Extraction of lanthanum, cerium, neodimium with
di-n-buthyldithiocarbamate (DBDTC) as chelating extractant and analysis
using inductively coupled plasma - optical emission spectroscopy
(ICP-OES)
Sistri Arda Afrillya*, Yayah Mulyasih, Iwan Hastiawan
Inorganic Laboratory, Department of Chemistry, Faculty of Mathematics and Natural Sciences, Padjadjaran University, Sumedang, West Java, Indonesia 45363
*e-mail: [email protected]
Abstract
Monazite is a reddish brown phosphate. Rare Earth Elements (REEs) exist in monazite and can be separated by using solvent extraction method. The objects of this research are to investigate the optimum condition to extract La, Ce and Nd complex with high recovery at pH 2.0, 3.5, 4.0, 4.5, 5.0, 5.5 and 6.0, in organic solvents toluene, diethyl ether and petroleum ether, and also to analyze the elements concentration of the aqueous phase with instrument inductively Couple Plasma – Optical Emission Spectroscopy (ICP-OES). This solvent extraction method used di-n-butyldithiocarbamat (DBDTC) as chelating extractant that the complex formed is extracted to organic phase. Di-n-butilditiocarbamat (DBDTC) was prepared by reacting carbon disulfide and di-n-buthylamine, then the La, Ce and Nd standard solution was prepared by diluting their oxides to diluted nitric acid and diluted to appropriate buffer solution. Each standard solution was extracted with organic solvents toluene/diethyl ether/petroleum ether by adding DBDTC 1% solution 1:3 in metal (mole) : ligand (mole) comparison. After that, the aqueous phases were analyzed using instrument ICP-OES. La/Nd-DBDTC complex can be best extracted using organic solvent diethyl ether at pH 5.5 with Kd for La-DBDTC and Nd-La-DBDTC are 1.1870 and 0.6274. Ce-La-DBDTC complex can be best extracted using solvent diethyl ether at pH 2.0 with Kd 0.9109. Instrument ICP-OES is good for standard solution and organic phase concentration analyzing.
Keywords: Di-n-buthyldithiocarbamate, ICP-OES, REE, Solvent Extraction, Diethyl ether
Introduction
Rare earth elements are a series of chemical elements of the periodic table, including the elements with atomic numbers 57 through 71, and, named in order, are lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu). Yttrium (Y, atomic no. 39) and scandium (Sc, atomic no. 21) are sometimes included in the group of rare earth elements. The elements cerium (Ce, atomic no. 58) through lutetium (Lu, atomic number 71) are commonly known as the lanthanide series (Krakew et al., 2005).
They are essential in many and relevant applications in chemical, metallurgical, optical, electronic and ceramic products. In several of these uses, the rare-earths are responsible for high technological performances, either by participating in the intermediate manufacturing processes or integrating the finished products (Masson & Osvaldo, 2002). Altec Lansing Technologies, Inc., a leader in the market for personal computer speakers, has
introduced two computer speaker systems that use neodymium and blend the art of sound with the beauty of design (Fox et al., 2001).
Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) is one of several techniques available in analytical atomic spectroscopy. ICP-OES utilizes a plasma as the atomization and excitation source. A plasma is an electrically neutral, highly ionized gas that consists of ions, electrons, and atoms. The sun, lightning, and the aurora borealis are examples of plasmas found in nature. The energy that maintains an analytical plasma is derived from an electric or magnetic field; they do not “burn” (Manning and Grow, 1997).
Materials and Method
Materials
La2O3, CeO2 and Nd2O3 (Aldrich, 99,99%)were used as REEs standard and diluted nitric acid was used to dilute them. CS2, di-n-buthylamine and ammonia were used to prepare DBDTC. Acetic acid, sodium acetic, hydrochloride acid and kalium chloride were used to prepared the varied buffer solutions. Toluene, diethyl ether and petroleum ether were used as organic solvents.
Apparatus
Digital pH-meter Mettler Toledo MP 220 was used for the pH measurements, spectrophotometer UV-Vis Ultrospec 3000 pro and spectrophotometer IR for spectrophotometric measurements, and ICP-OES Varian Vista MX for metal concentration analysis spectrometrically.
Methods
DBDTC was prepared by reacting CS2, di-n-buthylamine and ammonia at 0 0C, stirred continuously for about 30 minutes until permanent precipitate occurred. The precipitate was separated from the liquid and dried at room temperature.
La, Ce and Nd standard solutions were prepared by diluting their oxide in diluted nitric. Then, the extraction of each standard solution was carried out in separatory funnel containing 10 mL aqueous and organic phase. The samples were shaken for 10 minutes and stood for 10 minutes at room temperature. After the separation of the phases, the metal concentration in aqueous phase was determined spectrometrically using ICP-OES. UV-Vis spectra of DBDTC at 200-800 nm and Figure 2 shows IR spectra of ammonium-DBDTC.
The UV-Vis spectra show two peaks at 315 nm and 357 nm, which are considered as π to π* and n to π* transition absorption. The π to π* transition is appeared because of C=S double bond and n to π* transition is appeared because of lone pair electron in S atom that absorbs light at 250-380 nm.
The IR spectra show us several peaks. Peak at υ 3450 cm-1 is for N-H stretch from ammonium
fungsional group. Peaks at υ 2956 cm-1 - 2775 cm-1 is for C-H vibration from C4H9. Peak at υ 1554 cm-1 is for C=N stretch from electron delocalization with C=S fungsional group. Peak at υ 1462 cm-1 is from C=S
Extraction of La, Ce and Nd with DBDTC
Some researches of Krakew et al. (2005) and Atanassova et al. (2006-2007) about REEs extraction using mixtures of varied two extractants and several organic solvents, show that uses of certain chelating extractant and organic solvent need certain condition for each REEs in order to be extracted with high recovery. So, in this study, La, Ce and Nd standard solution were prepared in varied pH.
La and Nd extraction were carried out at pH 4.0; Ce and Nd complex are extracted to organic solvents. The mole comparison of metal and chelating extractant was 1:3 because it can best extract the light REEs complex. Toluene, diethyl ether and petroleum ether were used as organic solvents to solve the complex formed in extraction process. After separation of the phases, the metal concentration of aqueous phase was determined using ICP-OES.
La and Nd can be extracted with the highest elements extraction are shown in figure 3, 4 and 5.
Analysis using Inductively Coupled Plasma – Optical Emission Spectroscopy (ICP-OES)
Each atom has several energy levels. Atoms emit electromagnetic radiation as they relax from an excited state to their ground state. The emitted radiation can be easily detected when it is in the vacuum ultraviolet (VUV, 120-185 nm), ultraviolet (UV, 185-400 nm), visible (VIS, 400-700 nm), and near infrared regions (NIR, 700-850 nm).
li gan DBD TK Abs
Wa velengt h (nm)
20 0,0 40 0,0 60 0,0 80 0,0
0, 000 1, 000 2, 000 3, 000 4, 000
0, 000
1
Figure 1 UV-Vis Spectra of ammonium di-n-buthyldithiocarbamate in methanol
Figure 2 IR spectra of ammonium di-n-dibuthyldithiocarbamate
Figure 3 Kd of La-DBDTC extraction with toluene, diethyl ether and petroleum ether versus pH
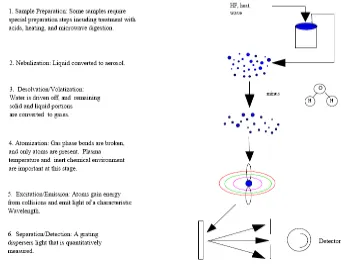
Figure 6 Steps involved in the analysis of aqueous samples by ICP-OES (Manning & Grow, 1997)
Every element absorbs light in more than one wave lengths, so we have to find the optimum wavelength to obtain the elements’ concentration using ICP-OES. This wavelength optimizing was done by observing the intensities of the elements’ standard solutions at 20 various wavelengths. In this study, lanthanum oxide, cerium oxide and neodymium oxide was used to prepare the standard solutions with various concentrations, 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50, and 100 ppm. The graphic of standard solution has to be linear in order to be able to use in the sample concentration determination. There are several wave lengths which give linear graphics, so we have to choose one of the wave lengths that give the highest intensities and less interferences for certain element. The optimum wave lengths for La, Ce and Nd are 408.671, 401.239 and 430,357 nm.
Coupled Plasma – Optical Emission Spectroscopy (ICP-OES) Principles
The ICP-OES principles are electric and magnetic fields, and detection of the radiation emissions by detector. A plasma is an electrically neutral, highly ionized gas that consists of ions, electrons, and atoms. Argon is usually used as plasma that is used as the atomization and excitation source (Gassing, 2005).
There are six steps involved in the analysis of aqueous samples by ICP-OES as shown in the figure 6. Plasma temperature used is 10.000 K because at this temperature chemical bonds of any compounds can be broken. There are only atoms that will excite
because they absorb energy from plasma. Then, the detector will detect the emission of radiation upon relaxation from an excited state of the atoms.
Conclusions
La and Nd can be extracted with the highest recovery at pH 5.5 using diethyl ether; Kd of La and Nd at this condition are 1.1870 and 0.6274. Ce can be extracted with the highest recovery at pH 2 using diethyl ether; Kd of Ce at this condition is 0.9109. ICP-OES is good for La, Ce and Nd extraction analysis.
Acknowledgements
The authors are grateful to Laboratory Sucofindo SBU JUM Cibitung for ICP-OES Analysis.
References
Atanassova, M. 2006. Effect of the 18-Crown-6 and Benzo-18-Crown-6 on the Solvent Extraction and Separation of Lanthanoids(III) Ions with 8-Hydroxyquinoline. Russian Journal of Inorganic Chemistry. 52 (8), 1304-1311.
Atanassova, M. & Dukov, I.L. 2006. Synergistic Solvent Extraction of Trivalent Lanthanoids with mixture of 1-Phenyl-3-methyl-4-benzoyl-5-pyrazolone and Crown Ethers. Acta Chim. 53, xxx-xxx.
Atanassova, M., Victoria L., Nikolay V., Sabi V., & Ivan D. 2006. Effect of p-tert-Butylcalix[4]arene Fitted with Phosphinoyl Pendant Arms as Synergistic Agent in the Solvent Extraction and Separation of some Trivalent Lanthanoids with 4-Benzoyl-3-methyl-1-phenyl-5-pyrazolone. Journal of Inclusion Phenomena and Macrocyclic Chemistry
Fox, G., Myra P., Louise R. & Rich V. 2001. Elements. High Tech Materials. Colorado. Gassing. 2005. Kajian Panjang Gelombang yang
Sesuai untuk Analisa 23 Unsur Logam secara Simultan dengan Menggunakan Instrument
ICP-OES Variant Vista MPX. Skripsi. Universitas Nusa Bangsa. Bogor.
Manning, T.J. & W.R. Grow. 1997. Inductively Coupled Plasma – Atomic Emissio Spectrometry. Springer-Verlag New York, Inc. New York. Masson, I.O.C. & Osvaldo G.C.C. 2002. Extraction
of Heavy Rare-Earths dan Yttrium with a Phosphonic Solvent. Contribuição Técnica aos Anais da Associação Brasileira de Química, 51(1),1-8.