Regular Article
Role of thrombophilic risk factors in children with
non-stroke cerebral palsy
Aviva Fattal-Valevski
a,b
,
*, Gili Kenet
b
,
c
, Michael J. Kupferminc
b
,
d
,
Ronit Mesterman
a
,
b
, Yael Leitner
a
,
b
, Eli Rimon
b
,
d
, Shaul Harel
a
,
b
,
Avi Hassner
b
,
e
a
The Institute for Child Development and Pediatric Neurology Unit, Tel Aviv Sourasky Medical Center,
Beit Habriut Strauss, 14 Balfour St., Tel Aviv 65211, Israel
b
Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
c
Pediatric Coagulation Service, the National Hemophilia Center and Institute of Thrombosis and
Hemostasis, Sheba Medical Center, Tel-Hashomer, Israel
d
The Department of Obstetrics and Gynecology, Lis Maternity Hospital,
Tel Aviv Sourasky Medical Center, Israel
e
Tel Aviv Sourasky Medical Center, Israel
Received 27 September 2004; received in revised form 16 November 2004; accepted 25 November 2004
Available online 29 December 2004
Abstract
Background:
Thrombophilic risk factors play an important role in the pathogenesis of
perinatal stroke and resultant cerebral palsy (CP). The association between
thrombophilia and CP caused by etiologies other than stroke is undetermined.
Methods:
We assessed three genetic thrombophilic markers (mutation of Factor V
Leiden [FV G1691A], 677T polymorphism of thermolabile methylenetetrahydrofolate
reductase [MTHFR] and G20210A mutation of the prothrombin gene) in 49 pediatric
patients with non-stroke CP and compared the findings with 118 apparently healthy
controls. CP in the study group was due to periventricular leukomalacia (
n
=27),
intraventricular hemorrhage (
n
=9), hypoxic ischemic encephalopathy (
n
=4),
pre-maturity with no apparent complication (
n
=8) and intrauterine growth retardation
(
n
=1). Twenty-five children had spastic diplegia, 20 had spastic quadriplegia and 4
had spastic hemiplegia. CP was graded as being severe in 26 children (53%).
Results:
No significant difference in the prevalence of thrombophilic risk factors was
found between the study and control groups. Twelve study children (24.5%) had at
least one of the three thrombophilic mutations compared with 27 controls (23%).
0049-3848/$ - see front matterD2004 Elsevier Ltd. All rights reserved. doi:10.1016/j.thromres.2004.11.022
* Corresponding author. The Institute for Child Development and Pediatric Neurology Unit, Tel Aviv Sourasky Medical Center, Beit Habriut Strauss, 14 Balfour St., Tel Aviv 65211, Israel. Tel.: +972 3 6423824; fax: +972 3 7441283.
E-mail address:[email protected] (A. Fattal-Valevski).
KEYWORDS
Thrombophilia;
Cerebral palsy;
MTHFR;
Factor V leiden;
Factor II
There was no significant difference in the prevalence of each thrombophilic risk
factor in the various etiologic groups and in the subgroups of mild/severe CP and
the control group.
Conclusion:
These findings support the notion that thrombophilia neither
contributes to the occurrence nor affects the clinical outcome and severity of
non-stroke CP.
D
2004 Elsevier Ltd. All rights reserved.
Introduction
Cerebral palsy (CP) is a common disorder in
child-hood, occurring in 2—2.5 per 1000 live births
[1].
Perinatal asphyxia and prematurity play an
impor-tant role in the pathogenesis of CP, but its
occur-rence is often triggered by other predisposing
factors, suggesting that more than a single risk
factor may be involved
[2,3]. Factors such as
intra-uterine exposure to infection, inflammation or
coagulation disorders have been suggested as
pre-disposing factors to brain injury
[4—6]. The relation
between CP and thrombophilia has not yet been
defined
[7]. It is well known that genetic
prothrom-botic risk factors play an important role in the
pathogenesis of infantile thrombosis
[8—10],
child-hood stroke
[11—13], perinatal stroke
[14,15]
and
resultant porencephaly
[16]
and hemiplegic CP
[17,18]. The largest known contributor reported is
Factor V Leiden mutation (FV G1691A)
[15—19].
Whether or not thrombophilia plays a role in the
pathogenesis of other brain lesions that result in CP
has not been established. Some case series have
demonstrated an increased risk of intraventricular
hemorrhage (IVH), a recognized cause for CP,
in preterm infants with thrombophilia
[20,21].
Another population-based study reported contrary
results, failing to demonstrate any increased risk of
IVH and periventricular leukomalacia (PVL) in
pre-mature infants with thrombophilia
[22]. Therefore,
the contribution of thrombophilia to the
develop-ment of non-stroke CP remains to be clarified.
Thrombophilia stems from acquired as well as
inherited causes
[23]. Several mutations in
coagu-lation proteins are associated with an increased risk
for thromboembolic complications. Resistance to
activated protein C caused by an adenine 506
guanine (A506G) mutation in FV G1691A has been
linked with an increased risk for venous
throm-boembolism
[24]
as well as childhood stroke
[13].
Homozygosity for the cytosine 677 thymine (C677T)
mutation in methylenetetrahydrofolate reductase
(MTHFR) may slightly increase the risk for venous
and arterial thrombosis
[25]
,and the guanine 20210
adenine mutation in prothrombin (FIIG20210A) has
been associated with increased risk for venous
thromboembolism and cerebral vein thrombosis
[26].
The present study aimed to determine the
prevalence of the abovementioned thrombophilic
risk factors in non-stroke pediatric CP patients
compared to those of a control group of apparently
normal children.
Subjects and methods
Subjects
Between September 2001 and September 2003, we
studied consecutive children with spastic CP who
were referred to a large pediatric CP referral center
in Israel. All children below 18 years of age who were
diagnosed as having CP were considered suitable
candidates for the study. Since the association of
genetic thrombophilia and childhood stroke is well
established, those children whose CP was caused by
ischemic stroke were excluded. Children with a
known familial thrombophilia, defined as either
family history positive for early (
b
50 years old)
thrombosis or presence of parental genetic
throm-bophilia, were excluded from our study as well.
Diagnosis of non-stroke CP was made by absence of a
past history of ischemic stroke and no evidence of
porencephaly on an imaging study (computerized
tomography [CT] or magnetic resonance imaging
[MRI]). Forty-nine children (mean age 4.8
F
2.9
years) defined as non-stroke CP who met the
inclusion criteria comprised the study group. All CP
patients were diagnosed at 6—12 months (median 8
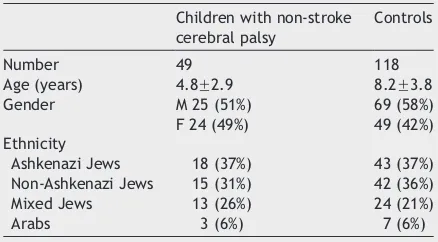
months). The children’s ethnic origin was classified
as Ashkenazi Jewish, non-Ashkenazi Jewish, mixed
Ashkenazi and non-Ashkenazi Jewish and Arabic
(Table 1). The mean gestational age in the study
group was 31.2
F
4.5 weeks and the mean birth
weight was 1.65
F
0.87 kg.
growth retardation (IUGR). PVL was defined by one
of two criteria: periventricular cystic lesions as
seen on neonatal brain ultrasound (US) or brain MRI
showing diminished periventricular white matter,
irregular shape of the lateral ventricles or
ven-tricular dilatation. IVH was defined according to
the grading system of Volpe
[27]
. Forty-two (86%)
children in our study group were preterm. In 7
children who were born at term, the etiology for
CP was HIE (
n
=4), IVH (
n
=2) and IUGR (
n
=1). The
study group included 25 children with spastic
diplegia, 20 with spastic quadriplegia and 4 with
spastic hemiplegia. The etiology in all children
with hemiplegic CP was IVH.
The level of severity of CP was defined according
to the functional ability and the type of CP.
Specifically, severe CP was defined as either spastic
quadriplegia and being either non-ambulatory or
mentally retarded. The remaining CP children who
were ambulatory without mental retardation were
defined as having mild CP.
The control group consisted of 118 apparently
healthy children who were recruited prior to
undergoing an elective surgical procedure or
routinely visiting ambulatory childcare clinics:
none had known familial thrombophilia defined
as either family history positive for early (
b
50
years old) thrombosis or presence of parental
genetic thrombophilia, history of previous
throm-boembolic events or neurological abnormalities.
The distribution of gender and ethnic origin was
similar in both groups (
Table 1
). The mean age of
the control group was 8.2
F
3.8 years (
Table 1
).
The difference in age between the two groups had
no impact upon our results since the studied
inherited mutations are genetically transmitted
and not age-dependent.
Methods
We evaluated three thrombophilic markers:
muta-tion of Factor V Leiden, C677T polymorphism of
thermolabile MTHFR and G20210A mutation of the
prothrombin gene. Citrated blood (0.38%) was
drawn for DNA analysis of the thrombophilic
mutations from all the children at the time of
study enrollment. Molecular diagnosis of the FV
G1691A mutation was performed as described by
Dalback
[28]
. The mutation in the MTHFR gene
was tested by the method of Frosst et al.
[25]
.
The mutation in the prothrombin gene was
detected by means of a slight modification of
the method of Poort et al.
[26]
as described by
Salomon et al.
[29]
. A questionnaire on birth
weight, perinatal history, family history, type and
severity of the CP, motor function, and cognitive
ability was filled out for all the children in the
study group. The information was obtained from
the medical charts and the children’s parents. The
study was approved by the Ethics Committee of
the Tel Aviv Sourasky Medical Center.
Statistical analysis
The unpaired
t
-test was used for between-group
comparison of birth weight and age. Prevalence of
prothrombotic risk factors in the patients and
control subjects was calculated by Fisher’s exact
test. The significance level was set at 0.05. The
odds ratios and 95% confidence intervals were
calculated in the study group compared with
controls, and in each of the three etiologic groups
of CP compared with controls. The same analysis
was performed to compare the mild with severe
CP patients.
Results
Prothrombotic risk factors in the study and
control groups
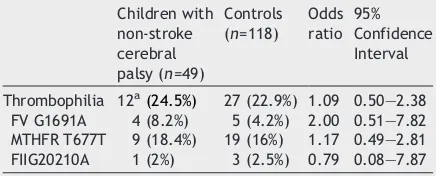
Overall, 12 of the 49 children with non-stroke CP
(24.5%) had at least one of the three
thrombo-philic mutations compared with 27 of the 118
normal controls (22.9%). Four study group
chil-dren had FV G1691A, 9 had an MTHFR mutation
and one had FIIG20210A mutation. Two children
had combined thrombophilic mutations: one FV
G1691A and MTHFR and one FIIG20210A and
MTHFR. Both were considered only once for the
statistical analysis. No children in the control
group had combinations of thrombophilia risk
factors. There was no significant difference in
the prevalence of each thrombophilic risk factor
among the study patients and the normal controls
(
Table 2
).
Table 1
Demographic data of the study and control
groups
Children with non-stroke cerebral palsy
Controls
Number 49 118
Age (years) 4.8F2.9 8.2F3.8
Gender M 25 (51%) 69 (58%)
F 24 (49%) 49 (42%)
Ethnicity
Ashkenazi Jews 18 (37%) 43 (37%)
Non-Ashkenazi Jews 15 (31%) 42 (36%)
Mixed Jews 13 (26%) 24 (21%)
Prothrombotic risk factors in the various
etiologic groups
There was no significant difference in the
preva-lence of each thrombophilic risk factor in the
various etiologic groups. Seven children in the PVL
group (26%) had at least one of the three
thrombo-philic mutations, 2 (22%) in the IVH group, 1 (25%)
in the HIE group and 2 (25%) in the prematurity
group.
Mild vs. severe CP
Twenty-six of the CP children (53%) were defined
as having severe CP and the remaining 23 children
(47%) as having mild CP. Seven of 26 children (27%)
with severe CP were found to have at least one
prothrombotic risk factor compared with 5
chil-dren (22%) in the mild CP group. There was no
significant difference in the prevalence of each
thrombophilic risk factor among the mild and
severe CP groups (odds ratio, 1.32; 95% CI, 0.35
to 4.94). Among the children with severe CP, 3 had
FV G1691A and 4 had an MTHFR mutation. Among
the children with mild CP, 1 had FV G1691A, 5 had
an MTHFR mutation and 1 had a FIIG20210A
mutation (two had combined mutations).
Discussion
In the present case-control study, we found no
significant difference in the prevalence of FV
G1691A, FIIG20210A and MTHFR T677T between
children with non-stroke CP and normal controls.
Previous case-series that demonstrated an
associ-ation between CP and thrombophilic risk factors,
mainly FV G1691A mutation, were confined to CP
that has been caused by ischemic stroke
[15—18]
.
In another case-study of hemiplegic CP patients,
thrombophilia was increased among the subgroup
of children with radiologically confirmed
ische-mia, however the overall rate of FV G1691A and
FIIG20210A was similar among patients and
pop-ulation, in concordance with our findings
[17]
.
These findings emphasize the importance of
defin-ing the underlydefin-ing brain lesion in studies of CP
patients.
In our study the prevalence of thrombophilia
markers was similar to that of the controls in each
etiologic subgroup of CP. The majority of our CP
patients were born prematurely (
n
=42) and
suf-fered from complications of prematurity, e.g. PVL
and IVH. Therefore, we conclude that
thrombo-philia did not contribute to the occurrence of CP in
premature infants nor in those with a history of PVL
or IVH. The fact that the majority of the study
group were premature should not affect the results
since the studied inherited mutations are
genet-ically transmitted and do not depend on gestational
age. Moreover, previous study demonstrated that
the prevalence of thrombophilia in preterm Israeli
newborns was similar to the healthy pediatric
Israeli population
[22]
. However, we cannot rule
out the impact of possible undiagnosed maternal
thrombophilia upon the occurrence of prematurity
in our study population.
The prevalence of thrombophilic markers did not
differ among patients with mild or severe CP.
Therefore, the presence of thrombophilic markers
had no impact upon the neurological outcome of
our patients. On the contrary two patients with
combined thrombophilic mutation had mild CP.
In summary, the results of the current study
support the notion that thrombophilia neither
contributes to the occurrence nor affects the
clinical outcome of CP that is not attributed to
stroke, in children.
Acknowledgment
Esther Eshkol is thanked for editorial assistance.
References
[1] Mutch L, Alberman E, Hagberg B, Kodama K, Perat MV. Cerebral palsy epidemiology: where are we now and where are we going?Dev Med Child Neurol1992;34:547 – 55. [2] Nelson KB, Grether JK. Causes of cerebral palsy.Curr Opin
Pediatr1999;11:487 – 91.
[3] Han TR, Bang MS, Lim JY, Yoon BH, Kim IW. Risk factors of cerebral palsy in preterm infants.Am J Phys Med Rehabil 2002;81:297 – 303.
[4] Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children.Ann Neurol 1998;44:665 – 75.
[5] Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O’Sullivan F, Burton PR, et al. Antepartum risk factors for newborn
Table 2
Thrombophilic risk factors in the study
versus the control groups
Children with
Thrombophilia 12a(24.5%) 27 (22.9%) 1.09 0.50—2.38
FV G1691A 4 (8.2%) 5 (4.2%) 2.00 0.51—7.82 MTHFR T677T 9 (18.4%) 19 (16%) 1.17 0.49—2.81 FIIG20210A 1 (2%) 3 (2.5%) 0.79 0.08—7.87
encephalopathy: the Western Australian case control study.
Br Med J1998;317:1549 – 53.
[6] Nelson KB. The epidemiology of cerebral palsy in term infants.Ment Retard Dev Disabil Res Rev 2002;8:146 – 50. [7] Smith RA, Skelton M, Howard M, Levene M. Is thrombophilia
a factor in the development of hemiplegic cerebral palsy?
Dev Med Child Neurol2001;43:724 – 30.
[8] Bonduel M, Sciuccati G, Hepner M, Torres AF, Pieroni G, Frontroth JP. Prethrombotic disorders in children with ischemic stroke and sinovenous thrombosis. Arch Neurol
1999;56:967 – 71.
[9] van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in child-hood: a prospective two-year registry in The Netherlands.
J Pediatr2001;139:676 – 81.
[10] Uttenreuther-Fischer MM, Vetter B, Hellmann C, Otting U, Ziemer S, Hausdorf G, et al. Pediatric thromboembolism; the influence of non-genetic factors and the role of activated protein C resistance and protein C deficiency.Eur J Pediatr
1996;156:227 – 81.
[11] De-Veber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, et al. Cerebral sinovenous thrombosis in children.N Engl J Med2001;345:417 – 423.
[12] Strater R, Becker S, von Eckardstein A, Heinecke A, Gutsche R, Junker R, et al. Prospective assessment of risk factors for recurrent stroke during childhood—a 5-year follow-up study.Lancet 2002;360:1540 – 5.
[13] Kenet G, Sadetzki S, Murad H, Martinowitz U, Rosenberg N, Gitel S, et al. Factor V Leiden and antiphospholipid anti-bodies are significant risk factors for ischemic stroke in children.Stroke2000;31:1283 – 8.
[14] Gunther G, Junker R, Strater R, Schobess R, Kurnik K, Heller C, et al. Childhood Stroke Study Group. Sympto-matic ischemic stroke in full-term neonates: role of acquired and genetic prothrombotic risk factors. Stroke
2000;31:2437 – 41.
[15] Harum KH, Hoon Jr AH, Kato GJ, Casella JF, Breiter SN, Johnston MV. Homozygous factor-V mutation as a genetic cause of perinatal thrombosis and cerebral palsy. Dev Med Child Neurol 1999;41:777 – 80.
[16] Debus OM, Kosch A, Strater R, Rossi R, Nowak-Gottl U. The factor V G1691A mutation is a risk for porencephaly: a case-control study.Ann Neurol2004;56:287 – 90.
[17] Halliday JL, Reddihough D, Byron K, Ekert H, Ditchfield M. Hemiplegic cerebral palsy and the factor V Leiden muta-tion.J Med Genet2000;37:787 – 9.
[18] Thorarensen O, Ryan S, Hunter J, Younkin DP. Factor V Leiden mutation: an unrecognized cause of hemiplegic cerebral palsy, neonatal stroke and placental thrombosis.
Ann Neurol1997;42:372 – 5.
[19] Lynch JK, Nelson KB, Curry CJ, Grether JK. Cerebrovas-cular disorders in children with the factor V Leiden mutation.J Child Neurol2001;16:735 – 44.
[20] Petaja J, Hiltunen L, Fellman V. Increased risk of intra-ventricular hemorrhage in preterm infants with thrombo-philia.Pediatr Res2001;49:643 – 6.
[21] Aronis S, Bouza H, Pergantou H, Kapsimalis Z, Platokouki H, Xanthou M. Prothrombotic factors in neonates with cerebral thrombosis and intraventricular hemorrhage. Acta Pae-diatr, Suppl2002;91:87 – 91.
[22] Kenet G, Maayan-Metzger A, Rosenberg N, Sela B, Mazkereth R, Ifrah A, et al. Thrombophilia does not increase risk for neonatal complications in preterm infants. Thromb Hae-most2003;90:823 – 8.
[23] Seligsohn U, Lubetsky A. Genetic susceptibility to venous thrombosis.N Engl J Med2001;344:1222 – 31.
[24] Bertina RM, Koeleman RPC, Koster T. Mutation in blood coagulation factor V associated with resistance to activated protein C.Nature1994;369:64 – 7.
[25] Frosst P, Bloom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylene-tetra-hydrofolate reductase.Nat Genet1995;10:111 – 3. [26] Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A genetic
variation in the 3V-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis.Blood1996; 88:3698 – 703.
[27] Volpe JJ. Neurobiology of the newborn. Philadelphia7 Saunders; 2001. p. 217 – 497.
[28] Dalback B. New molecular insights of thrombophilia: resistance to activated protein C caused by Arg506 to Gln. Mutation in factor V as pathogenetic risk factor for venous thrombosis.Thromb Haemost1995;74:139 – 48. [29] Salomon O, Steinberg DM, Zivelin A, Gitel S, Dardik R,