REGULAR ARTICLE
Impact of phosphorus mineral source (Al
–
P or Fe
–
P)
and pH on cluster-root formation and carboxylate exudation
in
Lupinus albus
L.
M. W. Shane&H. Lambers&G. R. Cawthray& A. J. Kuhn&U. Schurr
Received: 28 September 2007 / Accepted: 20 December 2007 / Published online: 10 January 2008
#Springer Science + Business Media B.V. 2007
Abstract Lupinus albusL. were grown in rhizoboxes containing a soil amended with sparingly available Fe–P or Al–P (100μg P g−1soil/resin mixture). Root halves of individual plants were supplied with nutrient solution (minus P) buffered at either pH 5.5 or 7.5, to assess whether the source of mineral-bound P and/or pH influence cluster-root growth and carboxylate exudation. The P-amended soil was mixed 3:1 (w/w) with anion-exchange resins to allow rapid fixation of carboxylates. Treatments lasted 10 weeks. Forty percent and 30% of the root mass developed as cluster roots in plants grown on Fe–P and Al–P respectively, but cluster-root growth was the same on root-halves grown at pH 5.5 or 7.5. Mineral-bound P source (Al–or Fe–P) had no influence on the types of carboxylates measured in soil associated with cluster roots—citrate (and trace amounts of malate
and fumarate) was the only major carboxylate detected. The [citrate] in the rhizosphere of cluster roots decreased with increased shoot P status (sug-gesting a systemic effect) and also, only for plants grown on Al–P, with decreased pH in the root environment (suggesting a local effect). In a separate experiment using anion exchange resins pre-loaded with malate or citrate, we measured malate (50%) and citrate (79%) recovery after 30 days in soil. We therefore, also conclude that measurements of [citrate] and [malate] at the root surface may be underesti-mated and would be greater than the 40- and 1.6-μmol g−1root DM, respectively estimated by us and others because of decomposition of carboxylates around roots prior to sampling.
Keywords Citrate . P-deficiency . Proteoid roots . Split-root design . Systemic signal . White lupin
Introduction
Phosphorus (P) deficiency induces formation of dense numbers of closely spaced, short-lived, determinate lateral roots (rootlets) termed‘cluster’or‘cluster-like’ roots (e.g., L. cosentinii, Trinick 1977; L. albus, Gardner et al. 1981; Clements et al. 1993; Skene
1998,2000) in two-thirds of the‘old-world’Lupinus
species (8 of the 11 species; Fabaceae, Longnecker et al. 1998). In L. albus, cluster roots can represent a significant proportion of the plant’s investment in
DOI 10.1007/s11104-007-9535-7
Responsible Editor: P. Randall.
M. W. Shane (*)
:
H. Lambers:
G. R. Cawthray School of Plant Biology,Faculty of Natural and Agricultural Sciences, The University of Western Australia, 35 Stirling Highway,
Crawley, WA 6009, Australia e-mail: mshane@cyllene.uwa.edu.au
biomass (i.e. 30% to 80% of the total root mass, Keerthisinghe et al.1998; Skene 2000; Hocking and Jeffery2004). Cluster roots acquire highly immobile phosphate and micronutrients that need to be chem-ically extracted from soils (Braum and Helmke1995), and cluster-root producing Lupinus species are well known for their capacity to grow on soils where P is highly unavailable (Trinick 1977; Neumann and Martinoia2002).
The P status of shoots has a strong systemic effect on cluster-root formation and the amount of citrate released from cluster roots inL. albus(Marschner et al. 1986,
1987; Gilbert et al.1997; Shane et al.2003a) and in other cluster-root forming species (e.g., Fabaceae,
Viminaria juncea, Walker and Pate1986; Proteaceae,
Hakea prostrata and Grevillea crithmifolia; Lamont
1972; Shane and Lambers 2006). There is also some evidence that a low supply of N, Fe, K or Zn (Lamont
1972; Hagström et al. 2001; Liang and Li 2003; McCluskey et al. 2004) can increase cluster-root formation in L. albus. Furthermore, Peiter et al. (2001) reported that pH of the root environment can influence cluster-root development. They found that cluster-root numbers, at a constant P supply, doubled in L. albus grown in nutrient solution buffered at pH 7.7 as compared with that at pH 5.5.
The release of exudates (e.g., carboxylates such as citrate, Jones 1998) from cluster roots of L. albus
mobilises sparingly available mineral-bound inorgan-ic and organinorgan-ic P as well as minorgan-icronutrients (e.g., Mn and Zn, Gardner et al. 1982a, b; Dinkelaker et al.
1989; Vance et al. 2003). In the case of Lupinus albus, leaf Mn accumulation are frequently observed as a consequence of Mn co-mobilization associated with rhizosphere-chemical changes involved in P acquisition (Shane and Lambers2005a; Dinkelaker et al.1995). Citrate is almost always observed to be the most abundant carboxylate found in cluster-root exudates (Jones 1998). Similar findings have been made for specialised root clusters developed in species from other families [e.g., Proteaceae (Dinkelaker et al.
1997; Shane et al.2004), Cyperaceae (Playsted et al.
2006; Shane et al. 2006)]. Root growth and turnover of new cluster roots provides further P mobilisation by the root system (e.g., proteoid roots inBanksia, Pate and Dell1984).
The carboxylate composition (e.g., citrate, malate etc.) and concentrations in root exudates have been found to vary amongst some species as related to soil
factors (Jones 1998). Investigations by Ström et al. (1994) discovered that nine species adapted to calcareous soils released more citrate and oxalate compared to nine species adapted to acidic soils. Furthermore, cluster roots of Proteaceae (i.e. Banksia grandisnative to soils of extremely low fertility) have been shown to release mainly di- and tri-carboxylates when supplied with Al–P but released additional mono-carboxylates when Fe–P was supplied (Lambers et al. 2002). Although it is not known if carboxylate exudation by cluster roots of L. albusvaries with the type ‘form’ of P (e.g., Al–P or Fe–P) supplies in the soil, Veneklaas et al. (2003) found that the proportion of malate to citrate in the rhizosphere varied as a function of soil pH: more malate at pH<6, and more citrate at pH>6.5.
The aim of the present study was to assess whether cluster-root development, and net accumulation of individual carboxylates in the rhizosphere ofL. albus
are affected by the‘form’of P supplied and/or pH of the root environment.
Materials and methods
Experimental design and split-root culture
A nutrient-deficient soil (pH 6 in water, Jülich, Germany) (acid-washed with 0.5 M HCl and rinsed several times in DI water, and dried) was mixed with anion exchange resin (Amberlite IRA-400, Amberjet 4200, Sigma-Aldrich, Deisenhofen, Germany) in the Cl− form (using 0.5 M NaCl) in a 3:1 ratio (soil/
resin). The soil/resin mixture was amended with 100 mg P kg−1 supplied as either Al
2O3P2O5 or FePO4
(both AR grade). The P-amended soil/resin mixture was added to each root compartment (9.5 cm wide and 33 cm in height) of the split-root rhizoboxes (inner thickness 1 cm) until half-filled. The remaining upper halves of the rhizoboxes were filled with the unamended, acid-washed soil, without added P and without anion exchange resin.
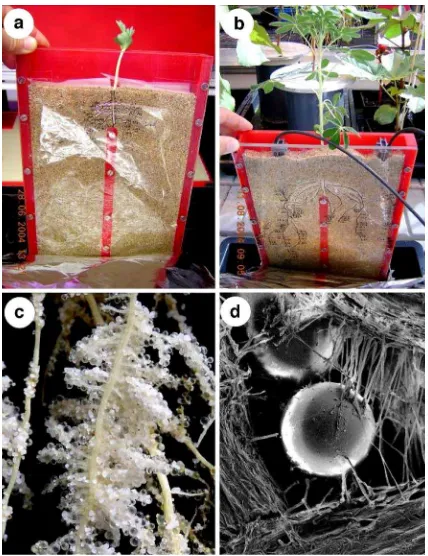
Fig. 1 Lupinus albusL. in split-root rhizoboxes grown on Al– P or Fe–P. a Individual plants were transferred to split-root rhizoboxes 7 days after germination. Roots grow-out into separate root compartments.bL. albusafter 6 weeks treatment. Root halves of individual plants were fertilised daily and automatically by drip irrigation with nutrient solution. One root
Plants were positioned in special trays so that each rhizobox was at approximately 30° relative to the vertical axis. For the first 2 weeks the plants were grown in a controlled environment chamber at 400 μmol quanta m2 s−1 (PAR) and received approximately 25 ml nutrient solution (to each root half every 2nd day) of the following composition (in μM): 1,200 NO3, 600, Ca2+, 600 K+, 678 SO24 , 378 Mg2+, 1.9 Mn2+, 0.8 Zn2+, 0.14 Cu2+, 19.2 H3BO3,
0.24 Mo, 25 Fe2+and 20 Cl−(pH 5.8). Subsequently,
and for the remaining 10 weeks of the experiment, the plants were grown in a glasshouse, where they received natural daylight supplemented by growth lamps (Son-T-Agro 400W (Philips, Köln, Germany) and HQI-Lamps (Osram, München, Germany); ap-proximately 400–500μmol m2s−1(PAR) at the level of the leaves). An automated watering system was used (Fig. 1b) so that each root-half received 15 ml nutrient solution at 6, 12, 18 and 24 h (as above without P) that had been buffered (using 1 M MES) to pH 5.5 or buffered to pH 7.5.
Plant harvest and chemical analyses of tissues
After 10 weeks of treatment (13 weeks growth), the plants were harvested and fresh mass determined for stems, and young, mature and older leaves. The rhizoboxes were carefully opened, soil samples taken (see below), and the roots gently removed and washed with DI water to remove soil. The fresh mass was determined for cluster and non-cluster roots on each root half.
Tissues were dried (to constant mass) at 70°C for 1 week, and ground in a ball-mill. Samples of ground tissues were acid-digested in HNO3/H2O2 (3:1 v/v).
Concentrations of macro-nutrients (P, Mg, K, Ca), and micronutrients (Mn, Fe, Zn and Cu) were determined by inductively coupled plasma with mass spectrosco-py (ICP-MS, ELAN 6100 Perkin Elmer). Concen-trations of N and S were determined after combustion of dried, homogenised, plant material at 1,000°C in flowing oxygen using a LECO CHNS-932 (Leco Corporation, St Joseph, MI, USA).
Solubilities of Al–P and Fe–P
Samples of Al–P or Fe–P were shaken for 24 h in nutrient solutions (as above) buffered at pH 5.5 or 7.5, and then filtered (0.45 μm). Total P concentration was
deter-mined using the Malachite green method (Motomizu et al.1983).
Carboxylate analysis by HPLC
Samples of soil/resin were taken from the bulk soil in each rhizobox compartment where no roots were growing and within 3 mm around cluster roots. Carboxylates were eluted from sub-samples by tumbling for 3 h in 0.3 ml of 1 M HCl. Samples were filtered to 0.45 μm and stored at 4°C until analysis. Carboxylates were separated on an Alltima C-18 column (250 mm long × 4.6 mm internal diameter with 5μm diameter packing), and identified using Waters® HPLC (600E pump, 717 auto injector and 996 photodiode-array detector, Milford, MA, USA). The mobile phase was a mixture of 25 mM KH2PO4 (pH 2.50) and MeOH (i.e. 93%:7%)
(pH 2.50) at a flow rate of 1 ml min−1. Detection
was at 210 nm, but data from 195 to 400 nm were collected and used for spectrum matching and peak purity analysis according to Cawthray (2003). The sample injection volume was generally 100 μl, but was reduced for samples with very high citrate concentrations. The column was completely flushed (gradient elution using 60% (v/v) methanol) after every 10 samples to eliminate the transfer of highly nonpolar compounds. Data acquisition and processing was with Millennium© software (Waters, Milford MA, USA). Retention times of organic acid standards including malic, iso-citric, malonic, lactic, acetic, maleic, citric, succinic, fumaric, cis-aconitic and trans-aconitic acids were used to identify carboxylates in rhizosphere extracts.
Measurements of malate and citrate decomposition
In a separate experiment (at the University of Western Australia), white lupin (n=12) were grown from seed in acid-washed quartz sand mixed 3:1 (as above) with anion-exchange resin in the Cl− from. Small nylon
extractions were carried out on each sub-sample as follows, (1) in 5 ml of DI water for 1.5 h on a shaker, (2) in 5 ml 0.5 M HCl for 1 h, and (3) again in 5 ml 0.5 M HCl for 1 h. After each extraction a 1-ml sample was filtered for HPLC analysis. Between extractions the remaining extraction solution was removed by filtration using a syringe and syringe filter (0.45 μ). The plants were also harvested and resin/soil that was not tightly bound to the cluster roots (Fig.1c) was removed by gentle shaking. Only resin beads strongly attached to cluster rootlets by root hairs (Fig.1d) were collected and extracted twice in 1.5 ml of 0.5 M HCl for 1 h on a shaker, filtered and a sample taken for HPLC analysis. The dry mass for each cluster root associated with resin beads collected for carboxylate extraction (24 roots from 12 plants) was determined. All samples were analysed for carboxylates as described above.
Statistics
Data were analysed with two-way analysis of variance (GenStat 7.1, Lawes Agricultural Trust; Rothamsted Experimental Station). Tukey’s pair-wise multiple comparison tests were used to determine which levels differed significantly (α=0.05).
Results
Solubility of Al–P and Fe–P
The solubility of both phosphorus sources was very low (i.e. <0.2% of the total amount of P added as either Al–P or Fe–P). However, Al–P was more soluble (0.17% and 0.12% at pH 5.5 and 7.5, respectively) than Fe–P (0.09% and 0.07% at pH 5.5 and pH 7.5, respectively).
Plant growth and cluster-root formation
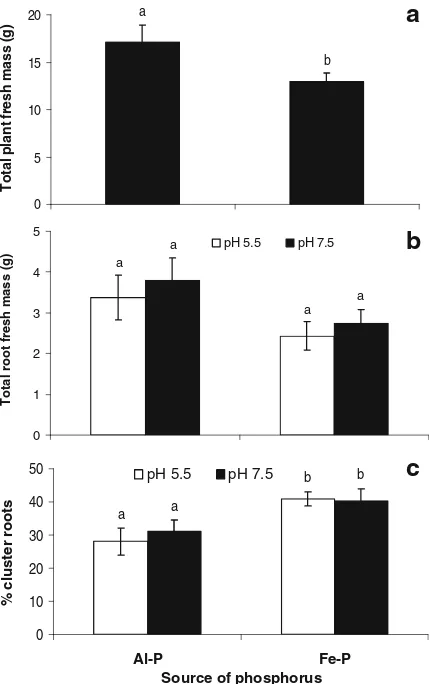
At harvest, after 10 weeks treatment, plants grown on Al–P were significantly larger than plants grown on Fe–P (Fig. 2a). Root fresh mass was greater for the plants supplied with Al–P but there was no difference in mass between root halves grown on pH 5.5 or 7.5 (Fig. 2b). The root mass ratio (RMR) (ca. 0.40, data not shown) was the same for both P treatments. The proportion of cluster roots (expressed as cluster-root
fresh mass as a percentage of total root mass) was significantly greater for plants supplied with Fe–P (Fig. 2c), but there was no difference between root halves grown on pH 5.5 or 7.5.
Carboxylates in the cluster-root rhizosphere
For plants supplied with Al–P or Fe–P, citrate was the only carboxylate detected above trace amounts (e.g., malate) in soil/resin within 3 mm of the cluster roots (not shown). A significantly greater [citrate] was recovered from around the cluster roots of plants grown on the less soluble source of P, i.e. Fe–P (up to 2.3μmol citrate g−1
a
b
0 5 10 15 20
Total plant fresh mass (g)
a
a
a a
a
0 1 2 3 4 5
Total root fresh mass (g)
pH 5.5 pH 7.5
b
a
b a
b
0 10 20 30 40 50
Al-P Fe-P
Source of phosphorus
% cluster roots
pH 5.5 pH 7.5
c
rhizosphere soil) compared with that of plants grown on Al–P (up to 1.2 μmol citrate g−1 soil) (Fig. 3). Plants grown on Fe–P had the same [citrate] around the cluster roots grown at pH 7.5 or 5.5. Interestingly, plants grown on Al–P had significantly greater [citrate] (ca. double) around the cluster-roots on root halves supplied with nutrient solution buffered at pH 7.5 compared with that of the other root half supplied with nutrient solution buffered at pH 5.5 (Fig. 3). Anion exchange resin beads in close proximity to the cluster roots were sometimes observed to be dark-brown and black. We collected 68 mg of these dark resin beads
and measured citrate at ca. 12.8μmol citrate g−1resin bead, compared with 2.5μmol citrate g−1resin for the non-discoloured beads (Table1).
Leaf and stem nutrients
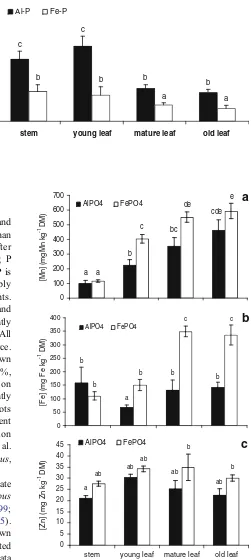
Macronutrients Plants supplied with Al–P had ap-proximately double the tissue [P] compared with that of plants supplied with Fe–P. Leaf [P] tended to decrease with leaf age (Fig.4). In contrast to [P], the leaf concentrations of [N], [S], [Ca], [K] and [Mg] were similar for plants supplied with Fe–P or Al–P, and nutrient levels were considered sufficient for adequate growth (data not shown; Marschner 1995).
Micronutrients The [Mn] (Fig. 5a), [Cu] (not shown) and [Fe] (Fig. 5b) in stems was similar for plants supplied with Fe–P or Al–P. The [Mn], [Cu] (not shown), [Fe] and [Zn] tended to be greater in leaves (young, mature and older) of plants supplied with Fe–P compared with those of plants supplied with Al–P (Fig. 5a,b,c). The leaf [Mn] (Fig. 5a) and leaf [Fe] (Fig.5b) increased significantly with increasing leaf age, whereas leaf [Zn] was unchanged. The levels of tissue micronutrients (except leaf [Mn] below) of all plants were considered neither deficient nor toxic, and suffi-cient for adequate growth (data not shown, Marschner
1995). However, the leaf [Mn] (up to 600 mg Mn kg−1
dry mass), which are considered extremely high for many plant species (Marschner1995), is characteristic in leaves of many cluster-root forming species, including
L. albus(see the“Introduction”section).
Recovery of malate or citrate from anion exchange resins
After 4 weeks buried in soil, we recovered 50% and 79% of the malate and citrate, respectively, which had been pre-loaded onto the anion exchange resin beads (Table 1). Of the total amounts of citrate and malate, 0.2% was extracted in water, 83% in the first HCl extraction and 17% in the second HCl extraction.
Both citrate and malate were detected in extracts of anion exchange resin beads that had been strongly bound to branch rootlets of cluster roots. However, the [citrate] was 10–20 times greater compared with malate (expressed either on a resin basis or a root dry weight basis, see Table1).
Table 1 Concentration of malate and citrate eluted from anion exchange resin beads that had been adhering to cluster rootlets when collected, and the percentage of malate and citrate recovered from anion exchange resin after pre-loading the resin with malate or citrate and burying resin bags in soil for 4 weeks
Malate Citrate
Cluster roots
Resin mass basis (μmol g−1) 0.22 (0.04) 2.5 (0.5) Cluster root dry mass basis (μmol g−1) 1.6 (0.3) 40 (8)
Percent recovery 50 (3) 79 (2)
Carried out at the University of Western Australia in summer/ autumn 2007; n=24 cluster roots and n=6 malate andn=5 citrate resin bags. Standard errors in parentheses.
a
c
b
c
0 0.5 1 1.5 2 2.5 3
Al-P Fe-P
Source of phosphorus
µ
mol citrate g
-1 rhizosphere soil
pH 5.5 pH 7.5
Fig. 3 Concentration of citrate in the rhizosphere of cluster roots from root compartments supplied with nutrient solution buffered at pH 5.5 or 7.5 forLupinus albusL. grown on Al–P or Fe–P.Barsare SE,n=15 (3 cluster roots on each root half).
Different letters above each barindicate significant differences
Discussion
Influence of P-form
Plants grown on Al–P had the largest mass and accumulated 3 times more P (14.6 mg total P) than plants grown on Fe–P (4.7 mg total P) after accounting for initial seed P reserves (ca. 1 mg P seed−1DM, Shane et al.2003a). The fact that Al–P is
ca. 1.8 times more soluble than Fe–P probably accounts for most of the increase in P in those plants. When the P source was Fe–P, the [P] in stems and leaves of L. albus plants was always significantly lower than that of plants grown on Al–P (Fig.4). All plants developed cluster roots, regardless of P source. However, in the relatively lower P-status plants grown on Fe–P the cluster-root proportions were ca. 40%, while in the relatively less P-stressed plants grown on Al–P the proportion of cluster roots was significantly lower (ca. 30%). A reduction in cluster roots concomitant with increased shoot [P] is consistent with systemic regulation of cluster-root formation (e.g., L. albus, Gilbert et al. 1997; Marschner et al.
1986, 1987; Shane et al. 2003a; and L. luteus, Hocking and Jeffery2004).
Citrate is almost invariably the major carboxylate measured in exudates from cluster roots of L. albus
(Gardner et al. 1982a; Watt and Evans 1999; Neumann and Martinoia 2002; Zhu et al. 2005). Compared with Al–P grown plants, the Fe–P grown plants had significantly greater [citrate] associated with their cluster roots (Figs. 3 and 4). These data clearly confirm previous findings that the amount of citrate released by cluster roots is determined by shoot P status (Neumann et al.1999; Shane et al.2003a)–
stem young leaf mature leaf old leaf
Phosphorus (
µ
g P g
-1 DM)
Al-P Fe-P
Fig. 4 Phosphorus concen-trations in stems and leaves (young, mature and older) ofLupinus albusL grown on Al–P or Fe–P. Bars are SE,n=5.Different letters above each barindicate significant differences
stem young leaf mature leaf old leaf
[Zn] (mg Zn kg
-1 DM)
AlPO4 FePO4
c
most likely the influence is systemic, as discussed above for cluster-root growth.
A greater mobilisation of Mn (and Fe) often occurs concomitantly with citrate accumulation in the clus-ter-root rhizosphere of P-deficient L. albus (Gardner et al.1982b; Dinkelaker et al.1989) andH. prostrata
(Shane and Lambers 2005b). Therefore, it is not surprising that the present study also showed signif-icantly greater leaf [Mn] for Fe–P grown plants that had significantly greater [citrate] associated with their cluster roots (Fig.5a).
Influence of pH
The finding by Peiter et al. (2001) that cluster-root growth doubled inL. albusgrown in nutrient solution buffered at pH 7.7 as compared with that at pH 5.5 was interpreted as local root-growth regulation by pH, even though suppression of cluster-root growth due to shoot [P] differences was not ruled out. The evidence in Fig. 2c that equal proportions of cluster roots developed on root halves of individual plants grown at pH 5.5 or 7.5, contrasts with the finding by Peiter et al. (2001). The present data do, however, support previous findings that shoot [P] is strongly associated with the extent of cluster-root growth (Gardner et al.
1982b; Shane et al.2003a,b). On the other hand, the amount of P supplied to roots can locally influence cluster-root growth. Shu et al. 2007, using split-root studies withL. albus, showed that cluster-roots were significantly suppressed by 6–10% on root halves that had greater P supply. In the present study, given the significantly greater solubility of both P sources at pH 5.5, equal cluster-root proportions on root halves would mean that any local influence of pH on cluster-root growth was indirect, perhaps on shoot P though greater P availability in the root environment.
The exudation of citrate into the soil by cluster roots ofL. albusgrowing on 11 different types of soil was examined by Veneklaas et al. (2003). Whereas citrate and malate were the major carboxylates in the rhizosphere, they found that the proportion of malate to citrate varied as a function of soil pH: more malate at pH<6, and more citrate at pH>6.5. In the present study, [citrate] was significantly greater in soils associated with cluster roots at pH 5.5 compared with that at pH 7.5 (Fig. 3), although that was only significant for plants grown on Al–P. Split-root studies have shown that the amount of citrate released
from cluster roots can be increased locally by reducing [P] in the root environment (e.g., L. albus, Shu et al.2007andH. prostrata, Shane et al.2003a,
b). It is possible that soil pH influences [citrate] accumulation locally, but the influence would be indirect, and due to higher solution [P] at acidic pH, as found in the present study (i.e. increased solubility of Al–P at pH 5.5 as compared with pH 7.5).
Measurements of rhizosphere carboxylates: implications of decomposition in soil
The decomposition of carboxylates around roots prior to sampling root exudates is a major concern for identifying and estimating carboxylates in the rhizo-sphere (Jones 1998). There are few data available in the literature on carboxylate decomposition in soils, but Jones et al. (1996) found that malate degradation in acid soil can be rapid (half life approximately 1.7 h). However, decomposition was extremely slow where carboxylates were precipitated with earth metals (i.e. Ca-oxalate) or fixed to the soil’s exchange phase (Ström et al. 1994; Jones et al. 2003). Our finding that anion-exchange resins prevented com-plete degradation of citrate and malate, and that malate decomposed faster than citrate (under our growth conditions) may have important consequences for comparing [carboxylate] in soil. Although citrate is invariably present in large concentrations in the rhizosphere of cluster roots, faster decomposition rates of malate (as compared with citrate) in soil may result in underestimation of malate (and other carboxylates) in soil extracts.
Our finding of almost 80% citrate recovery (Table 1) allows for reasonable calculations to be made from the present data. Dinkelaker et al. (1989) estimated the dissolved citrate concentration associat-ed with cluster roots ofL. albusgrown in a calcareous soil to be ca. 1.1 μmol g−1soil. Our finding of 0.5– 2.5 μmol citrate g−1 soil/resin mixture for plants grown on Al–P or Fe–P fits well with values calculated from the literature (Dinkelaker et al.
1989; Gerke et al. 1994). For example, an average rate of citrate exudation, i.e. 100 pmol citrate g−1root
fresh mass s−1(from Table 3 in Jones1998) over 24 h
gives 8.6μmol, if the rates persist for 3–4 days. That equates to approximately the amount of citrate released from 1 g of cluster roots in our study. Table1
citrate g−1 root DM were present on resin beads
directly attached to the cluster rootlets. The finding that malate was 10–20 times less concentrated compared with that of citrate is perhaps consistent with at least some decomposition of the malate (and citrate) originally exuded.
Concluding remarks
Cluster-root growth and the amount of citrate released byL. albuscluster roots were influenced by P-source (Al–P or Fe–P) and rhizosphere pH (5.5 or 7.5). Cluster-root growth and exudation of citrate in L. albuswas strongly decreased when plants were grown on Al–P which was likely due to greater solubility of Al–P and hence greater shoot [P] in those plants. The P sources (Al–P and Fe–P) and pH (5.5 and 7.5) used were not associated with the release of different carboxylates. However, enhanced [P] in the root environment of plants grown on Al–P at pH 7.5 demonstrated some local influence on citrate accu-mulation in the cluster-root rhizosphere. Carboxylates in soil need further study; they differentially degrade and may not represent the suite of carboxylates initially released.
Acknowledgements Helpful comments by anonymous reviewers were greatly appreciated. MWS was the recipient of a Research Fellowship at the Institute Phytosphere Forschungs-zentrum, Jülich, Germany (kindly provided by Professor U. Schurr) and a Janice Klumpp award from the University of Western Australia. Many thanks to Carola Mohl for the ICP-MS analysis, Manfred Michulitz for the N and S measurements and to Marion Roeb for the help in setting-up the rhizoboxes and growing the plants. Also, thanks to Drs. Siegfried Jahnke, Peter Minchin, Björn Thiele and Achim Walter for the helpful discussions. Many thanks to Dr. Walter Schröder for help with the SEM image in Fig. 1 (all FZ Jülich). This research was supported in part by the Australian Research Council.
References
Braum SM, Helmke PA (1995) White lupin utilises soil phosphorus that is unavailable to soybean. Plant Soil 176:95–100
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromatogr A 1011:233–240
Clements JC, White PF, Buirchell BJ (1993) The root morphology ofLupinus angustifoliusin relation to other
Lupinusspecies. Aust J Agr Res 44:1367–1375
Dinkelaker B, Römheld V, Marschner H (1989) Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albusL.). Plant Cell Environ 12:285–292
Dinkelaker B, Hengeler C, Marschner H (1995) Distribution and function of proteoid root clusters and other root clusters. Bot Acta 108:183–200
Dinkelaker B, Hengeler G, Neumann G, Eltrop L, Marschner H (1997) Root exudates and mobilization of nutrients. In: Rennenberg H, Eschrich W, Ziegler H (eds) Trees— contributions to modern tree physiology. Backhuys, Leiden, The Netherlands, pp 441–452
Gardner WK, Parbery DG, Barber DA (1981) Proteoid root morphology and function in Lupinus albus. Plant Soil 60:143–147
Gardner WK, Parbery DG, Barber DA (1982a) The acquisition of phosphorus byLupinus albusL. I. Some characteristics of the soil/root interface. Plant Soil 68:19–32
Gardner WK, Parbery DG, Barber DA (1982b) The acquisition of phosphorus by Lupinus albus L. II. The effect of varying phosphorus supply and soil type on some characteristics of the soil/root interface. Plant Soil 68:33– 41
Gerke J, Römer W, Jungk A (1994) The excretion of citric and malic acid by proteoid roots ofLupinus albusL.: effects on soil solution concentrations of phosphate, iron, and alumi-num in the proteoid rhizosphere in samples of an oxisol and a luvisol. Z Pflanzenernähr Bodenkd 157:289–294 Gilbert GA, Allan DA, Vance CP (1997) Phosphorus deficiency
in white lupin alters root development and metabolism. In: Flores HE, Lynch JP, Eissenstat D (eds) Radical biology: advances and perspectives on the function of plant roots. American Society of Plant Physiologists, Rockville, USA, pp 92–103
Hagström J, James WM, Skene KR (2001) A comparison of structure, development and function in cluster roots of
Lupinus albusL. under phosphate and iron stress. Plant Soil 232:81–90
Hocking P, Jeffery S (2004) Cluster root production and organic anion exudation in a group of old world lupins and a new world lupin. Plant Soil 258:135–150
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Jones DL, Prabowo AM, Kochain LV (1996) Kinetics of malate transport and decomposition in acid soils and isolated bacterial populations: the effect of microorganisms on root exudation of malate under Al stress. Plant Soil 182:239– 247
Jones DL, Dennis PG, Owen AG, van Hees PAW (2003) Organic acid behavior in soils—misconceptions and knowledge gaps. Plant Soil 248:31–41
Keerthisinghe G, Hocking P, Ryan PR, Delhaize E (1998) Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albusL.). Plant Cell Environ 21:467–478
Lambers H, Juniper D, Cawthray GR, Veneklaas EJ, Martinez E (2002) The pattern of carboxylate exudation inBanksia grandis(Proteaceae) is affected by the form of phosphate added to the soil. Plant Soil 238:111–122
Liang R, Li C (2003) Differences in cluster-root formation and carboxylate exudation inLupinus albusL. under different nutrient deficiencies. Plant Soil 248:221–227
Longnecker N, Brennan R, Robson A (1998) Lupin nutrition. In: Gladstones JS, Atkins C, Hamblin J (eds) Lupin as crop plants. Biology, production and utilization. CAB International, Wallingford, Oxfordshire, UK, pp 121–148 Marschner H (1995) Mineral nutrition of higher plants, 2nd
edn. Academic, London, UK
Marschner H, Römheld V, Horst WJ, Martin P (1986) Root-induced changes in the rhizosphere: Importance for the mineral nutrition of plants. Z Pflanzenernähr Bodenkd 149:441–456
Marschner H, Römheld V, Cakmak I (1987) Root-induced changes of nutrient availability in the rhizosphere. J Plant Nutr 10:1175–1184
McCluskey J, Herdman L, Skene KR (2004) Iron deficiency induces changes in metabolism of citrate in lateral roots and cluster roots ofLupinus albus. Physiol Plant 121:586–594 Motomizu S, Wakimoto T, Toei K (1983) Spectrophotometric determination of phosphate in river waters with molybdate blue and malachite green. Analyst 108:361–367
Neumann G, Martinoia E (2002) Cluster roots—an under-ground adaptation for survival in extreme environments. Trends Plant Sci 7:162–167
Neumann G, Massonneau A, Martinoia E, Römheld V (1999) Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 208:373– 382
Pate JS, Dell B (1984) Economy of mineral nutrients in sandplain species. In: Pate JS, Beard JS (eds) Kwongan— plant life of the sandplain. University of Western Australia Press, Nedlands, Australia, pp 227–252
Peiter E, Yan F, Schubert S (2001) Proteoid root formation of
Lupinus albus L. is triggered by high pH of the root medium. J Appl Bot 75:50–52
Playsted CWS, Johnston ME, Ramage CM, Edwards DG, Cawthray GR, Lambers H (2006) Functional significance of dauciform roots: exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei(Cyperaceae). New Phytol 170:491–500
Shane MW, Lambers H (2005a) Cluster roots: a curiosity in context. Plant Soil 274:101–125
Shane MW, Lambers H (2005b) Manganese accumulation in leaves of Hakea prostrata R.Br. (Proteaceae) and the significance of cluster roots for micronutrient uptake as dependent on phosphorus supply. Physiol Plant 274:441– 450
Shane MW, Lambers H (2006) Systemic suppression of cluster-root formation and net P-uptake rates in Grevillea crithmifolia at elevated P supply: a proteacean with resistance for developing symptoms of ‘P toxicity’. J Exp Bot 57:413–423
Shane MW, de Vos M, de Roock S, Lambers H (2003a) Shoot P status regulates cluster-root growth and citrate exudation inLupinus albusgrown with a divided root system. Plant Cell Environ 26:265–273
Shane MW, De Vos M, De Roock S, Cawthray GR, Lambers H (2003b) Effect of external phosphorus supply on internal phosphorus concentration and the initiation, growth and exudation of cluster roots inHakea prostrataR.Br. Plant Soil 248:209–219
Shane MW, Cramer MD, Funayama-Noguchi S, Cawthray GR, Millar AH, Day DA, Lambers H (2004) Developmental physiology of cluster-root carboxylate synthesis and exudation in Harsh Hakea. Expression of phospho
enol-pyruvate carboxylase and the alternative oxidase. Plant Physiol 135:549–560
Shane MW, Cawthray GR, Cramer MD, Kuo J, Lambers H (2006) Specialised ‘dauciform’ roots of Cyperaceae are structurally distinct, but functionally analogous with ‘cluster’roots. Plant Cell Environ 29:1989–1999 Shu L, Shen JU, Rengel Z, Tang C, Zhang F, Cawthray GR
(2007) Formation of cluster roots and citrate exudation by
Lupinus albus in response to localised application of different phosphorus sources. Plant Sci 172:1017–1024 Skene KR (1998) Cluster roots: some ecological
considera-tions. J Ecol 86:1060–1064
Skene KR (2000) Pattern formation in cluster roots: some developmental and evolutionary considerations. Ann Bot 85:901–908
Ström L, Olsson T, Tyler G (1994) Differences between calcifuge and acidifuge plants in root exudation of low-molecular organic-acids. Plant Soil 167:239–245 Trinick MJ (1977) Vesicular–arbuscular infection and soil
phosphorus utilization in Lupinus spp. New Phytol 78:297–304
Vance CP, Uhde-Stone C, Allen DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a non-renewable source. New Phytol 157:423– 447
Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197
Walker BA, Pate JS (1986) Morphological variation between seedling progenies of Viminaria juncea (Schrad. & Wendl.) Hoffmans. (Fabaceae) and its physiological significance. Aust J Plant Physiol 13:305–319