Improved plant regeneration from cultured leaf segments in peanut
(
Arachis hypogaea
L.) by limited exposure to thidiazuron
Yoko Akasaka
a, Hiroyuki Daimon
b, Masahiro Mii
a,*
aLaboratory of Plant Cell Technology,Faculty of Horticulture,Chiba Uni6ersity,Matsudo,Chiba 271-8510, Japan bCollege of Agriculture,Osaka Prefecture Uni6ersity,Sakai,Osaka 599-8531, Japan
Received 1 November 1999; received in revised form 18 January 2000; accepted 6 March 2000
Abstract
Bud primordia were induced from leaf segments, which were harvested from young seedlings of Spanish type peanut (Arachis hypogaeaL. cv. Chico), on 0.8% agar-solidified medium containing Murashige and Skoog (MS) basal salts supplemented with B5 vitamins, 1 mg/l NAA and various cytokinins such as benzyladenine (BA), isopentenyladenine (2ip), kinetin (KIN), chloropy-ridylphenylurea (4PU), thidiazuron (TDZ), zeatin (ZTN) in different concentrations. Among the cytokinins tested, TDZ was found to be the most efficient for inducing bud primordia. However, continuous culture on TDZ-containing media induced abnormal development of these primordia, and they failed to grow into plantlets. Histological observations revealed that the malformation most often obtained was a shoot-like structure which lacked shoot apical meristem (SAM) and had disorganized vascular bundles. For normal shoot regeneration, it was necessary to limit the culture period of the explants on TDZ-containing medium to 7 days at 10 mg/l or 21 days at 1 mg/l and then transfer them onto plant growth regulator-free medium. The percentage of conversion from shoot buds to shoots was 34.7%. When shoots were removed from the explants and transferred onto basal medium containing 1 mg/l NAA, all regenerated shoots readily rooted and successfully acclimatized. All of the acclimatized plants produced viable seeds in the greenhouse condition. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Peanut; Plant regeneration; Shoot conversion; Thidiazuron
www.elsevier.com/locate/plantsci
1. Introduction
Peanut (Arachis hypogaea L.) is a grain legume
crop important for its high content of seed protein and oil. Due to increasing demand, it is expected that novel cultivars with improved agronomic characteristics such as resistance to diseases and pests and drought tolerance will be produced. Although there are some wild species which have
desirable characteristics for breeding Arachis,
ge-netic improvement by utilizing these wild relatives has been limited because of problems with sterile
genomic incompatibility between peanut and its
wild relatives. Consequently, unconventional
breeding methods such as genetic transformation are expected to be applied, and the establishment of a reliable plant regeneration system in peanut is now needed.
The cultivated peanut is known to be relatively recalcitrant for regenerating plants in tissue cul-ture, although some successful results in plantlet regeneration have been reported via organogenesis [1 – 4] and embryogenesis [5 – 9] from various ex-plants. Cheng et al. [3] reported that bud primor-dia were easily induced from cut surface of leaf segments although the primordia showed abnor-mal development and failed to grow into norabnor-mal shoots. Chengalrayan et al. [10] also reported that the shoot buds from leaf cultures of peanut re-mained stunted, resulting in poor plant regenera-Abbre6iations: BA, benzyladenine; 2ip, isopentenyladenine; KIN,
kinetin; PGR, plant growth regulator; 4PU, chloropyridylphenylurea (CPPU); SAM, shoot apical meristem; TDZ, thidiazuron; ZTN, zeatin.
* Corresponding author. Tel.: +81-47-3088854; fax: 81-47-3088852.
E-mail address:[email protected] (M. Mii).
tion. For genetic transformation studies, leaf seg-ments isolated from young seedlings have widely been used as the target materials. However, the frequency of plant recovery from shoots regener-ated from leaf segments so far reported are not so high (0.2 – 0.3%) due to the difficulty in conversion from bud primordia to shoots [11].
In this paper we report the effect of different cytokinins and duration of treatment with thidi-azuron (TDZ) on bud primordia formation and shoot conversion from leaf segments of peanut. We also report the results on histological and morphological observations of stunted shoot-like structures produced from the leaf segments. Con-sequently, we have succeeded in establishing an efficient protocol for plant regeneration from leaf segments in peanut, the efficiency of which is significantly higher than those previously reported.
2. Materials and methods
2.1. Plant materials and culture conditions
Explant tissues were leaflets of seedlings ob-tained from germinated embryo axes dissected from mature peanut seeds of ‘Chico’, a Spanish type cultivar. The seeds were surface-sterilized for 10 min with sodium hypochlorite solution (1% active chlorine) containing a drop of Tween 20 and rinsed three times with sterile deionized water. After removing seed coats, the seeds were
germi-nated in culture bottles (130×80 mm) containing
100 ml of 0.8% (w/v) agar-solidified Murashige
and Skoog [12] (MS) basal medium with B5
vita-mins [13] and 3% (w/v) sucrose by inoculating five
seeds for each bottle. Media were adjusted to pH 5.8 and autoclaved. All the cultures were kept at
25°C under continuous light condition (60 mmol
photons/m2 per s).
Leaflets excised from 6 – 8-day-old seedlings thus obtained were cut transversely into three pieces by intersecting the midvein. The explants were placed on media with the abaxial side down. The media used for inducing bud primordia were MS basal
medium containing 1 mg/l NAA and various
cy-tokinins of different concentrations. Each explant
was placed on 10 ml medium per test tube (100×
25 mm), which was sealed with aluminum foil. After culture on these media for different dura-tions, the explants were transferred to the basal
medium without plant growth regulators (PGRs) for development of shoots, and then transferred monthly to the same basal medium. Shoots were removed from shoot clumps and transferred onto 1% agar-solidified basal medium supplemented
with 1% sucrose and 1 mg/l NAA for rooting.
Regenerated plants were transferred to pots con-taining a 1:1 mixture of perlite: vermiculite wetted by water for acclimatization and grown in the greenhouse.
2.2. Effect of 6arious cytokinins on bud primordia formation
Induction of bud primordia formation from leaf segments was examined on media containing dif-ferent kinds and concentrations of cytokinins in-cluding benzyladenine (BA), isopentenyladenine
(2ip), kinetin (KIN), chloropyridylphenylurea
(4PU), thidiazuron (TDZ), zeatin (ZTN) (Table 1). All PGRs used, except for 2ip and 4PU, were obtained from Wako (Tokyo, Japan). 2ip and 4PU were obtained from Sigma (St Louis, USA) and Kyowa Hakko Kogyo (Tokyo, Japan), re-spectively. 2ip, 4PU, KIN and ZTN were filter-sterilized and added to medium after autoclaving. After 40 days of culture, the explants were trans-ferred onto basal medium. Each treatment con-sisted of 30 explants and the experiment was repeated three times. The frequency of bud pri-mordia formation was determined as the percent-age of cultures showing bud primordia after 3 weeks of culture. The frequency of shoot regenera-tion was determined as the percentage of cultures showing shoot elongation from bud primordia within 2 months after transfer to basal medium without PGRs.
2.3. Effect of the duration of exposure to TDZ on
shoot regeneration
For shoot regeneration from bud primordia, leaf segments were cultured for various periods (0, 1, 3, 5, 7, 14, 21 and 28 days) on media containing
0.1, 1 or 10 mg/l TDZ with 1 mg/l NAA. After the
determined as the percentage of cultures showing shoot elongation from bud primordia after 2 months of culture.
2.4. Histological obser6ation with light microscopy
Bud primordia and shoot samples were fixed
with 3% (w/v) paraformaldehyde and 2% (v/v)
glutaraldehyde in 0.2 M cacodylate buffer at pH 7.2, dehydrated through a graded ethanol series, infiltrated and embedded in Technovit 7100 (Her-aeus Kulzer, Germany). Embedded samples were
sectioned into 5 – 10-mm thick segments with a
microtome (RM 2135 Leica, Germany), stained with 0.05% toluidine blue, viewed, and pho-tographed under a light microscope (IX-70, Olym-pus, Japan).
3. Results and discussion
3.1. Bud primordia formation and plant
regeneration
Small outgrowths were induced at the cut end of
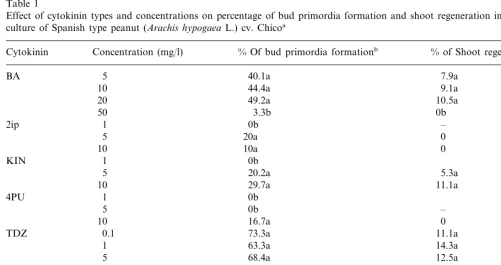
the midvein after 2 weeks of culture on medium containing any of the cytokinins tested. They eventually formed bud primordia (Fig. 1A), simi-lar to those described as ‘protuberances’ by Cheng et al. [3]. The bud primordia formed as a mass and it was difficult to count the number of buds per explant. There were differences in percentages of bud primordia formation after 3 weeks of culture among the cytokinin types tested (Table 1). High frequency bud primordia formation (40 – 80%) was obtained at all concentrations of BA and TDZ
except for 50 mg/l BA, at which almost all the
explants turned brown. The size of bud primordia produced on medium containing TDZ was larger than that in medium containing BA, and the time required for producing bud primordia in TDZ-containing medium was shorter than that in BA-containing medium. In other cytokinin treatments such as 2ip, KIN, 4PU and ZTN, the frequency of bud primordia formation was low. When bud primordia were transferred onto basal medium without PGRs after 40 days of culture, some of them developed into shoots with leaf segments
Table 1
Effect of cytokinin types and concentrations on percentage of bud primordia formation and shoot regeneration in leaf segment culture of Spanish type peanut (Arachis hypogaeaL.) cv. Chicoa
% Of bud primordia formationb
Concentration (mg/l) % of Shoot regenerationc
Cytokinin
aEach value represents the average of three replications. All values followed by the same letters are not significantly different
according to Tukey test atP=0.05. –, no value.
b% Of total explants showing bud primordia formation after 3 weeks of culture.
Fig. 1. Plant regeneration from leaf segments of Spanish type peanut (Arachis hypogaea L.) cv. Chico. (A) Bud primordia formation on basal medium containing 1 mg/1 NAA and 1 mg/1 TDZ after 21 days of culture (bar=l mm). (B) Differentiation of leaflets from leaf segment-derived bud primordia on basal medium containing no PGRs after 1 month of culture (bar=1 mm). (C) Shoot elongation from bud primordia 2 months after transfer onto basal medium containing no PGRs (bar=1 cm). Note the sequential development of leaves. (D) Root induction on the shoot 2 weeks after transfer onto basal medium containing 1 mg/1 NAA (bar=1 cm). (E) A regenerated plant in pot established under greenhouse condition. Arrow indicates the gynophore.
after 30 days after the transfer (Fig. 1B,C). The highest frequency of shoot regeneration 2 months
after the transfer was 14.3% at 1 mg/l TDZ (Table
1), although the value was not so high as those reported previously [4,10], suggesting that some buds failed to grow into normal shoots and plants.
3.2. Shoot regeneration by limited exposure with TDZ
Although high percentages of bud primordia formation were obtained by treatment with BA or TDZ in the present study, they did not always develop into normal shoots. To improve the fre-quency of plant regeneration, effects of duration and concentration of TDZ treatments on shoot
regeneration from the bud primordia were
examined.
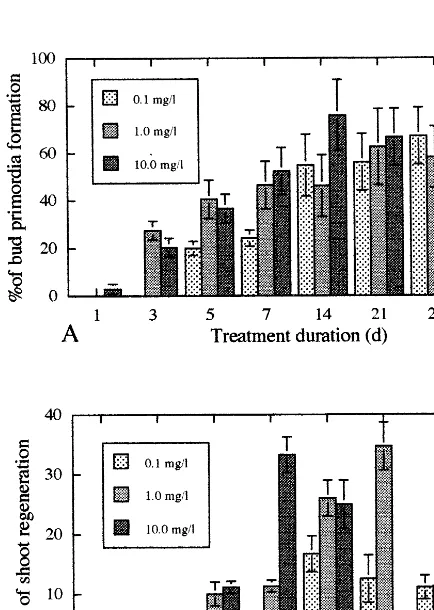
Both the concentration of TDZ in the induction medium and the period of culture on this medium influenced the frequencies of both bud primordia formation after 3 weeks of culture and shoot regeneration after 2 months of culture (Fig. 2A,B). The frequency of bud primordia formation in-creased with increasing concentration of TDZ and duration of treatment (Fig. 2A). When explants were cultured on induction medium supplemented
with TDZ either at 10 mg/l for 7 days or at 1 mg/l
for 21 days, bud primordia regenerated into shoots at the highest frequencies, 33.3 and 34.7%, respectively, and eventually grew into shoot
clumps (Fig. 2B). By the treatment with 1 mg/l
TDZ for 21 days, the highest number of elongated shoots (5.6) were produced per shoot clump 2 months after the transfer. Shoot elongation from shoot clumps continuously occurred on medium
shoots per shoot clump were obtained 4 months after the transfer on this medium. When the shoot clumps had been cultured for more than 21 days
on medium containing 10 mg/l TDZ, they did not
show any regeneration despite bud primordia for-mation at high frequency (Fig. 2A). These results indicate that the optimum level of TDZ for bud induction differed from that for shoot regenera-tion. Shoots normally developed and then
occa-sionally formed flower buds in vitro 2 months
after the transfer.
The normal shoots were detached from the shoot clumps and transferred to the basal medium
containing 1 mg/l NAA for further development
and rooting. Almost all the detached shoots rooted within 14 days after the transfer (Fig. 1D). Regenerated plantlets showed vigorous growth and normal phenotypes under the greenhouse con-dition. They produced gynophore which subse-quently formed pods with seeds (Fig. 1E).
3.3. Morphological and histological obser6ations on stunted shoot-like structures
In this study, normal development of shoots and buds was markedly inhibited and various abnormal morphologies such as embryoid, fused shoot or leaf without shoot apex were observed (Fig. 3A – D) in the culture with continuous expo-sure to cytokinins. The abnormal shoot morpholo-gies were classified into three types: (1) somatic embryo-like structure (Fig. 3A), (2) fused shoots which elongated slightly (Fig. 3B), and (3) ‘stalk’-like structure with expanded leaves, but lacking shoot apex (Fig. 3C and D). Although somatic embryo formation from cotyledonary node of peanut has previously been reported by treatment with TDZ [9], the embryo-like structures produced in the present study lacked root meristems and failed to germinate. Fused shoot formation ob-served in the present study has also been reported in other plant species associated with the use of TDZ [14]. The ‘stalk’-like structure lacking shoot apex was observed at higher frequencies than two other abnormal structures. Histological observa-tions showed that the ‘stalk’-like structure lacked shoot apical meristem (SAM) in longitudinal sec-tion (Fig. 3E). Furthermore, there were approxi-mately seven to eight vascular elements in the ‘stalk’-like structure without a regular arrange-ment (Fig. 3F), which was different from the vascular system of peanut epicotyl, since the epi-cotyl in peanut has been characterized by a large pith in the central area completely surrounded with vascular bundles [15]. As a similar structure, a complete single leaf with four to five leaflets (Fig. 3D) without differentiation of SAM at the base was occasionally formed with the ‘stalk’-like structure, these morphological and histological ob-servations suggest that the ‘stalk’-like structure was not the stem of the stunted shoot but the petiole of abnormal leaf.
In conclusion, we have succeeded in increasing the frequency of shoot regeneration by limiting the period of TDZ treatment to induce normal bud primordia from leaf segments. Furthermore, we revealed the nature of stunted shoot by histologi-cal and morphologihistologi-cal observations. We are now using this regeneration system in attempts to
transform peanut via Agrobacterium-mediated
gene transfer.
Fig. 3. Morphological and histological observations of abnormal shoot-like structures produced on leaf segments in continuous culture for 2 months on medium containing 1 mg/1 TDZ. (A) Embryo-like structures (bar=l mm). (B) Fused shoots (bar=l mm). (C) ‘Stalk’-like structure without shoot apex (bar=l mm). An arrow indicates ‘stalk’. (D) Differentiation of a tetrafoliated single leaf with a ‘stalk’ (arrow) (bar=l mm). (E) Longitudinal section of a stunted shoot showing the lack of SAM (bar=200 gm). (F) Transverse section of the ‘stalk’ which showed disorganized vascular elements (bar=500 gm). Arrows indicate vascular elements.
References
[1] L.A. Mroginski, K.K. Kartha, J.P. Shyluk, Regeneration of peanut (Arachis hypogaea) plantlets by in vitro cul-ture of immacul-ture leaves, Can. J. Bot. 59 (1981) 826 – 830. [2] H. Daimon, M. Mii, Multiple shoot formation and plantlet regeneration from cotyledonary node in peanut (Arachis hypogaeaL.), Jpn. J. Breed. 41 (1991) 461 – 466.
[3] M. Cheng, D.C.H. Hsi, G.C. Phillips, In vitro regenera-tion of Valencia-type peanut (Arachis hypogaeaL.) from cultured petioles, epicotyl sections and other seedling explants, Peanut Sci. 19 (1992) 82 – 87.
[5] P. Ozias-Akins, Plant regeneration from immature em-bryos of peanut, Plant Cell Rep. 8 (1989) 217 – 218. [6] C.M. Baker, H.Y. Wetzstein, Somatic embryogenesis
and plant regeneration from leaflets of peanut,Arachis hypogaea, Plant Cell Rep. 11 (1992) 71 – 75.
[7] K. Chengalrayan, S.S. Sathaye, S. Hazra, Somatic em-bryogenesis from mature embryo-derived leaflets of peanut (Arachis hypogaeaL.), Plant Cell Rep. 13 (1994) 578 – 581.
[8] C.M. Baker, R.E. Durham, J. Austin Burns, W.A. Par-rott, H.Y. Wetzstein, High frequency somatic embryoge-nesis in peanut (Arachis hypogaeaL.) using mature, dry seed, Plant Cell Rep. 15 (1995) 38 – 42.
[9] B.N.S. Murthy, S.J. Murch, P.K. Saxena, Thidiazuron-induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): endogenous growth regula-tor levels and significance of cotyledons, Physiol. Plant. 94 (1995) 268 – 276.
[10] K. Chengalrayan, V.B. Mhaske, S. Hazra, In vitro regu-lation of morphogenesis in peanut (Arachis hypogaea
L.), Plant Sci. 110 (1995) 259 – 268.
[11] M. Cheng, R.L. Jarret, Z. Li, A. Xing, J.W. Demski, Production of fertile transgenic peanut (Arachis hypo
-gaea L.) plants using Agrobacterium tumefaciens, Plant Cell Rep. 15 (1996) 653 – 657.
[12] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassay with tobacco tissue cultures, Phys-iol. Plant. 15 (1962) 473 – 497.
[13] O.L. Gamborg, R.A. Miller, K. Ojima, Nutrient require-ments of suspension cultures of soybean root cells, Exp. Cell Res. 50 (1968) 151 – 158.
[14] C.A. Huetteman, J.E. Preece, Thidiazuron: a potent cy-tokinin for woody plant tissue culture, Plant Cell Tiss. Org. Cult. 33 (1993) 105 – 119.
[15] J.A. Yarbrough, Arachis hypogaea. The seedling, its cotyledons, hypocotyl and roots, Am. J. Bot. 36 (1949) 758 – 772.