www.elsevier.com/locate/ibmb
A review of the role of neurosecretion in the control of juvenile
hormone synthesis: a tribute to Berta Scharrer
Barbara Stay
*Department of Biological Sciences, University of Iowa, Iowa City, IA 52242, USA
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
In the 1950s, Berta Scharrer predicted that neurosecretions from the brain regulated corpus allatum activity based upon the
observation of the change in localization of neurosecretory material in the brain and change in gland activity after severance of nerves
between the brain and corpus allatum. Isolation and characterization of neuropeptide regulators of juvenile hormone production by
the corpora allata in the late 1980s has confirmed this prediction. Both a stimulatory allatotropin and an inhibitory allatostatin have
been isolated from moth brains. Two families of allatostatins, both quite different from each other and that of moths, have been
isolated from cockroaches and crickets.
The wide distribution of these peptides in the nervous system, in nerves to visceral muscle, in endocrine cells of the midgut and
in blood cells, indicate multifunctions in the insects in which they are allatoregulatory. Some of these other functions have been
demonstrated in these insects and in insects in which these neuropeptides occur but do not act as corpus allatum regulators. For
the latter group, the neuropeptide regulators of the corpora allata have yet to be isolated. The families of neurosecretory regulators
will continue to grow.
2000 Elsevier Science Ltd. All rights reserved.
Keywords: Allatostatin; Allatotropin; Corpora allata; Juvenile hormone; Neurosecretion
1. Berta Scharrer’s experiment showing inhibitory
action of brain on corpora allata
Berta and Ernst Scharrer were founders of the concept
of neurosecretion. Their studies of invertebrates and
ver-tebrates, respectively, provided evidence for the
impor-tance and long evolutionary history of neuropeptides
(Scharrer and Scharrer, 1945, 1963). Berta Scharrer
demonstrated the neurohormonal regulation of the
corpora allata (CA) in experiments on the cockroach
Leucophaea maderae (Scharrer, 1952). After unilateral
severance of the nervi corporis cardiaci emanating from
the pars intercerebralis of the brain, she observed after
several
days:
(1)
accumulation
of
neurosecretory
material proximal to the cut, (2) disappearance of it
dis-tal to the cut and (3) enlargement of the corpus allatum
on the operated side. In the discussion of this paper she
noted that supernumerary molts of last instar nymphs
* Fax:+1-319-335-1069.
E-mail address: [email protected] (B. Stay).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 3 6 - 9
following this operation suggested brain regulation of
corpus allatum hormone. Furthermore, she assumed
from physiological effects and morphological change of
the glands that the “severance constitutes an increase
rather than a decrease in glandular activity.” In these
experiments Scharrer paved the way for the isolation and
characterization of the neurosecretory material in the
brain that could restrain, as in her experiment, or
stimu-late the production of juvenile hormone (JH).
2. Brain innervation of the corpora allata
clearer picture of the axon pathways from the brain to
the CA. In the cockroach Periplaneta americana this
method showed cells located in the contralateral pars
intercerebralis and the ipsilateral pars lateralis projecting
to the CA through the nervi corporis cardiaci (NCC) 1
and 2, respectively (Pipa, 1978). More recently,
Virant-Doberlet et al. (1994) found that two of the lateral cells
project contralaterally in the NCC2 to the CA in this
cockroach and in the cricket Gryllus bimaculatus. A
similar projection of protocerebral medial and lateral
cells was shown for the moth of the tobacco hornworm,
Manduca sexta, in which the NCC1 and 2 leave the brain
as one nerve (Copenhaver and Truman, 1986). These and
other retrograde diffusion studies identified the brain
cells whose products might influence juvenile hormone
synthesis by the CA.
3. Isolation of the neuropeptides affecting JH
production by the CA
This endeavor was much facilitated by the
measure-ment of JH production by CA in vitro, a method
developed by Pratt and Tobe (1974) after the structure
of JH was discovered (Ro¨ller et al., 1967) and methods
for purification of small amounts of material became
available. It was also spurred on by numerous
experi-mental demonstrations of brain regulation of the CA. For
all of the novel allatoactive peptides so far isolated, the
short-term radiochemical assay has been used to identify
the active fractions. However, identification of similar
peptides whether active in the species in which they were
found or not could then be achieved by immunological
or molecular biological techniques.
4. One identified allatotropin
The first allatal-active neuropeptide isolated was the
allatotropin of M. sexta (Kataoka et al., 1989), for which
the gene has now been cloned (Taylor et al., 1996). This
peptide has been isolated from and shown to be active
in a second (Spodoptera frugiperda) and possibly a third
lepidopteran, the tomato moth Lacanobia oleracea
(Table 1). The Mas-allatotropin has also been cloned in
Pseudaletia unipuncta and their CA show allatostatin
immunoreactivity to this peptide (Truesdell et al., 2000).
These data strongly suggest that the Mas-allatotropin is
also a true allatotropin in P. unipuncta. Originally found
to stimulate only adult CA (Audsley et al., 1999a,b), this
peptide is now shown, under favorable assay conditions,
to be active on larval CA of L. oleracea (Audsley et al.,
2000) and on CA of certain larval stages in the honey
bee Apis mellifera (Rachinsky and Feldlaufer, 2000;
Rachinsky et al., 2000). To date, this 13 amino acid
ami-dated peptide is the only allatotropin to be identified. It
occurs in M. sexta larval brain in one or two pairs of
ipsilateral lateral cells (1a
1), two pairs of contralateral
lateral cells (III) and numerous axons in the corpora
car-diaca and corpora allata as shown by immunoreactivity
to antibodies against this peptide (Zitnan et al., 1995).
Immunoreactivity and in situ hybridization indicate that
the allatotropin occurs in other nerves of the central
ner-vous system and in frontal ganglion cells projecting to
the gut (Bhatt and Horodyski, 1999).
4.1. Allatotropins of unknown structure
Clearly there are other allatotropic neurosecretions to
be discovered. Some examples which have the potential
for success in isolation of allatotropins are given in
Table 2. The Galleria mellonella peptide has been
par-tially purified and an antibody against it developed
(Bogus and Scheller, 1996). The G. bimaculatus putative
allatotropin was extracted from subesophageal ganglia
(SOG) and is not species specific; it acts also on the
cricket Acheta domesticus (Lorenz and Hoffmann,
1995). As was found with the known allatotropin, the
actions of these putative allatotropins are stage specific
in their stimulation of the CA, and the CA of Locusta
migratoria require special incubation treatment for
suc-cessful stimulation by the allatotropin (Table 2).
4.2. Allatotropins from non-neural sources
Factors other than neuropeptides are known to
stimu-late JH synthesis. In the cockroach Diploptera punctata
the ovary stimulates increased JH production (Rankin
and Stay, 1984). Also, the ovary of G. bimaculatus
implanted into a male resulted in increased hormone
pro-duction by the CA, but extract of ovaries did not
stimu-late CA in vitro (Hoffmann et al., 1996). It remains to
be determined whether ovaries produce allatotropins that
act directly on the CA.
The male accessory sex gland of Drosophila
mel-anogaster produces a 36 amino acid peptide of known
sequence that, when transferred to the female during
mating, stimulates the CA, and does so also when CA
from virgin flies are incubated with this sex peptide in
vitro (Moshitzky et al., 1996). This peptide also acts as
an allatotropin on CA of the noctuid moth Helicoverpa
armigera (Fan et al., 1999). However, in the medfly
Cer-atitis capitata the Drosophila sex peptide stimulates CA
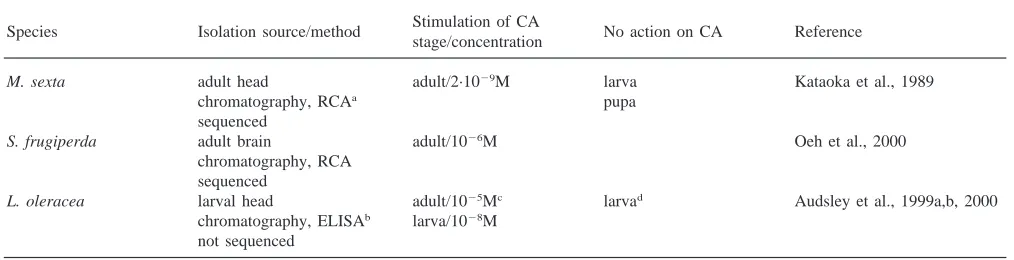
Table 1
The allatotropin of Manduca, Spodoptera, and Lacanobia: Gly–Phe–Lys–Asn–Val–Glu–Met–Met–Thr–Ala–Arg–Gly–Phe–NH2 Stimulation of CA
Species Isolation source/method No action on CA Reference
stage/concentration
M. sexta adult head adult/2·1029M larva Kataoka et al., 1989
chromatography, RCAa pupa
sequenced
S. frugiperda adult brain adult/1026M Oeh et al., 2000
chromatography, RCA sequenced
L. oleracea larval head adult/1025Mc larvad Audsley et al., 1999a,b, 2000 chromatography, ELISAb larva/1028M
not sequenced aRadiochemical assay for JH synthesis. b ELISA with antibody for Manduca allatotropin. cAudsley et al., 1999a.
d Audsley et al., 1999b.
Table 2
Examples of allatotropins of unknown structure
Species Source Stimulation of CA in vitro Reference
Galleria mellonella larval brain larval Bogus and Scheller, 1996
Pyrrhocoris apterus adult brain – SOGa–CC–CA adult, long day Hodkova´ et al., 1996 Gryllus bimaculatus
adult SOG adult, 0-day Lorenz and Hoffmann, 1995
Acheta domesticus
Locusta migratoria adult brain – CC adult, chilled 4°C, 24 h Lehmberg et al., 1992 aSubesophageal ganglion.
5. Three identified allatostatins
Mating in Drosophila stimulates JH production by the
female corpus allatum as a result of the transfer of
allato-tropic sex peptide. Mating in the cockroach D. punctata
also stimulates JH production, but it does so,
presum-ably, by signaling the brain not to release inhibitory
neu-ropeptide. Allatostatin (AST) is the name given to such
peptides and the first ones were isolated from D.
punctata brains (Woodhead et al., 1989; Pratt et al.,
1991). The three types of ASTs isolated thus far are
sum-marized in Table 3.
The M. sexta AST is a 15 amino acid non-amidated
pep-tide, completely different from the cockroach ASTs
(Kramer et al., 1991). The M. sexta AST inhibited CA
of adult Heliothis virescens (Kramer et al., 1991) and
also inhibited adult CA of S. frugiperda only after the
glands were stimulated by M. sexta allatotropin (Oeh et
al., 2000). A peptide that co-eluted with and had a
mol-ecular mass consistent with Mas-AST was isolated from
another lepidopteran, L. oleracea, but whether it is a true
AST in this insect is questionable because a much higher
concentration is required for its action than in M. sexta
(Audsley et al., 1998).
The cockroach allatostatins are a family of peptides with
a common pentapeptide amidated C-terminal sequence
and a variable number and sequence of amino acids at
the N-terminus. Several members of this family have
been isolated from other cockroaches in several different
families (Weaver et al., 1994; Belle´s et al., 1994) and
molecular characterization of the AST genes in these and
other cockroaches revealed that the gene sequence
con-tains 13 or 14 ASTs (Belle´s et al., 1999). Two members
of this Tyr–Xxx–Phe–Gly–Leu–NH
2(YXFGL-amide)
family were isolated from the cricket G. bimaculatus
(Lorenz et al., 1995a) and a different family of ASTs,
four nonapeptides, were also found in this cricket
(Lorenz et al., 1995b).
The allatostatins have been shown to act on adult and
larval stages, and in D. punctata in embryos (Holbrook
et al., 1996). Several studies have shown that even
within one stage, the effect of the AST varies with the
physiological state of the CA (Pratt et al., 1990; Stay et
al., 1991a; Weaver, 1991). This is an important
consider-ation when assaying material for allatostatic or
allato-tropic action.
5.1. Localization of allatostatin in brain and other
tissues
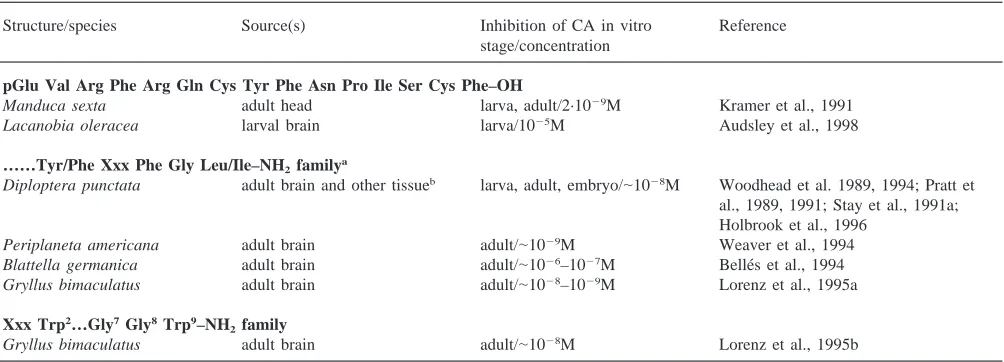
Table 3
Isolated allatostatins
Structure/species Source(s) Inhibition of CA in vitro Reference
stage/concentration
pGlu Val Arg Phe Arg Gln Cys Tyr Phe Asn Pro Ile Ser Cys Phe–OH
Manduca sexta adult head larva, adult/2·1029M Kramer et al., 1991
Lacanobia oleracea larval brain larva/1025M Audsley et al., 1998
……Tyr/Phe Xxx Phe Gly Leu/Ile–NH2familya
Diploptera punctata adult brain and other tissueb larva, adult, embryo/
|1028M Woodhead et al. 1989, 1994; Pratt et al., 1989, 1991; Stay et al., 1991a; Holbrook et al., 1996
Periplaneta americana adult brain adult/|1029M Weaver et al., 1994 Blattella germanica adult brain adult/|1026–1027M Belle´s et al., 1994 Gryllus bimaculatus adult brain adult/|1028–1029M Lorenz et al., 1995a
Xxx Trp2…Gly7Gly8Trp9–NH 2family
Gryllus bimaculatus adult brain adult/|1028M Lorenz et al., 1995b
aThe cDNA for these cockroach species and three others indicates families of 13–14 peptides (Donly et al., 1993; Ding et al., 1995; Belle´s et al., 1999).
b CA, muscle extract, and hemolymph showed peptide coeluting with synthetic allatostatin (Stay et al., 1991b; Woodhead et al., 1992, 1993); midgut cells (Reichwald et al., 1994; Yu et al., 1995b) and hemocytes (Skinner et al., 1997) contain allatostatin mRNA.
immunocytochemically to be lateral protocerebral cells.
In M. sexta they are 1a
2and 1b cells that project axons
to the ipsilateral CA with branching in the corpora
car-diaca and within the CA (Zitnan et al., 1995). In D.
punctata and G. bimaculatus it is also the lateral
proto-cerebral cells and the NCC2 that show allatostatin
immu-noreactivity which is seen in nerve branches in the
corpora cardiaca and the CA (Stay et al., 1992;
Neu-ha¨user et al., 1994). In addition to these cells that project
to the CA, there are interneurons in the brain (Zitnan et
al., 1995; Stay et al., 1992). As is indicated in Table 3,
the allatostatins of D. punctata were shown by isolation
or by the presence of mRNA in many tissues other than
the brain. Immunocytochemistry for these various
allato-statins show that they are widely distributed not only in
the central nervous system but also in peripheral nerves
to visceral muscle (e.g. Lange et al., 1993), in midgut
cells (e.g. Audsley et al., 1998) and in hemocytes
(Audsley et al., 1998; Skinner et al., 1997). Such a wide
distribution of the peptides suggests that they have
mul-tiple functions and some of these have been
demon-strated (see Section 6).
5.2. Peptides similar to allatostatins but inactive on
CA in the insect of origin
In view of the occurrence of ASTs in many tissues of
an insect in addition to brain cells innvervating the CA,
it is not surprising that peptides similar or identical to
those that do act as ASTs are found in insects, even other
arthropods, in which they do not act as allatostatins.
Examples of these are given in Table 4.
The M. sexta allatostatin is found in P. unipuncta, a moth
of a different family, but it is believed not to function
as a true allatostatin because at 10
26M it inhibits JH
synthesis by CA of 5-day adult females only 60% and
does not inhibit larval CA (Jansons et al., 1996). It is
less surprising that M. sexta allatostatin does not inhibit
the CA of a dipteran, e.g. Drosophila (M.D. Price, R.
Nichols, P.M. Koladich, J. Merte, S.S. Tobe, W.G.
Bendena, unpublished). Also insensitive to the
cock-roach allatostatin-like peptides, YXFGL-amide, are the
CA of the dipteran Calliphora vomitoria (Duve et al.,
1993) and another fly Neobelleria bullata (Veelaert et
al., 1995) that are not innervated with allatostatin
immu-noreactive neurons. In general, it appears that CA
lack-ing innervation by nerves containlack-ing the allatostatin
being tested are not inhibited by that peptide. The CA of
the earwig Euborellia annulipes is another such example
(Rankin et al., 1998b). However, the locusts L.
migratoria and Schistocerca gregaria are exceptions to
this generality in that their CA do have allatostatin
immunoreactive neurons (Veelaert et al., 1995). Perhaps
assay conditions and/or CA stage were not ideal for
demonstrating an allatostatic effect of the schistostatins
(Veelaert et al., 1996).
5.3. Allatinhibins and allatostatins of unknown
structure
vitel-Table 4
Isolated peptides similar to allatostatins (AST), inactive on CA in insect of origin
Species Structure/name Reference
M. sexta AST
Pseudaletia unipunctaa identical Jansons et al., 1996 Drosophila melanogastera one a. a. different Price et al., unpublishedc Periplaneta americana coelutes with Audsley et al., 1998
– Tyr Xxx Phe Gly Leu–NH2
Calliphora vomitoria Leu/Met callatostatins Duve et al., 1993; Duve and Thorpe, 1994
Lucilia cuprinaa,b Leu-allatostatins East et al., 1996
Aedes aegyptib allatostatins Veenstra et al., 1997
Schistocerca gregaria schistostatins Veelaert et al., 1996; Vanden Broeck et al., 1996
Helicoverpa armigera helicostatins Duve et al., 1997a
Cydia pomonella cydiastatins Duve et al., 1997a
Carausius morosus cricket – type A Lorenz et al., 2000
Xxx Trp2– Gly7Gly8/Ala8Trp9–NH 2
Carausius morosus cricket – type B Lorenz et al., 2000 acDNA identification.
b Presumed to be inactive on CA of this species.
cM.D. Price, R. Nichols, P.M. Koladich, J. Merte, S.S. Tobe, W.G. Bendena, unpublished.
logenic cycles, for example in the viviparous cockroach
D. punctata (Rankin and Stay, 1985a), or at the end of
the last larval stadium, for example in M. sexta
(Bhaskaran et al., 1990). Bhaskaran and coworkers have
partially identified a factor from this stage larva of M.
sexta that provides stable inhibition until after
metamor-phosis, when glands are first treated in vitro with a brain
factor and then implanted into penultimate instar larvae
(Unni et al., 1993). They have called this brain factor
allatinhibin to distinguish it from the fast, reversible
action of allatostatins. The complete identification of this
factor will be important for understanding neuropeptide
regulation of the CA. Table 5 gives some examples for
other insects in which a search has begun for other
alla-tostatins. It will be of particular interest to learn what
this factor is in Drosophila since it has already been
demonstrated that M. sexta allatostatin is not active in
this species (M.D. Price, R. Nichols, P.M. Koladich, J.
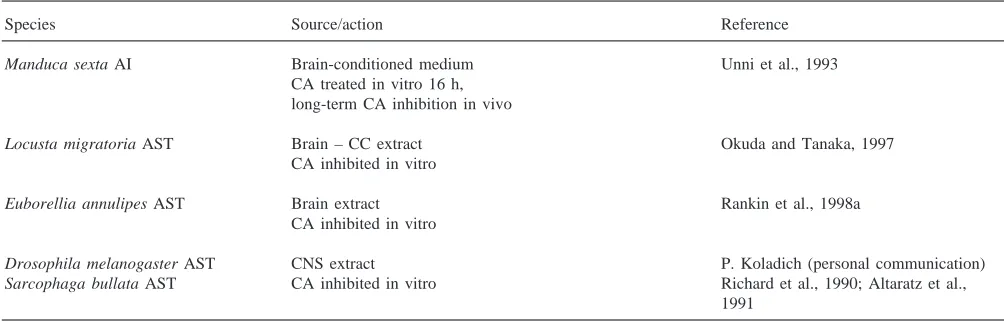
Table 5
Examples of long-acting allatinhibin (AI) and short-acting allatostatins (AST) of unknown structure
Species Source/action Reference
Manduca sexta AI Brain-conditioned medium Unni et al., 1993
CA treated in vitro 16 h, long-term CA inhibition in vivo
Locusta migratoria AST Brain – CC extract Okuda and Tanaka, 1997
CA inhibited in vitro
Euborellia annulipes AST Brain extract Rankin et al., 1998a
CA inhibited in vitro
Drosophila melanogaster AST CNS extract P. Koladich (personal communication)
Sarcophaga bullata AST CA inhibited in vitro Richard et al., 1990; Altaratz et al., 1991
Merte, S.S. Tobe, W.G. Bendena, unpublished) and the
cockroach allatostatin is also likely not to be active since
it is inactive in the blow fly C. vomitoria (Duve et al.,
1993). Thus the Drosophila allatostatin may be a new
allatostatin.
5.4. Allatostatins of unknown structure, from
non-neural sources
syn-thesis by CA in vitro (Hoffmann et al., 1996). This was
reversible, dose-dependent and showed differential
potency on CA from different aged animals (Hoffmann
et al., 1996). In L. migratoria a partially purified ovarian
allatostatic peptide, with an apparent molecular weight
of 1–1.3 kDa, was also found to inhibit JH synthesis by
CA in vitro in a dose-dependent manner (Ferenz and
Aden, 1993). Further purification of this ovarian
allatos-tatin is underway (H.-J. Ferenz, H. Weinert, personal
communication).
6. Other functions of allatoactive peptides
Because the allatoactive peptides were isolated by
their action on JH production by the CA in vitro they
have been called allatostatins and allatotropins.
How-ever, demonstration of their distribution throughout the
insect of origin, in other insects and in other
invert-ebrates, clearly indicates that their function is not limited
to modulation of JH synthesis. Nerve terminals in
neu-rohemal organs, for example in M. sexta, allatostatin and
allatotropin immunopositive nerves in the corpus
car-diacum (Zitnan et al., 1995) and allatotropin
immunore-activity in the neurohemal transverse nerve (Veenstra et
al., 1994), indicate that these peptides could act
humor-ally. Direct innervation of the CA and muscles of the
gut suggest local action of the peptides in these locations
(see Section 5.1). Numerous examples of interneurons
and neuropile showing allatostatin immunoreactivity
suggest direct neuromodulatory function in the central
nervous system (e.g. Davis et al., 1997; Veelaert et al.,
1996; Yoon and Stay, 1995).
Demonstrated actions of allatoactive peptides other
than those on the CA are summarized in Table 6. These
include inhibition of muscle contraction by members of
the YXFGL-amide peptide family, shown in diverse
insects for several parts of the digestive tract and for
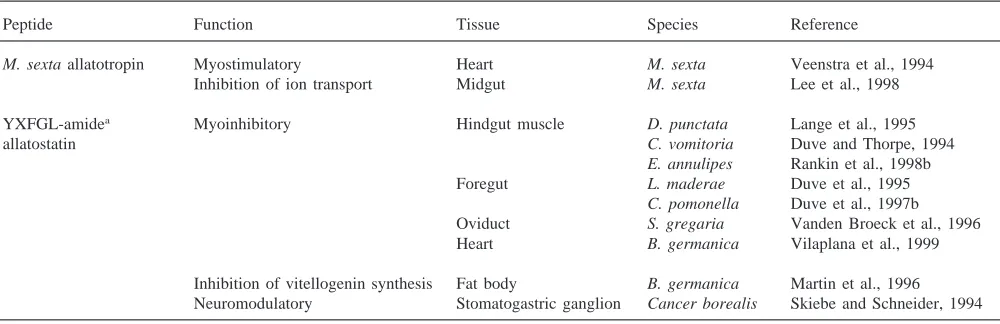
Table 6
Other functions of allatoactive peptides
Peptide Function Tissue Species Reference
M. sexta allatotropin Myostimulatory Heart M. sexta Veenstra et al., 1994
Inhibition of ion transport Midgut M. sexta Lee et al., 1998
YXFGL-amidea Myoinhibitory Hindgut muscle D. punctata Lange et al., 1995
allatostatin C. vomitoria Duve and Thorpe, 1994
E. annulipes Rankin et al., 1998b
Foregut L. maderae Duve et al., 1995
C. pomonella Duve et al., 1997b
Oviduct S. gregaria Vanden Broeck et al., 1996
Heart B. germanica Vilaplana et al., 1999
Inhibition of vitellogenin synthesis Fat body B. germanica Martin et al., 1996 Neuromodulatory Stomatogastric ganglion Cancer borealis Skiebe and Schneider, 1994 aTyr–Xxx–Phe–Gly–Leu–NH
2.
oviducts; and stimulation of heart muscle contraction by
M. sexta allatotropin reported for that insect (Table 6).
The M. sexta allatotropin has also been shown to rapidly
and reversibly inhibit ion transport across the midgut
epithelium in vitro in larvae of M. sexta (Lee et al.,
1998). A quite different function of YXFGL-amide
reported in Blattella germanica was inhibition of
vitello-genin production by fat body, apparently by inhibition
of glycosylation of the vitellogenin (Martin et al., 1996).
Previously reported putative allatostatin receptors in fat
body of D. punctata suggested action of allatostatin on
this tisuse (Cusson et al., 1991). The only experiments
demonstrating neuromodulatory action of an allatoactive
peptide were done with the crab Cancer borealis.
Anti-bodies against D. punctata allatostatin demonstrate the
presence of allatostatin-positive cells in the commisural
ganglion that send axons to the neuropile of the
stomato-gastric ganglion, where they ramify around the neurons
that control the rhythmic movements of the pyloric
stom-ach (Skiebe and Schneider, 1994). Allatostatin applied
to this ganglion strongly decreased the rhythmic
move-ments of the stomach in a dose-dependent manner
(Skiebe and Schneider, 1994). Members of the
YXFGL-amide family of peptides have been isolated and
sequenced from the crab Carcinus maenas (Duve et al.,
1997c). The distribution of allatostatin immunoreactivity
in the stomatogastric nervous system of several decapod
crustaceans suggests that these peptides function as
neu-rohormones as well as neuromodulators in these
crus-taceans (Skiebe, 1999).
7. Important on-going investigations
investi-gation in order to provide a complete understanding of
the regulation of the CA by neurosecretions.
Identification of the receptors for these peptides will
be important in demonstrating whether there are multiple
receptors and to what extent regulation of the receptors
conditions the sensitivity of the corpora allata to
allatos-tatins and allatotropins. Search for allatostatin receptors
has only begun (Cusson et al., 1991; Yu et al., 1995a).
The signal transduction pathways for the action of
allatoactive peptides will require further investigation.
Only a few studies have been done. The activation of
the inositol triphosphate pathway is implicated in the
action of M. sexta allatotropin on CA of M. sexta
(Reagan et al., 1992), as well as in the action of D.
punctata allatostatin on CA of D. punctata (Rachinsky
et al., 1994).
The target of action of allatoactive peptides in the
pathway of JH synthesis is also an important area of
ongoing research. Many studies have shown that
YXFGL-amide allatostatins act before the final steps in
JH synthesis (e.g. Pratt et al., 1989). It could be that the
transfer of two-carbon units from the mitochondria to
the cytoplasm is the target for allatostatin action in the
CA of D. punctata (Sutherland and Feyereisen, 1996).
Regulation of release of the allatoactive peptides is a
challenging area that will require investigating the roles
of internal and external signals that impinge on the
func-tioning of the CA. The interaction of many factors will
undoubtedly be revealed. For example, Berta Scharrer’s
severance of the nerve from the pars intercerebralis
released the corpus allatum from inhibition (Scharrer,
1952), yet the allatostatins of the cockroach are delivered
to the CA by lateral neurosecretory cells (Stay et al.,
1992). This suggests multiple neurosecretory influences
on JH production which could be regulated by different
signals. Much remains to be learned about the extent to
which neuropeptides participate in the regulation of JH
synthesis by the CA.
8. Conclusion
Only one allatotropin and three kinds of allatostatins
have been found, and only in Manduca sexta have both
an allatotropin and an allatostatin been identified.
Undoubtedly more stimulatory and inhibitory peptides
will be found. In the search for them it will be important
to consider the physiological state of the CA as well as
the conditions for assaying activity.
The allatoactive neuropeptides are not limited to
action on the CA. They occur not only in the brain but
throughout the nervous system, in the midgut cells and
in blood cells. This is not unexpected since, as Scharrer
pointed out in a retrospective review, a neuroendocrine–
gut axis is well known for vertebrates (Scharrer, 1990).
Clearly, the neuroactive peptides have multiple functions
and many of these have yet to be well characterized.
The peptides that have allatoactive function in one
insect may occur in others, but not act on CA in these
insects. These “false allatostatins” and “false
allatotrop-ins” could in some cases change their status under
differ-ent assay conditions.
Finally it is important to note that the allatoactive
pep-tides of insects are not limited to insects. They have been
found in crustaceans and will undoubtedly be found in
phyla other than arthropods. The relationship of these
molecules in their long evolutionary history will be an
interesting story which Berta Scharrer started, and in
which she took a keen interest throughout her long and
distinguished career. She would, I believe, be even more
delighted to learn of a worldwide study of neuropeptides,
including cockroach allatostatin, in crustacean ganglia,
an investigation being utilized to shed light on how the
human brain functions highlighted in the 1998 report of
Human Frontier Science Program (Strasbourg, France).
Berta Scharrer was a strong proponent of relating her
research on insects to a broad understanding of the
func-tion of neuropeptides.
References
Altaratz, M., Applebaum, S.W., Richard, D.S., Gilbert, L.I., Segal, D., 1991. Regulation of juvenile hormone synthesis in wild-type and
apterous mutant Drosophila. Molecular and Cellular
Endocrin-ology 81, 205–216.
Audsley, N., Weaver, R.J., Edwards, J.P., 1998. Enzyme linked immu-nosorbent assay for Manduca sexta allatostatin (Mas-AS), isolation and measurement of Mas-AS immunoreactive peptide in Lacanobia
oleracea. Insect Biochemistry and Molecular Biology 28, 775–784.
Audsley, N., Weaver, R.J., Edwards, J.P., 1999a. Juvenile hormone synthesis by corpora allata of tomato moth, Lacanobia oleracea (Lepidoptera: Noctuidae) and the effects of allatostatins and allato-tropin in vitro. European Journal of Entomology 96, 287–293. Audsley, N., Weaver, R.J., Edwards, J.P., 1999b. The significance of
Manduca sexta allatostatin in the tomato moth Lacanobia oleracea.
Annals of the New York Academy of Sciences 897, 330–341. Audsley, N., Weaver, R.J., Edwards, J.P., 2000. Juvenile hormone
biosynthesis by corpora allata of larval tomato moth, Lacanobia
oleracea, and regulation by Manduca sexta allatostatin and
allato-tropin. Insect Biochemistry and Molecular Biology 30, 681–689. Belle´s, X., Maestro, J.-L., Piulachs, M.-D., Johnsen, A.H., Duve, H.,
Thorpe, A., 1994. Allatostatic neuropeptides from the cockroach
Blattella germanica (L.) (Dictyoptera, Blattellidae). Identification,
immunolocalization and activity. Regulatory Peptides 53, 237–247. Belle´s, X., Graham, L.A., Bendena, W.G., Ding, Q., Edwards, J.P., Weaver, R.J., Tobe, S.S., 1999. The molecular evolution of the allatostatin precursor in cockroaches. Peptides 20, 11–22. Bhaskaran, G., Dahm, K.H., Barrera, P., Pacheco, J.L., Peck, K.E.,
Muszynksa-Pytel, M., 1990. Allatinhibin, a neurohormonal inhibi-tor of juvenile hormone biosynthesis in Manduca sexta. General and Comparative Endocrinology 78, 123–136.
Bhatt, T.R., Horodyski, F.M., 1999. Expression of the Manduca sexta allatotropin gene in cells of the central and enteric nervous systems. Journal of Comparative Neurology 403, 407–420.
Copenhaver, P.F., Truman, J.W., 1986. Metamorphosis of the cerebral neuroendocrine system in the moth Manduca sexta. Journal of Comparative Neurology 249, 186–204.
Cusson, M., Prestwich, G.D., Stay, B., Tobe, S.S., 1991. Photoaffinity labeling of allatostatin receptor proteins in the corpora allata of the cockroach, Diploptera punctata. Biochemical and Biophysical Research Communications 181, 736–742.
Davis, N.T., Veenstra, J.A., Feyereisen, R., Hildebrand, J.G., 1997. Allatostatin-like-immunoreactive neurons of the tobacco hornworm, Manduca sexta, and isolation and identification of a new neuropeptide related to cockroach allatostatins. Journal of Comparative Neurology 385, 265–284.
Ding, Q., Donly, B.C., Tobe, S.S., Bendena, W.G., 1995. Comparison of the allatostatin neuropeptide precursors in the distantly related cockroaches Periplaneta americana and Diploptera punctata. Eur-opean Journal of Biochemistry 234, 737–746.
Donly, B.C., Ding, Q., Tobe, S.S., Bendena, W.G., 1993. Molecular cloning of the gene for the allatostatin family of neuropeptides from the cockroach Diploptera punctata. Proceedings of the National Academy of Science USA 90, 8807–8811.
Duve, H., Thorpe, A., 1994. Distribution and functional significance of Leu-callatostatins in the blowfly Calliphora vomitoria. Cell and Tissue Research 276, 367–379.
Duve, H., Johnsen, A.H., Scott, A.G., Yu, C.G., Yagi, K.J., Tobe, S.S., Thorpe, A., 1993. Callatostatins: neuropeptides from the blowfly
Calliphora vomitoria with sequence homology to cockroach
allato-statins. Proceedings of the National Academy of Science USA 90, 2456–2460.
Duve, H., Wren, P., Thorpe, A., 1995. Innervation of the foregut of the cockroach Leucophaea maderae and inhibition of spontaneous contractile activity by callatostatin neuropeptides. Physiological Entomology 20, 33–44.
Duve, H., Johnsen, A.H., Maestro, J.-L., Scott, A.G., Winstanley, D., Davey, M., East, P.D., Thorpe, A., 1997a. Lepidopteran peptides of the allatostatin superfamily. Peptides 18, 1301–1309.
Duve, H., Johnsen, A.H., Maestro, J.-L., Scott, A.G., Crook, N., Win-stanley, D., Thorpe, A., 1997b. Identification, tissue localisation and physiological effect in vitro of a neuroendocrine peptide ident-ical to a dipteran Leu-callatostatin in the codling moth Cydia
pomonella (Trotricidae: Lepidoptera). Cell and Tissue Research
289, 73–83.
Duve, H., Johnsen, A.H., Maestro, J.-L., Scott, A.G., Jaros, P.P., Thorpe, A., 1997c. Isolation and identification of multiple neuro-peptides of the allatostatin superfamily in the shore crab Carcinus
maenas. European Journal of Biochemistry 250, 727–734.
East, P., Tregenza, K., Duve, H., Thorpe, A., 1996. Identification of the dipteran Leu-callatostatin peptide family: characterization of the prohormone gene from Calliphora vomitoria and Lucilia
cup-rina. Regulatory Peptides 67, 1–9.
Fan, Y., Rafaeli, A., Gileadi, C., Kubli, E., Applebaum, S., 1999.
Dro-sophila melanogaster sex peptide stimulates juvenile hormone
syn-thesis and depresses sex pheromone production in Helicoverpa
armigera. Journal of Insect Physiology 45, 127–133.
Ferenz, H.-J., Aden, E., 1993. Ovarian control of juvenile hormone biosynthesis in Locusta migratoria (Orthoptera: Acrididae). Entom-ologia Generalis 18, 9–17.
Hodkova´, M., Okuda, T., Wagner, R., 1996. Stimulation of corpora allata by extract from neuroendocrine complex; comparison of reproducing and diapausing Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). European Journal of Entomology 93, 535–543. Hoffmann, K.H., Lorenz, M.W., Klein, P.M., 1996. Ovarian control
of juvenile hormone-III biosynthesis in the cricket species Gryllus
bimaculatus (Ensifera: Gryllidae). Entomologia Generalis 20,
157–167.
Holbrook, G.L., Chang, A.-S., Schal, C., 1996. Allatostatin inhibition and farnesol stimulation of corpus allatum activity in embryos of
the viviparous cockroach Diploptera punctata. Archives of Insect Biochemistry and Physiology 32, 341–352.
Jansons, I.S., Cusson, M., McNeil, J.N., Tobe, S.S., Bendena, W.G., 1996. Molecular characterization of a cDNA from Pseudaletia
unipuncta encoding the Manduca sexta allatostatin peptide
(Mas-AST). Insect Biochemistry and Molecular Biology 26, 767–773. Kataoka, H., Toschi, A., Li, J.P., Carney, R.L., Schooley, D.A.,
Kramer, S.J., 1989. Identification of an allatotropin from adult
Manduca sexta. Science 243, 1481–1483.
Kramer, S.J., Toschi, A., Miller, C.A., Kataoka, H., Quistad, G.B., Li, J.P., Carney, R.L., Schooley, D.A., 1991. Identification of an allatostatin from the tobacco hornworm Manduca sexta. Proceed-ings of the National Academy of Science USA 88, 9458–9462. Lange, A.B., Chan, K.K., Stay, B., 1993. Effect of allatostatin and
proctolin on antennal pulsatile organ and hindgut muscle in the cockroach, Diploptera punctata. Archives of Insect Biochemistry and Physiology 24, 79–92.
Lange, A.B., Bendena, W.G., Tobe, S.S., 1995. The effect if the thir-teen dip-allatostatins on myogenic and induced contractions of the cockroach (Diploptera punctata) hindgut. Journal of Insect Physi-ology 41, 581–588.
Lee, K.-Y., Horodyski, F.M., Chamberlin, M.E., 1998. Inhibition of midgut ion transport by allatotropin (Mas-AT) and Manduca FLRFamides in the tobacco hornworm Manduca sexta. Journal of Experimental Biology 201, 3067–3074.
Lehmberg, E., Ferenz, H.-J., Applebaum, S.W., 1992. Maturation and responsiveness to extracts of corpora allata from male Locusta
migratoria containing allatotropic factors. Verlag der Zeitschrift fu¨r
Naturforschung 47C, 449–452.
Lorenz, M.W., Hoffmann, K.H., 1995. Allatotropic activity in the suboesophageal ganglia of crickets, Gryllus bimaculatus and
Ach-eta domesticus (Ensifera: Gryllidae). Journal of Insect Physiology
41, 191–196.
Lorenz, M.W., Kellner, R., Hoffmann, K.H., 1995a. Identification of two allatostatins from the cricket, Gryllus bimaculatus de Geer (Ensifera, Gryllidae): additional members of a family of neuropep-tides inhibiting juvenile hormone biosynthesis. Regulatory Pepneuropep-tides 57, 227–236.
Lorenz, M.W., Kellner, R., Hoffmann, K.H., 1995b. A family of neuro-peptides that inhibit juvenile hormone biosynthesis in the cricket,
Gryllus bimaculatus. Journal of Biological Chemistry 270,
21103–21108.
Lorenz, M.W., Kellner, R., Hoffmann, K.H., Gade, G., 2000. Identifi-cation of multiple allatostatic peptides isolated from stick insect brains. Insect Biochemistry and Molecular Biology 30, 711–718. Martin, D., Piulachs, M.D., Belle´s, X., 1996. Inhibition of vitellogenin
production by allatostatin in the German cockroach. Molecular and Cellular Endocrinology 121, 191–196.
Moshitzky, P., Fleischmann, I., Chaimov, N., Saudan, P., Klauser, S., Kubli, E., Applebaum, S.W., 1996. Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus alla-tum. Archives of Insect Biochemistry and Physiology 32, 363–374. Neuha¨user, T., Sorge, D., Stay, B., Hoffmann, K.H., 1994. Responsive-ness of the adult cricket (Gryllus bimaculatus and Acheta
domesticus) retrocerebral complex to allatostatin-1 from a
cock-roach, Diploptera punctata. Journal of Comparative Physiology B 164, 23–31.
Oeh, U., Lorenz, M.W., Dyker, H., Loesel, P., Hoffmann, K.H., 2000. Interaction between Manduca allatotropin and Manduca allatostatin in the fall armyworm Spodoptera frugiperda. Insect Biochemistry and Molecular Biology 30, 719–727.
Okuda, T., Tanaka, S., 1997. An allatostatic factor and juvenile hor-mone synthesis by corpora allata in Locusta migratoria. Journal of Insect Physiology 43, 635–641.
Periplaneta americana (L.). Cell and Tissue Research 193, 443–
445.
Pratt, G.E., Tobe, S.S., 1974. Juvenile hormones radiobiosynthesized by corpora allata of adult female locusts in vitro. Life Sciences 14, 575–586.
Pratt, G.E., Farnsworth, D.E., Siegel, N.R., Fok, K.F., Feyereisen, R., 1989. Identification of an allatostatin from adult Diploptera
punctata. Biochemical and Biophysical Research Communications
163, 1243–1247.
Pratt, G.E., Farnsworth, D.E., Feyereisen, R., 1990. Changes in the sensitivity of adult cockroach corpora allata to a brain allatostatin. Molecular and Cellular Endocrinology 70, 185–195.
Pratt, G.E., Farnsworth, D.E., Fok, K.F., Siegel, N.R., McCormack, A.L., Shabanowitz, J., Hunt, D.F., Feyereisen, R., 1991. Identity of a second type of allatostatin from cockroach brains: an octadeca-peptide amide with tyrosine-rich address sequence. Proceedings of the National Academy of Science USA 88, 2412–2416.
Rachinsky, A., Feldlaufer, M.F., 2000. Responsiveness of honey bee (Apis mellifera L.) corpora allata to allatoregulatory peptides from four insect species. Journal of Insect Physiology 46, 41–46. Rachinsky, A., Tobe, S.S., Feldlaufer, M.F., 2000. Terminal steps in
JH biosynthesis in the honey bee (Apis mellifera L.): developmental changes in sensitivity to JH precursor and allatotropin. Insect Bio-chemistry and Molecular Biology 30, 729–737.
Rachinsky, A., Zhang, J., Tobe, S.S., 1994. Signal transduction in the inhibition of juvenile hormone biosynthesis by allatostatins: roles of diacylglycerol and calcium. Molecular and Cellular Endocrin-ology 105, 89–96.
Rankin, S.M., Stay, B., 1984. The changing effect of the ovary on rates of juvenile hormone synthesis in Diploptera punctata. General and Comparative Endocrinology 54, 382–388.
Rankin, S.M., Stay, B., 1985a. Regulation of juvenile hormone syn-thesis during pregnancy in the cockroach, Diploptera punctata. Journal of Insect Physiology 31, 145–157.
Rankin, S.M., Stay, B., 1985b. Ovarian inhibition of juvenile hormone synthesis in the viviparous cockroach, Diploptera punctata. Gen-eral and Comparative Endocrinology 59, 230–237.
Rankin, S.M., Garside, C.S., Christopher, C.A., Tobe, S.S., 1998a. Par-tial characterization and isolation of earwig “allatostatins”: biologi-cal activities in earwigs and cockroaches. Comparative Biochemis-try and Physiology A 121, 395–403.
Rankin, S.M., Stay, B., Chan, K., Jackson, E.S., 1998b. Cockroach allatostatin-immunoreactive neurons and effects of cockroach alla-tostatin in earwigs. Archives of Insect Biochemistry and Physi-ology 38, 155–165.
Reagan, J.D., Miller, W.H., Kramer, S.J., 1992. Allatotropin-induced formation of inositol phosphates in the corpora allata of the moth,
Manduca sexta. Archives of Insect Biochemistry and Physiology
20, 145–155.
Reichwald, K., Unnithan, G.C., Davis, N.T., Agricola, H., Feyereisen, R., 1994. Expression of allatostatin gene in endocrine cells of the cockroach midgut. Proceedings of the National Academy of Science USA 91, 11894–11898.
Richard, D.S., Applebaum, S.W., Gilbert, L.I., 1990. Allatostatic regu-lation of juvenile hormone production in vitro by the ring gland of
Drosophila melanogaster. Molecular and Cellular Endocrinology
68, 153–161.
Ro¨ller, H., Dahm, K.H., Sweeley, C.C., Trost, B.M., 1967. The struc-ture of the juvenile hormone. Angewant Chemie International Edi-tion English 6, 179–180.
Scharrer, B., 1952. Neurosecretion. XI. The effects of nerve section on the intercerebralis–cardiacum–allatum system of the insect
Leu-cophaea maderae. Biological Bulletin Woods Hole 102, 261–272.
Scharrer, B., 1990. The neuropeptide saga. American Zoologist 30, 887–895.
Scharrer, E., Scharrer, B., 1945. Neurosecretion. Physiological Reviews 25, 171–181.
Scharrer, E., Scharrer, B., 1963. Neuroendocrinology. Columbia Uni-versity Press, New York.
Skiebe, P., 1999. Allatostatin-like immunoreactivity in the stomato-gastric nervous system and the pericardial organs of the crab
Can-cer pagurus, the lobster Homarus americanus, and the crayfish Cherax destructor and Procambarus clarkii. Journal of
Compara-tive Neurobiology 403, 85–105.
Skiebe, P., Schneider, H., 1994. Allatostatin peptides in the crab stoma-togastric nervous system: inhibition of the pyloric motor pattern and distribution of allatostatin-like immunoreactivity. Journal of Experimental Biology 194, 195–208.
Skinner, J.R., Fairbairn, S.E., Woodhead, A.P., Bendena, W.G., Stay, B., 1997. Allatostatin in hemocytes of the cockroach Diploptera
punctata. Cell and Tissue Research 290, 119–128.
Stay, B., Friedel, T., Tobe, S.S., Mundall, E.C., 1980. Feedback control of juvenile hormone synthesis in cockroaches: possible role for ecdysterone. Science 207, 898–900.
Stay, B., Joshi, S., Woodhead, A.P., 1991a. Sensitivity to allatostatins of corpora allata from larval and adult female Diploptera punctata. Journal of Insect Physiology 37, 63–70.
Stay, B., Woodhead, A.P., Joshi, S., Tobe, S.S., 1991b. Allatostatins, neuropeptide inhibitors of juvenile hormone synthesis in brain and corpora allata of the cockroach Diploptera punctata. In: Menn, J.J., Kelly, T.J., Masler, E.P. (Eds.) Insect Neuropeptides. ACS Sym-posium Series, 453. American Chemical Society, Washington, DC, pp. 164–176.
Stay, B., Chan, K.K., Woodhead, A.P., 1992. Allatostatin-immunore-active neurons projecting to the corpora allata of adult Diploptera
punctata. Cell and Tissue Research 270, 15–23.
Sutherland, T.D., Feyereisen, R., 1996. Target of cockroach allatostatin in the pathway of juvenile hormone biosynthesis. Molecular and Cellular Endocrinology 120, 115–123.
Taylor, P.A., Bhatt, T.R., Horodyski, F.M., 1996. Molecular charac-terization and expression analysis of Manduca sexta allatotropin. European Journal of Biochemistry 239, 588–596.
Truesdell, P.F., Koladich, P.M., Kataoka, H., Kojima, K., Suzuki, A., McNeil, J.N., Mizoguchi, A., Tobe, S.S., Bendena, W.G., 2000. The molecular characterization of Pseudaletia unipuncta allatotro-pin. Insect Biochemistry and Molecular Biology (in press). Unni, B., Barrera, P., Muszynska-Pytel, M., Bhaskaran, G., Dahm,
K.H., 1993. Partial characterization of allatinhibin, a neurohormone of Manduca sexta. Archives of Insect Biochemistry and Physiology 24, 173–185.
Vanden Broeck, J., Veelaert, D., Bendena, W.G., Tobe, S.S., De Loof, A., 1996. Molecular cloning of the precursor cDNA for schistostat-ins, locust allatostatin-like peptides with myoinhibiting properties. Molecular and Cellular Endocrinology 122, 191–198.
Veelaert, D., Schoofs, L., Tobe, S.S., Yu, C.G., Vullings, H.G.B., Couillaud, F., De Loof, A., 1995. Immunological evidence for an allatostatin-like neuropeptide in the central nervous system of
Schi-stocerca gregaria, Locusta migratoria and Neobelleria bullata.
Cell and Tissue Research 279, 601–611.
Veelaert, D., Devreese, B., Schoofs, L., Van Beeumen, J., Vanden Broeck, J., Tobe, S.S., De Loof, A., 1996. Isolation and characteriz-ation of eight myoinhibiting peptides from the desert locust,
Schis-tocerca gregaria: new members of the cockroach allatostatin
fam-ily. Molecular and Cellular Endocrinology 122, 183–190. Veenstra, J.A., Lehman, H.K., Davis, N.T., 1994. Allatotropin is a
cardioacceleratory peptide in Manduca sexta. Journal of Experi-mental Biology 188, 347–354.
Veenstra, J.A., Noriega, F.G., Graf, R., Feyereisen, R., 1997. Identifi-cation of three allatostatins and their cDNA from the mosquito
Aedes aegypti. Peptides 18, 937–942.
Vilaplana, L., Maestro, J.L., Piulachs, M.-D., Belle´s, X., 1999. Modu-lation of cardiac rhythm by allatostatins in the cockroach Blattella
germanica (L.) (Dictyoptera, Blattellidae). Journal of Insect
Virant-Doberlet, M., Horseman, G., Loher, W., Huber, F., 1994. Neu-rons projecting from the brain to the corpora allata in orthopteroid insects: anatomy and physiology. Cell and Tissue Research 277, 39–50.
Weaver, R.J., 1991. Profile of the responsiveness of corpora allata from virgin female Periplaneta americana to an allatostatin from
Diploptera punctata. Journal of Insect Physiology 37, 111–118.
Weaver, R.J., Freeman, Z.A., Pickering, M.G., Edwards, J.P., 1994. Identification of two allatostatins from the CNS of the cockroach
Periplaneta americana: novel members of a family of neuropeptide
inhibitors of insect juvenile hormone biosynthesis. Comparative Biochemistry and Physiology 107C, 119–127.
Woodhead, A.P., Stay, B., Seidel, S.L., Khan, M.A., Tobe, S.S., 1989. Primary structure of four allatostatins: neuropeptide inhibitors of juvenile hormone synthesis. Proceedings of the National Academy of Science USA 86, 5997–6001.
Woodhead, A.P., Stoltzman, C.A., Stay, B., 1992. Allatostatins in the nerves of the antennal pulsatile organ muscle of the cockroach
Diploptera punctata. Archives of Insect Biochemistry and
Physi-ology 20, 253–263.
Woodhead, A.P., Asano, W.Y., Stay, B., 1993. Allatostatins in the
haemolymph of Diploptera punctata and their effect in vivo. Jour-nal of Insect Physiology 39, 1001–1005.
Woodhead, A.P., Khan, M.A., Stay, B., Tobe, S.S., 1994. Two new allatostatins from the brains of Diploptera punctata. Insect Bio-chemistry and Molecular Biology 24, 257–263.
Yoon, J.G., Stay, B., 1995. Immunocytochemical localization of
Diploptera punctata allatostatin-like peptide in Drosophila mel-anogaster. Journal of Comparative Neurology 363, 475–488.
Yu, C.G., Hayes, T.K., Strey, A., Bendena, W.G., Tobe, S.S., 1995a. Identification and partial characterization of receptors for allatostat-ins in brain and corpora allata of the cockroach Diploptera punctata using a binding assay and photoaffinity labeling. Regulatory Pep-tides 57, 347–358.
Yu, C.G., Stay, B., Ding, Q., Bendena, W.G., Tobe, S.S., 1995b. Immunochemical identification and expression of allatostatins in the gut of Diploptera punctata. Journal of Insect Physiology 41, 1035–1043.
Zitnan, D., Kingan, T.G., Kramer, S.J., Beckage, N.E., 1995. Accumu-lation of neuropeptides in the cerebral neurosecretory system of