis, R.E. Coggeshall, Sensory Mechanisms of the Spinal Neurosci. 21 (1998) 273–278. Cord, 2nd Edition, Plenum Press, New York, 1991.
[79] J.P. Pin, R. Duvoisin, The metabotropic glutamate receptors: struc- [99] S. Yoshida, Tetrodotoxin-resistant sodium channels, Cell Mol. tures and functions, Neuropharmacology 34 (1995) 1–26. Neurobiol. 14 (1994) 227–244.
[80] P.C. Pook, D.C. Sunter, P.M. Udvarhelyi, J.C. Watkins, Evidence for [100] M. Yoshimura, T.M. Jessell, Amino acid-mediated EPSPs at presynaptic depression of monosynaptic excitation in neonatal rat primary afferent synapses with substantia gelatinosa neurones in motoneurones by (1S,3S )- and (1S,3R)-ACPD, Exp. Physiol. 77 the rat spinal cord, J. Physiol. Lond. 430 (1990) 315–335. (1992) 529–532. [101] M. Yoshimura, S. Nishi, Primary afferent-evoked glycine- and [81] L.D. Pozzo-Miller, J.J. Petrozzino, J.A. Connor, G protein-coupled GABA-mediated IPSPs in substantia gelatinosa neurones in the rat
receptors mediate a fast excitatory postsynaptic current in CA3 spinal cord in vitro, J. Physiol. Lond. 482 (1995) 29–38. pyramidal neurons in hippocampal slices, J. Neurosci. 15 (1995) [102] M.R. Young, S.M. Fleetwood-Walker, R. Mitchell, F.E. Munro, 8320–8330. Evidence for a role of metabotropic glutamate receptors in
sus-´
[103] M.R. Young, S.M. Fleetwood-Walker, R. Mitchell, T. Dickinson, spinal nociceptive transmission, J. Neurosci. 18 (1998) 10180– The involvement of metabotropic glutamate receptors and their 10188.
intracellular signalling pathways in sustained nociceptive transmis- [106] F. Zheng, J.P. Gallagher, Metabotropic glutamate receptors are sion in rat dorsal horn neurons, Neuropharmacology 34 (1995) required for the induction of long-term potentiation, Neuron 9
1033–1041. (1992) 163–172.
[104] M.R. Young, S.M. Fleetwood-Walker, T. Dickinson, G. Blackburn- [107] F. Zheng, J.P. Gallagher, Pharmacologically distinct, pertussis Munro, H. Sparrow, P.J. Birch, C. Bountra, Behavioural and toxin-resistant inward currents evoked by metabotropic glutamate electrophysiological evidence supporting a role for group I receptor (mGluR) agonists in dorsolateral septal nucleus (DLSN) metabotropic glutamate receptors in the mediation of nociceptive neurons, J. Neurosci. 15 (1995) 504–510.
´