Solvent extraction of gadolinium as a complex with
di-n-butyldithiocarbamates
Mohammad Taufan*, Iwan Hastiawan, Yayah Mulyasih
Department of Chemistry, Padjadjaran University, 45363 Jatinangor, Sumedang, West Java, Indonesia *e-mail: [email protected]
Abstract
Gadolinium (Gd) is one of rare earth metals that can be collected in minerals such as monazite. This element using as contrast agents in medicinal application as a chelate with diethylenetriaminepentaacetic acid (DTPA). The effect of different pH values, ratio mole metals with ligand, and organic solvent in extraction gadolinium as a complex with di-n-butyldithiocarbamate (DBDTC) have been investigated. Separatory funnel was used for this experiment. Extraction was shaked by hand for about 10 minutes with amount of organic solvent 2 x 5 mL. DBDTC was added into Gd solution in separatory funnel before adding organic solvent. Concentration of gadolinium in liquid phase analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES). From this resesarch showed that optimum condition extraction are pH 6, petroleum ether as solvent of extraction, and ratio mole metal with ligand 1:3. In this condition, extraction of Gd obtained maximum extraction 20,8 %. As a conclusion DBDTC is less efficiency as an alternative chelating extractants for Gd separation.
Keywords: Di-n-butyldithiocarbamate, gadolinium, ICP-AES, rare earths, solvent extraction
Introduction
Interest in the production of individual rare earth (RE) elements has increased in the last years, due to the advances in technological applications of RE. Gadolinium is employed in the medical field as a contrasting agent in images obtained by magnetic resonance, as well as in the nuclear area, as a thermal neutrons absorber (Morais and Ciminelli, 2007).
The RE elements occur together in nature in some minerals like bastnasite, monazite, xenotime and others (Morais and Ciminelli, 2004). The high value of these elements depends on their effective separation into high purity compounds. The separation of the natural RE mixtures into the individual elements is very difficult to achieve, due to the very low separation factors involving the adjacent RE elements. The extraction behavior of rare earths has been studied since the 1950s. Liquid-liquid extraction is a separation technique based on the distribution of a solute between two phases: aqueous and organic, practically immiscible with each other. The organic phase generally contains a diluent and an extractant capable of combining differently with RE elements, then forming compounds that are more soluble, i.e., show greater affinity with the organic phase.
There are several reports on the separation of rare earth elements in different media and extractants, such as: phosphoric, phosphonic and phosphinic acids, with 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester (HEH(EHP)) also known as ethylhexyl 2-ethylhexylphosphonic acid (EHEHPA) and DEHPA
being the most used (Xu et al., 1992); neutral phosphate, such as tri-n-butylphosphate (TBP) and trinoctylphosphine oxide (TOPO) (Mathur and Choppin, 1998); carboxylic acid derivative (Du Preez and Preston, 1992); amines (Gorski, et al., 1991). The majority of these studies focus on the fundamentals, which include the determination of separation and
extraction parameters in synthetic solutions,
evaluation of kinetics and reaction mechanism, among others. There is little information in the literature regarding process development, mainly for proprietary reasons (Morais and Ciminelli, 2004). Complex rare earths element with ligand dithiocarbanate have been studied in several reports. Su et.al. in 1996 reported synthesis, structure and spectroscopic properties of
dimethylammonium lanthanide
tetrakis(N,N-dimethyldithiocarbamate). Raya et.al. in 2006-2007 reported complexes of samarium (III) also gadolinium (III) with dithiocarbamates and 1,10-phenantroline.
DBDTC chelating metal to make complex compound. In view of this, the objective of the present work was to identify the most favorable conditions for the separation of a Gd with DBDTC as chelating
extractants using organic solvents. Several
Materials and Method
Materials
Gd2O3 (Aldrich, 99,99%) were used as standard and
diluted nitric acid was used to dilute them. CS2,
di-n-butylamine and ammonia were used to prepare DBDTC. Acetic acid and sodium acetic were used to prepared the varied buffer solutions. Diethyl ether and petroleum ether were used as organic solvents.
Apparatus
Digital pH meter Mettler Toledo MP 220 was used for the pH measurements, spectrophotometer UV-Vis
Ultrospec 3000 pro, spectrophotometer FTIR
IRPrestige-21 Shimadzu, and ICP-AES Jobin Yvon Emission JY 38 S for metal concentration analysis spectrometrically.
Methods
DBDTC was prepared by reacting CS2,
di-n-butylamine and ammonia at 0 0C, stirred continuosly
for about 30 minutes until permanent precipitate occurred. The precipitate was separated from the liquid and dried at room temperature.
Gd standard solution were prepared by diluting their oxide in diluted nitric acid. Then, the extraction of each standard solution was carried out in separatory funnel containing 10 mL aqueous and organic phase. The samples were shaken for 10 minutes and stood for 10 minutes at room temperature. After separation of liquid phase and organic phase, metal concentration in liquid phase was determined spectrometrically using ICP-AES.
Results and Discussion
DBDTC Characterization
DBDTC was synthesized chelating agent from its
ammonium salt. This compound melted at 42-44 0C.
In table 1 shows the solubility of DBDTC in common solvents.
Table 1 Solubility of DBDTC in common solvents.
Solvent Observation
Water Solved slighty
Methanol Solved
Ethanol Solved
Chloroform Solved
Petroleoum Ether Solved
Diethyl Ether Solved
Figure 1 shows the UV-Vis spectra of DBDTC at 200-600 nm. The UV-Vis spectra show two peaks at
302 nm and 354 nm, which are considered as π to π*
transition absorption from C=S double bond and n to
π* transition absorption from lone pair electron in S
atom.
li gan DBDTK Abs
Wa velength (nm)
20 0,0 30 0,0 40 0,0 50 0,0 60 0,0 0, 000
1, 000 2, 000 3, 000
0, 000
2
The IR spectra in figure 2 show us that peak at υ and DBDTC was 1:3 with petroleum ether as organic solvent
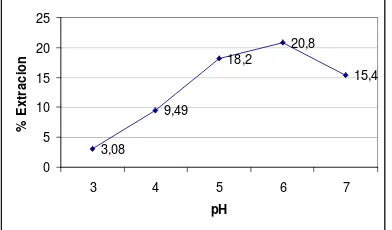
Under ratio mole metal and chelating extractans was 1:3 with petroleum ether as organic solvent were studied. Gd extraction were carried out at pH 3.0; 4.0; 5.0; 6.0; and 7.0. The result in figure 3 shows that pH involved toward distribution metal complex in organic
phase, in other words H+ concentration gives role in
distribution ratio metal complex between liquid phase and organic phase. Optimum pH for extraction in this condition reached at pH 6 and obtained % E values is 20.8 %. DBDTC was examined. Mole Gd : DBDTC were 1:1, 1:2, and 1:3. The result in figure 4 shows that % E from Gd, raising with ratio moles. At ratio mole 1:3, it means that 1 mole Gd reacting with 3 moles DBDTC to forms complex Gd-DBDTC and solved in organic extraction Gd at pH 6 with petroleum ether.
Effect of organic solvent on extraction Gd under constant pH and ratio mole Gd : DBDTC
Under constant pH and ratio mole Gd : DBDTC, the effect of organic solvent (petroleum ether and diethyl ether) were studied. Metal, Gd, with chelate extractants, DBDTC, makes complex compound Gd-DBDTC. The result in figure 5 shows that % E values on petroleum ether higher than diethyl ether. Its indicate that in petroleum ether, complex more soluble than in diethyl ether. From like dissolved like theory, it mention that compound is soluble in solvent by relatively same polarity. The polarity of Gd-DBDTC is relatives same with petroleum ether. Thus, Gd-DBDTC complex more distributed in petroleum ether than diethyl ether.
Figure 5 Effect of organic solvent on extraction Gd at pH 6 and ratio mole Gd : DBDTC 1:3
Complex Gd-DBDTC characterization in organic phase after extraction
Complex that formed in organic phase has been investigated. Complex were carried out from extraction product in at optimum condition extraction, and the highest recovery of Gd (at pH 6, mole ratio 1:3, and petroleum ether as organic solvent).
Gd-DBDTC complex were analyzed by
Figure 6 IR spectra of complex Gd-DBDTC after extraction at optimum condition. after extraction at optimum condition.
Figure 5 showed spectrum UV-Vis of complex. From spectrum UV-Vis DBDTC compared with spectrum Gd-DBDTC complex, shows that there is chemical shift on spectrum. On DBDTC, there is 2
peaks at λ 302 and 354 nm, but in complex just 1 peak
at λ 233,6 nm. It comes from transisition n to π* from
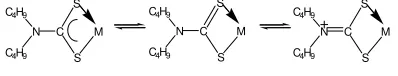
lone electron at sulfur atom. Transition π to π* doesn’t
appear because double bond in C=S broken, made coordinative bond with metal.
Figure 6 showed spectrum IR of complex. There was several differences between spectrum DBDTC with Gd-DBDTC complex. Spectrum IR of complex stretch from electron delocalized with C=S groups.
Peak at υ 1382 cm-1 from bending symmetry aliphatic
C4H9. Peak at υ 780-468 cm-1, its finger prints region
from C-S stretch, this is the different between IR spectra from Gd-DBDTC complex and DBDTC.
Ammonium group at IR spectra complex doesn’t
1
doesn’t appear in spectrum IR complex. In this case, atom S in C=S groups makes covalent coordinative efficiency as an alternative chelating extractants for Gd separation.
Acknowledgements
The authors are grateful to Chemical Mineral and Coal Testing Laboratory Center Geological Resources Bandung for ICP-AES analysis.
References
Du Preez, A. C., & J. S. Preston. 1992. The solvent extraction of rare earth metals by carboxylic acids. Solv. Extr. Ion Exch. 10:207–230.
Gorski, B., N. Gorski, M. Beer. 1991. Extraction of Sc, Y, and lanthanides by quaternary ammonium salts. Solv. Extr. Ion Exch. 9:623– 635.
Mathur, J. N., & G. R. Choppin. 1998. Parafin
WAX-TOPO, an extractant for actinides and
lanthanides.Solv. Extr. Ion Exch. 16:739–749. Morais, C. A., & V. S. T. Ciminelli. 2007. Selection
of solvent extraction reagent for the separation of
europium(III) and gadolinium(III). Minerals
Engineering. 20:747–752.
Morais, C. A., & V. S. T. Ciminelli. 2004. Process development for the recovery of high-grade lanthanum by solvent extraction. Hydrometallurgy. 73:237–244.
Raya, I., I. Baba, & B.M. Yamin. 2006. New Mixed Ligands Complexes of Samarium(III) with
Dithiocarbamates and 1,10-Phenantroline.
Malaysia Journal of Anakytical Sciences. 10: 93-98.
Raya, I., I. Baba, & B. M. Yamin. 2007. Mixed Ligands Complexes of Gadolinium(III) with
Dithiocarbamates and 1,10-Phenantroline.
Malaysia Journal of Anakytical Sciences. 7: 15-20. Su, C., M. Tan, N. Tang, X. Gan, Z. Zhang, Q. Xue, & K. Yu. 1996. synthesis, structure and spectroscopic properties of dimethylammonium
lanthanide
tetrakis(N,N-dimethyldithiocarba-mate). Polyhedron. 16:1643-1650.