L
Journal of Experimental Marine Biology and Ecology 246 (2000) 53–67
www.elsevier.nl / locate / jembe
The effects of sea urchin grazing and drift algal blooms on a
subtropical seagrass bed community
* ´ Silvia Macia
Center for Marine and Environmental Analyses, University of Miami, Rosenstiel School of Marine and
Atmospheric Science, 4600 Rickenbacker Causeway, Miami, FL 33149, USA
Received 27 January 1999; received in revised form 18 November 1999; accepted 25 November 1999
Abstract
Subtropical seagrass beds can be subject to relatively high levels of direct herbivory and large blooms of drift algae, both of which can have important effects on the floral and faunal components of the community. Caging experiments were used to investigate these factors in a Thalassia
tes-tudinum bed in Biscayne Bay, Florida. Abundance of sea urchins, Lytechinus variegatus, and drift
algae was manipulated within the cages. Naturally occurring levels of urchin grazing do not appear to affect the T. testudinum population. With experimentally increased urchin densities in the winter, seagrass shoot density and aboveground biomass decreased significantly. Similar effects were not detected in the summer, indicating that the impact of grazing on T. testudinum is lessened during this time of year. Shoot density was more vulnerable to grazing than aboveground biomass. This may be a result of grazing-induced increases in seagrass productivity, in which the remaining shoots produce more or longer leaves. In the winter, drift algal blooms form large mats that cover the sea-grass canopy. Under the normal grazing regime these algal blooms do not have significant negative effects on the seagrass. With increased grazing pressure, however, there is a synergistic effect of
22 grazing and drift algae on seagrass shoot density. At intermediate urchin density (10 per m ), cages without algae did not undergo significant decreases in shoot density, while those with algae did. At the high density of urchins, the number of seagrass shoots in cages both with and without algae de-creased, but the effect was more pronounced for cages with algae. Invertebrate abundance at the field site was low relative to other seagrass beds. There were no discernible effects, either positive or negative, of urchin and algae manipulations on the sampled invertebrate community. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Drift algae; Grazing; Seagrass community; Sea urchin; Thalassia
*Tel.: 11-305-361-4833; fax: 11-305-361-4077. ´
E-mail address: [email protected] (S. Macia)
1. Introduction
Seagrass beds are complex ecosystems composed of many interacting species of plants, invertebrates, and fishes. Tropical and subtropical seagrass beds in particular have an added level of complexity resulting from the direct grazing of seagrasses, which is more prevalent than in temperate areas (Ogden, 1976, 1980; Zieman, 1982). The seasonal occurrence of large blooms of drift algae also contributes to the complexity of subtropical seagrass meadows (Holmquist, 1994, 1997; Bell and Hall, 1997). These two factors can have major effects on the structure and function of warm-water seagrass beds, yet they have rarely been studied in concert.
Several studies have documented blooms of drift algae in subtropical seagrass beds (Gore et al., 1981; Virnstein and Carbonara, 1985; Holmquist, 1994, 1997; Bell and Hall, 1997). These blooms can result in very large mats of algae that compete with the seagrass for light and form bare patches void of seagrass (Hull, 1987; Short et al., 1995; Holmquist, 1997). Dense accumulations of algae can also decrease the flux of oxygen to the sediment, thereby lowering the amount of oxygen available to animals living in or beneath the algae (Hull, 1987; Hansen and Kristensen, 1997). Conversely, drift algal mats greatly increase the structural complexity of the seagrass community and can provide superior habitat for invertebrates (Gore et al., 1981; Holmquist, 1994, 1997). Thus, the effects of drift algae on the floral and faunal components of seagrass beds can be very complex, and may depend on the physical and biological characteristics specific to the particular seagrass bed in question (Hull, 1987).
Sea urchins are the main invertebrate grazers of live seagrass material (Greenway, 1976; Zieman, 1982; Valentine and Heck, 1991). The urchin Lytechinus variegatus (Lamarck) is commonly found in seagrass beds throughout the western Atlantic and Gulf of Mexico. Much work has been devoted to the ecology of this important grazer,
¨
especially in turtlegrass (Thalassia testudinum Banks ex Konig) beds, where L.
variegatus most commonly occurs at population densities less than 10 individuals per 22
m (Moore et al., 1963; Engstrom, 1982; Keller, 1983; Oliver, 1987; Montague et al., 1988; McGlathery, 1995). Larger urchin populations, however, have been reported and can have serious impacts on the seagrass community in which they reside. The most dramatic example of this is the formation of grazing fronts — extremely dense
22
aggregations (up to 600 individuals m ) of urchins capable of completely denuding ´ areas of seagrass as large as thousands of square meters (Camp et al., 1973; Macia and Lirman, 1999). However, even populations of urchins far smaller than those of the
22
grazing fronts (20–40 urchins m ) can remove a large proportion of the seagrass standing crop (Greenway, 1976, 1995; Valentine and Heck, 1991).
2. Methods and materials
2.1. Field site
The field site, West Point, is a dense subtidal Thalassia testudinum bed in northern Biscayne Bay, Florida, USA (Fig. 1). Temperature at the field site ranges annually from 18 to 318C and salinity from 30 to 43‰, with a water depth of approximately 1 m. The sea urchin Lytechinus variegatus is the most conspicuous invertebrate member of the seagrass community. Monthly surveys were conducted at the site from September 1995 to September 1998. At each survey five parallel 50-m transects were laid out 5 m apart,
2
and five 1-m quadrats were randomly selected along each transect. All urchins within these 25 quadrats were counted (except for the first two surveys, in which only 15 and ten total quadrats (i.e. three and two transects), respectively, were counted).
2.2. Cage design
2
Circular cages with an area of 2 m were used. Cages were constructed of 5 m lengths of stiff polyethylene netting (Vexar) with a mesh size of 3.233.2 cm. The sides of the cages were 60 cm high. Each cage had four 90-cm long pieces of steel reinforcement bar (rebar) attached with cable ties at regular distances from each other. The rebar protruded
approximately 25 cm from the bottom of the netting for burial in the sediment. Cage tops were made of thin (0.5 mm diameter) flexible polypropylene netting with a mesh size of 3.233.8 cm. The thinnest possible netting was used for the cage tops in order to minimize shading effects. This netting was sewn onto the top of the cage side with plastic weed trimmer line.
Several control treatments were employed to test for caging artifacts. Partial cages were constructed from 3.8 m lengths of netting and four pieces of rebar, as above. These partial cage controls did not form a completely enclosed circle, having instead an opening of approximately 1.2 m (25% of the circumference). Two types of partial cage controls were used: with a top as described above, and with no top. Top controls were also deployed; these were constructed by attaching a top to four pieces of rebar alone (i.e. no cage sides). Unmanipulated controls consisted of plots marked only by rebar. To test for cage effects on water movement, current speed immediately above the seagrass canopy (approximately 30 cm from the bottom) was measured both inside and outside of the full cages. Red dye was released into the water column and the time to travel 50 cm was measured. Dye within the cages was released just inside the cage and allowed to travel towards the opposite side. There was no significant difference between current speed inside and outside of full cages (ln-transformed data: t51.75; df510; p50.11). 2.3. Experimental design
Caging experiments were performed twice, in winter and summer. Experimental
22 22 22
treatments were 0 urchins per m , 10 urchins m (20 / cage), and 20 urchins m (40 / cage). During the winter and fall months, the field site experiences a bloom of drift algae (primarily Laurencia spp. and Dictyota spp.). These algae disappear in the warmer months (Irlandi, unpublished data). To account for the effects of these drift algae, the winter experiment included algae and no-algae treatments (2algae) for each of the experimental urchin densities. Thus, the total number of treatments were: for the
22
summer, seven (0, 10, and 20 urchins per m , unmanipulated control, partial cage1
22
top, partial cage2top, top only); for the winter, ten (0 per m 1algae, 02algae, 101algae, 102algae, 201algae, 202algae, unmanipulated control, partial cage1
top, partial cage2top, top only).
There were six replicates for each treatment. The cages were allocated, using a random number generator, to positions on a rectangular grid (winter: 1036 points; summer: 736) with 1-m intersection intervals. After the cages and control treatments were set out, preliminary surveys were conducted within each replicate. Epifaunal invertebrates visible to the naked eye were visually identified and counted over a period
2
addition or removal of urchins and algae. Summer cages were checked every 2–4 days during the experiment. Winter cages could not be visited as often because of weather conditions, but were checked approximately once a week. Most cages had fewer than five escaped urchins at any given inspection, but at six inspections there was one cage with more than 15 urchins missing. All missing urchins were replaced as necessary. Invading algae in the no-algae treatments were also removed as necessary (approximate-ly once every 10 days). Replacement of algae in 1 algae treatments was required in only five instances.
Each experiment ran for 6 weeks: August–September 1997 (summer) and January– February 1998 (winter). Cages were established in the same general area on both occasions. At the end of the 6 weeks, each plot was resurveyed. Visual counts of epifaunal invertebrates were repeated, as well as shoot counts within the permanently marked quadrat. Within each plot a core (15 cm diameter) was taken to a depth of 25 cm. Prior to penetrating the sediment the shoots to be included in the sample were manipulated into the corer to ensure effective collection of all aboveground biomass. Core samples were sieved through a 0.5-mm mesh sieve. Seagrass samples were separated into live below- and above-ground biomass (including epiphytic algae), dried to constant weight at 708C, and weighed. All infaunal macroinvertebrates collected in the cores were identified and counted.
2.4. Data analysis
The methods employed for surveying invertebrates were effective only for infauna and for large, slow-moving epifauna (e.g. gastropods, sponges, echinoderms); highly mobile species, such as crustaceans, were not included in the study. The four most common species were chosen as representative of the invertebrate population. Because of the low population densities found for most species (see Results), the non-parametric Kruskal–Wallis test was used to compare abundances among treatments at the end of the experiment. Given the lack of mobility of sponges, their abundance was counted at the beginning and end of the experiment and compared for each treatment using a paired
t-test.
Assumptions of normality and homoscedasticity were tested with the Shapiro–Wilk test and the Bartlett test, respectively (a 50.05), prior to all parametric statistical analyses. Because shoot counts were conducted within the same permanently marked quadrat at the beginning and end of the experiments, these data were compared individually for each treatment with a paired t-test. To test for cage effects, above- and below-ground seagrass biomass of the unmanipulated control and the three cage control treatments were compared with a one-way ANOVA. Because lack of a significant difference among the cage controls indicated that there was no cage effect on seagrass biomass (see Results), the cage control treatments were not included in further analyses. For the summer experiment, a one-way ANOVA comparing biomass in unmanipulated control plots and the three urchin density treatments was performed. For the winter
22
appropriate replication for the algae factor. Because no algal effects were detected (see Results), the 1 and 2 algae treatments were pooled into their respective urchin densities, and a one-way ANOVA was then used to compare these to the unmanipulated controls.
3. Results
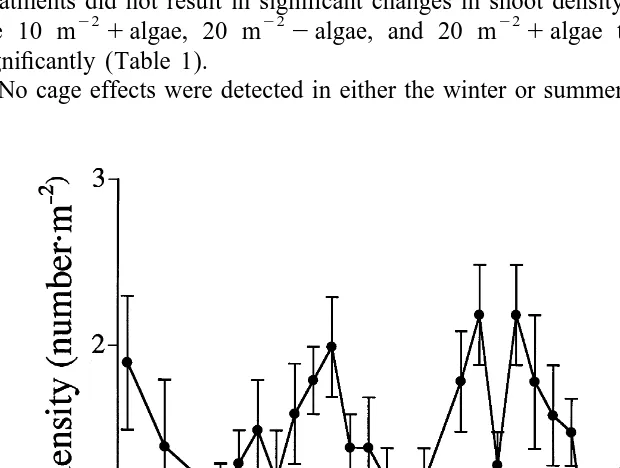
The long-term average population density of L. variegatus at the field site (September
22
1995–September 1998) was 1.460.08 (S.E.) urchins per m (Fig. 2). Average monthly
22
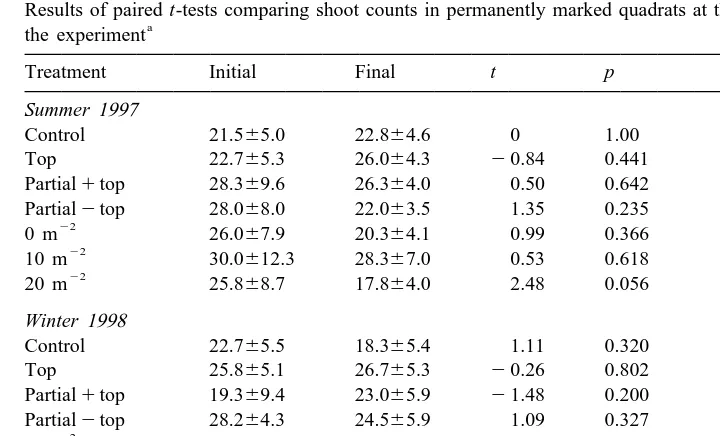
urchin density at the site ranged from 0.5 to 2.2 m . There was no significant difference in shoot density before and after the experiment in any of the cage control treatments, indicating that there was no cage effect for this variable (Table 1). This was true for both winter and summer experiments. In the summer none of the urchin treatments showed significant differences in shoot density before and after the 6 weeks (Table 1). The
22
20-urchins m treatment, however, was only marginally non-significant, with a p value
22 22
of 0.056. In the winter, the urchin exclusion (0 m 6algae) and 10 m 2algae treatments did not result in significant changes in shoot density, while shoot density in
22 22 22
the 10 m 1algae, 20 m 2algae, and 20 m 1algae treatments did decrease significantly (Table 1).
No cage effects were detected in either the winter or summer experiments for
Table 1
Results of paired t-tests comparing shoot counts in permanently marked quadrats at the beginning and end of
a
Initial and final shoot count data presented as number of shoots per 0.04 m (6S.E.). See text for explanation of treatments. Assumptions of normality and homoscedasticity were met. All df55.
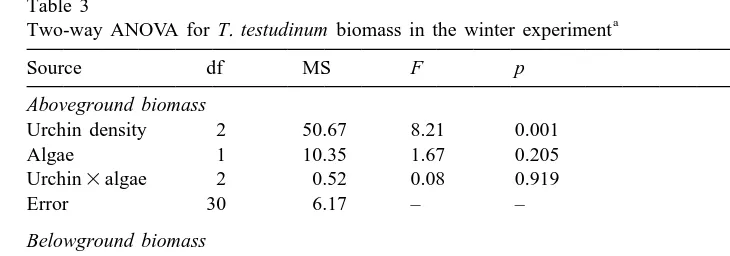
or below-ground seagrass biomass (Table 2). In the winter, higher urchin density significantly decreased aboveground T. testudinum biomass, but there was no effect of algae on seagrass biomass, either above- or ground (Table 3). Winter below-ground biomass was not affected by urchin grazing. Because there was no effect of algae, the 1 and 2 algae treatments were combined into their respective urchin
22
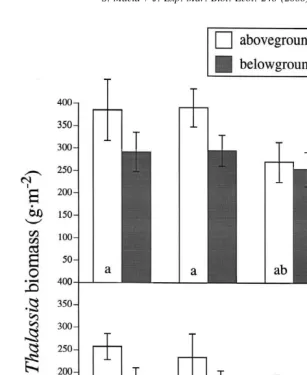
densities and the three treatments (0, 10, and 20 urchins m ) were compared to the unmanipulated controls with a one-way ANOVA. This comparison showed a significant difference in aboveground biomass among the four treatments (df541; F56.42;
22
p50.001). The 20 m treatment had significantly lower biomass than the control and 0
22
m treatments (Fig. 3a; Tukey–Kramer test). In the summer, urchin grazing, regardless
Table 2
a
One-way ANOVA for cage effects for both above- and below-ground seagrass biomass
Control Top Partial1top Partial2top F p
Aboveground, winter 382.9667.8 383.4696.2 371.46126.5 363.4639.2 0.40 0.752 Aboveground, summer 266.4629.7 190.2664.0 234.0661.9 171.8647.6 0.36 0.786 Belowground, winter 302.4645.3 312.7642.5 201.3643.2 339.9626.1 2.29 0.109 Belowground, summer 184.5633.0 120.8622.9 143.4620.7 207.1635.3 1.85 0.170
a 22
Table 3
a
Two-way ANOVA for T. testudinum biomass in the winter experiment
Source df MS F p
Aboveground biomass
Urchin density 2 50.67 8.21 0.001
Algae 1 10.35 1.67 0.205
Urchin3algae 2 0.52 0.08 0.919
Error 30 6.17 – –
Belowground biomass
Urchin density 2 2.41 0.54 0.590
Algae 1 9.35 2.08 0.160
Urchin3algae 2 1.65 0.37 0.697
Error 30 4.50 – –
a
Cage control treatments were not included in the analysis because no cage effects were detected (see Table 2). Assumptions of both normality and homoscedasticity were met.
of density, had no significant effect on seagrass biomass (Fig. 3b; aboveground: df523;
F51.34; p50.289; belowground: df523; F50.13; p50.939).
A total of 34 macroinvertebrate species were identified from the site, but the vast
22
majority were present in very small numbers (<1 per m ). Overall macrofaunal abundance (including echinoderms, molluscs, polychaetes and sponges) was 144.5615.3
22
individuals m in the summer, and 117.7612.0 in the winter. The four most common species were the epifaunal gastropod Lithopoma americanum (Turbinidae), the sponge
Haliclona permollis (Haliclonidae), and the infaunal polychaetes Marphysa sanguinea
(Eunicidae) and Asychis elongata (Maldanidae). The density of H. permollis, averaged
22 22
over all treatments, was 0.24 m 60.07 in the summer and 0.31 m 60.07 in the winter. Results of the paired t-tests indicated that for no treatment, in either winter or summer, was there a change in the density of H. permollis (all p.0.05). Similarly, no significant differences were found in numbers of L. americanum, A. elongata, or M.
sanguinea (Table 4).
4. Discussion
4.1. Effects of urchin grazing on seagrass population
The seagrass bed at West Point harbors a small, but relatively stable, population of L.
variegatus. Exclusion of urchins had no effect on either the shoot density or biomass of T. testudinum, indicating that the natural levels of grazing at West Point are not sufficient to have a significant impact on the biomass or shoot density of the seagrass
´
Fig. 3. Biomass of Thalassia after 6 weeks at experimental urchin densities. (a) Winter experiment (August– September 1997): bars represent pooled 1and 2algae treatments. Aboveground values with different letters are significantly different from each other. Belowground biomass did not differ among treatments. (b) Summer experiment (January–February 1998): no significant differences were detected among any of the treatments for either above- or below-ground biomass.
period of only 6 weeks. Further experiments are needed to determine if the pattern found ´
here holds true over longer time periods (Macia, manuscript in preparation).
Two seagrass population variables, biomass and shoot density, were used to assess the effects of urchin grazing and drift algae at the study site. These two variables, however, did not necessarily react similarly to the experimental treatments. In the summer, experimentally increased urchin grazing did not significantly affect shoot density. At 20
22
Table 4
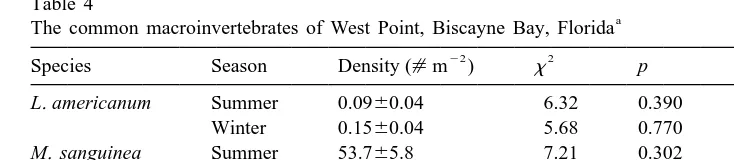
a
The common macroinvertebrates of West Point, Biscayne Bay, Florida 22 2
Species Season Density ([m ) x p
L. americanum Summer 0.0960.04 6.32 0.390
Winter 0.1560.04 5.68 0.770
M. sanguinea Summer 53.765.8 7.21 0.302
Winter 67.369.7 6.41 0.698
A. elongata Summer 33.067.6 12.38 0.054
Winter 61.9611.4 16.77 0.053
a
Lithopoma americanum is an epifaunal gastropod; Marphysa sanguinea and Asychis elongata are infaunal
polychaetes. Population densities are averaged over all treatments, and given in mean6S.E. Results of the Kruskal–Wallis test comparing final invertebrate densities among all treatments are presented.
22
( p50.056). This suggests that, for this time of year, 20 urchins m is the critical urchin density above which the seagrass shoot density will be negatively affected. During the winter months, this critical density is less than that of the summer. Shoot
22
count data indicate that at 10 urchins m , in the presence of the naturally occurring algae, shoot density is significantly decreased. Winter critical urchin density for negative
22
effects on shoot number is therefore approximately 10 urchins m , half of the corresponding summer value. Thus, the effects of grazing on T. testudinum shoot density are more pronounced in the winter than in the summer.
Estimated critical urchin densities for negative effects on biomass were higher than those for shoot density. Aboveground biomass was affected by urchin grazing only in
22
the winter, and then only at the highest density tested (20 urchins m ). Biomass at this urchin density, however, was not significantly different than the biomass at 10 urchins
22
m , indicating that critical urchin density for biomass effects in the winter is
22
somewhere between 10 and 20 urchins m . Biomass in the summer did not decline
22
significantly even at 20 urchins m . Therefore, critical urchin density for biomass
22
effects in the summer must be at some value higher than 20 per m . Although the critical urchin densities for shoot number and aboveground biomass differ, both variables indicate that grazing on T. testudinum has a more pronounced effect in the winter. This conclusion is similar to that found by Valentine and Heck (1991), who used cages to study L. variegatus grazing effects in the northern Gulf of Mexico. Their study indicated that approximately twice as high an urchin abundance is required to overgraze
22
aboveground T. testudinum biomass in the summer (.40 urchins m ) than in the
22
winter (,20 urchins m ). The results of Valentine and Heck (1991) pertain to complete defoliation of the seagrasses, however; even their lowest urchin density (10
22
urchins m ) was enough to cause significant decreases in biomass at all times of the year.
At West Point, the critical urchin density required for negative effects on aboveground
22
biomass (420 and 10–20 urchins m in the summer and winter, respectively)
22
Increased productivity could manifest itself as longer or more numerous leaves per shoot, either of which would produce greater amounts of biomass. In this way, aboveground biomass may remain unaffected by grazing even though shoot density is decreasing. A grazing-induced increase in productivity has in fact been documented for a
T. testudinum population in the Caribbean island of St. Croix, which exhibits increased specific growth rate when subject to moderate levels of turtle or urchin grazing (Zieman et al., 1984). Studies from terrestrial grasslands suggest several mechanisms through which grazing can increase plant productivity. These mechanisms include: increased rates of photosynthesis stimulated by the loss of photosynthetic products; reallocation of nutrient reserves from the root system; increased light penetration resulting from decreased self-shading effects; and nutrient input in the form of grazer feces / urine (McNaughton, 1979 and references therein; Milchunas and Lauenroth, 1993). Of the mechanisms mentioned above, fertilization of seagrasses by urchin feces may be
´
particularly important to T. testudinum (Macia, manuscript in preparation).
The results of this study suggest that the vulnerability of seagrasses to grazing depends on the level of grazing pressure that the seagrass population normally experiences. Seagrass beds exposed to relatively low grazing rates, such as at West Point, may be more resistant to short-term increases in grazing rates. Critical urchin density for negative effects on seagrass biomass estimated from this study is higher than
22
the 10 urchins m found by Valentine and Heck (1991) in the Gulf of Mexico. This contrast may be a result of the different grazing pressures exerted naturally on these two seagrass beds. In the Gulf of Mexico site, urchin density ranged from 12 to 25 urchins
22 22
m , whereas in Biscayne Bay the urchin density is only 1.4 per m . Field studies from Jamaica show that T. testudinum subjected to repeated cropping every 70 days exhibits a decline in biomass after the sixth and seventh harvests (Greenway, 1974). Valentine and Heck (1991) stated that conditions for overgrazing are common at their site. Periodic overgrazing may make this T. testudinum population more susceptible to grazing effects than the West Point population, which appears to suffer virtually no impact from the naturally low grazing pressure. Using caging experiments, Keller (1983) investigated another Jamaican population of L. variegatus with a relatively low population density
22
(3.7 per m ). On all but one of the sampling dates, experimental densities of 16 urchins
22
m had no significant effect on aboveground T. testudinum biomass. This situation is similar to the present study, where a seagrass population with low natural urchin abundance appears to be relatively resistant to experimentally increased grazing pressure.
4.2. Effects of drift algae and interaction with grazing
Removal of algae in the urchin exclusion cages did not have a positive effect on seagrass shoot density, indicating that drift algae alone do not appear to negatively affect this parameter. Similarly, drift algae did not affect seagrass biomass. For shoot density, however, there was an interaction between drift algae and increased urchin density. Removal of algae had a positive effect on T. testudinum shoot density in the 10 urchins
22
22
without algae in the 20 urchins m treatment, the effect was more pronounced for the
1 algae treatment, as shown by the lower p-value in the paired t-test.
Hull (1987) discussed the potential effects, both positive and negative, of benthic macroalgal mats on the surrounding community. Among these are reduced water velocity, flushing and oxygen exchange between the sediment and water column. While these factors may not be of great importance to T. testudinum, which thrives in anoxic sediments (Zieman, 1982), competition for light can be very important (Short et al., 1995). The drift algal mats at the study site are very thick and can completely obscure large seagrass patches of many square meters in area (personal observation). Neverthe-less, the results of the present study suggest that the annual algal bloom alone has no negative effects on T. testudinum shoot density or biomass. Other studies, however, have shown that if such patches remain in place for sufficiently long periods of time (e.g. 6 months) they can cause substantial loss of aboveground biomass and create bare patches (Holmquist, 1997).
Although the naturally-occurring drift algal blooms do not appear to have significant effects on the T. testudinum population, when combined with increased grazing pressure there are synergistic effects that can greatly decrease seagrass shoot density. Increased grazing pressure exacerbates the negative effects of the drift algae (or vice versa). When subject to both algal competition and increased urchin grazing, T. testudinum shoot density experiences a greater decline than when there is only an increase in grazing activity. Thus it appears that the non-significant impact of the naturally occurring urchin population is important to the persistence of the West Point seagrass community. Increased grazing pressure from a larger urchin population, coupled with the negative effects of algae, could be so detrimental as to prevent full recovery of the seagrass population from the annual algal bloom.
4.3. Effects on the invertebrate population
The numbers of invertebrates found in this study are relatively low in comparison to other seagrass bed studies, which often report invertebrate abundances in the thousands
22
per m (Santos and Simon, 1974; Brook, 1978; Gore et al., 1981; Greening and Livingston, 1982; Virnstein et al., 1983; Bauer, 1985; Virnstein and Howard, 1987; Valentine and Heck, 1993; Greenway, 1995). One factor undoubtedly contributing to the low observed faunal numbers in the present study is sampling design. The sampling protocol used in this study could not effectively include crustaceans, which are among the most common inhabitants of seagrass beds (Orth, 1973; Gore et al., 1981; Lewis, 1984; Holmquist et al., 1989). Even with the absence of crustacean numbers taken into account, however, the abundance of invertebrates at the study site is still considerably lower than that found in other studies. The reasons for this are unclear, and await the results of further studies.
the seagrass blades, presumably feeding on epiphytes (Emerson and Jacobson, 1976). As there was no decrease in aboveground biomass of T. testudinum in the summer experiment, the lack of an effect on L. americanum is not surprising. In the winter, however, there was a decrease in seagrass biomass in the high urchin density treatment. Nevertheless, a concurrent decline in the abundance of L. americanum did not occur. The population density of L. americanum found during the winter was very low 2
22
0.1560.04 individuals m . It is possible that such a small population could maintain itself on what seagrass remained, despite the significant losses in aboveground biomass. The polychaetes Marphysa sanguinea and Asychis elongata feed primarily on detritus (Day, 1967; Fauchald and Jumars, 1979; Prevedelli, 1992). Neither species was affected by the experimental treatments. As part of the infauna, these animals would not experience any direct impact from the algae or the urchins, which are primarily aboveground grazers. It is also unlikely that, over the short duration of the experiment (6 weeks), increased grazing pressure would affect the amount of detritus available below the surface of the sediment, where these species feed. Long-term exposure to increased urchin grazing activity, however, could potentially increase the amount of organic
´
material in the sediment via deposition of feces (Macia, manuscript in preparation). These could eventually be worked into the sediment and benefit the infaunal detritivores.
5. Conclusions
The T. testudinum population at West Point in Biscayne Bay, Florida, experiences low levels of grazing from a small but relatively stable population of the sea urchin
Lytechinus variegatus. This natural grazing pressure does not appear to have significant
consequences for either the biomass or shoot density of the seagrass population. Resistance of T. testudinum to grazing is higher in the summer than in the winter. Shoot density is more vulnerable to urchin grazing than aboveground biomass, possibly as a result of grazing-induced increases in seagrass productivity. The overall vulnerability of a seagrass population to grazing may depend on the level of grazing to which it is normally exposed. Seagrass beds, such as at West Point, with small herbivore populations may be more resistant to temporarily increased grazing than beds that normally experience higher grazing pressure. The normal presence of drift algal blooms does not appear to be detrimental to T. testudinum, but when combined with increased grazing pressure can have serious negative effects on the shoot density of the seagrass. At West Point, a site with relatively low faunal abundances, increased urchin grazing appears to have neither positive nor negative effects on the sampled invertebrate community.
Acknowledgements
forever satisfied: B. Orlando, P. Biber, L. Kaufman, T. Jones, E. Irlandi, D. Lirman, M. Brown, A. Morales, and J. Wiley. The comments of M. Harwell, E. Irlandi, J. Leal, A. Szmant, N. Voss, an anonymous reviewer and especially M. Robinson helped greatly in shaping my rough early drafts into this final version. I am also very grateful to S. Schultze for his help in the identification of polychaetes. Financial support for this work was provided by NOAA Coastal Ocean Program Award[NA37RJ0149, The
Sanibel-Captiva Shell Club, and the RSMAS Anonymous Donor Award. This research is submitted in partial fulfillment of the requirements for the PhD degree at the University of Miami. [RW]
References
Bauer, R.T., 1985. Diel and seasonal variation in species composition and abundance of caridean shrimps (Crustacea, Decapoda) from seagrass meadows on the north coast of Puerto Rico. Bull. Mar. Sci. 36, 150–162.
Bell, S.S., Hall, M.O., 1997. Drift macroalgal abundance in seagrass beds: investigating large-scale associations with physical and biotic attributes. Mar. Ecol. Prog. Ser. 147, 277–283.
Brook, I.M., 1978. Comparative macrofaunal abundance in turtlegrass (Thalassia testudinum) communities in South Florida characterized by high blade density. Bull. Mar. Sci. 28, 212–217.
Camp, D.K., Cobb, S.P., van Breedveld, J.F., 1973. Overgrazing of seagrasses by a regular urchin, Lytechinus
variegatus. Bioscience 23, 37–38.
´
Cebrian, J., Duarte, C.M., Agawin, N.S.R., Merino, M., 1998. Leaf growth response to simulated herbivory: a comparison among seagrass species. J. Exp. Mar. Biol. Ecol. 220, 67–81.
Day, J.H., 1967. A Monograph of the Polychaeta of Southern Africa. Part 1. Errantia, British Museum of Natural History, London.
Emerson, W.K., Jacobson, M.K., 1976. The American Museum of Natural History Guide to Shells, Knopf, New York.
Engstrom, N.A., 1982. Immigration as a factor in maintaining populations of the sea urchin Lytechinus
variegatus (Echinodermata: Echinoidea) in seagrass beds on the southwest coast of Puerto Rico. Stud.
Neotrop. Fauna Environ. 17, 51–60.
Fauchald, K., Jumars, P., 1979. The diet of worms: a study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 17, 193–284.
Gore, R.H., Gallaher, E.E., Scotto, L.E., Wilson, K.A., 1981. Studies on decapod crustacea from the Indian River Region of Florida. Estuar. Coast. Shelf Sci. 12, 485–508.
Greening, H.S., Livingston, R.J., 1982. Diel variation in the structure of seagrass-associated epibenthic macroinvertebrate communities. Mar. Ecol. Prog. Ser. 7, 147–156.
¨
Greenway, M., 1974. The effects of cropping on the growth of Thalassia testudinum (Konig) in Jamaica. Aquaculture 4, 199–206.
Greenway, M., 1976. The grazing of Thalassia testudinum in Kingston Harbour. Jamaica. Aquat. Bot. 2, 117–126.
Greenway, M., 1995. Trophic relationships of macrofauna within a Jamaican seagrass meadow and the role of the echinoid Lytechinus variegatus (Lamarck). Bull. Mar. Sci. 56, 719–736.
Hansen, K., Kristensen, E., 1997. Impact of macrofaunal recolonization on benthic metabolism and nutrient fluxes in a shallow marine sediment previously overgrown with macroalgal mats. Estuar. Coast. Shelf Sci. 45, 613–628.
Holmquist, J.G., 1994. Benthic macroalgae as a dispersal mechanism for fauna: influence of a marine tumbleweed. J. Exp. Mar. Biol. Ecol. 180, 235–251.
Holmquist, J.G., Powell, G.V.N., Sogard, S.M., 1989. Decapod and stomatopod assemblages on a system of seagrass-covered mud banks in Florida Bay. Mar. Biol. 100, 473–483.
Hull, S.C., 1987. Macroalgal mats and species abundance: a field experiment. Estuar. Coast. Shelf Sci. 25, 519–532.
Keller, B.D., 1983. Coexistence of sea urchins in seagrass meadows: an experimental analysis of competition and predation. Ecology 64, 1581–1598.
Lewis, F.G., 1984. Distribution of macrobenthic crustacean associated with Thalassia, Halodule and bare sand substrata. Mar. Ecol. Prog. Ser. 19, 101–113.
´
Macia, S., Lirman, D., 1999. Destruction of Florida Bay seagrasses by a grazing front of sea urchins. Bull. Mar. Sci. 65, 593–601.
McGlathery, K.J., 1995. Nutrient and grazing influences on a subtropical seagrass community. Mar. Ecol. Prog. Ser. 122, 239–252.
McNaughton, S.J., 1979. Grazing as an optimization process: grass–ungulate relationships in the Serengeti. Am. Nat. 113, 691–703.
Milchunas, D.G., Lauenroth, W.K., 1993. Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol. Monogr. 63, 327–366.
Montague, J.R., Ortiz, L., Arguelles, A., Millan, J.M., Cardoch, L., 1988. Density and dispersion estimates for sea urchins in a south Florida seagrass community. Fla. Sci. 51, 19–22.
Moore, H.B., Jutare, T., Bauer, J.C., Jones, J.A., 1963. The biology of Lytechinus variegatus. Bull. Mar. Sci. 13, 23–53.
Ogden, J.C., 1976. Some aspects of herbivore–plant relationships on Caribbean reefs and seagrass beds. Aquat. Bot. 2, 103–116.
Ogden, J.C., 1980. Faunal relationships in Caribbean seagrass beds. In: Phillips, R.C., McRoy, C.P. (Eds.), Handbook of Seagrass Biology, Garland Press, New York, pp. 173–198.
Oliver, G.D., 1987. Population Dynamics of Lytechinus variegatus. MS thesis, University of Miami. Orth, R.J., 1973. Benthic infauna of eelgrass, Zostera marina, beds. Chesapeake Sci. 14, 258–269. Prevedelli, D., 1992. Influence of temperature and diet on the larval development and growth of juveniles
Marphysa sanguinea (Montagu) (Polychaeta, Eunicidae). In: Dauvin, J.C., Laubier, L., Reish, D.J. (Eds.),
Proceedings of the Fourth International Polychaete Conference, pp. 521–526.
Santos, S.L., Simon, J.L., 1974. Distribution and abundance of the polychaetous annelids in a south Florida estuary. Bull. Mar. Sci. 24, 669–689.
Short, F.T., Burdick, D.M., Kaldy, J.E., 1995. Mesocosm experiments quantify the effects of eutrophication on eelgrass, Zostera marina. Limnol. Oceanogr. 40, 740–749.
Valentine, J.F., Heck, K.L., 1991. The role of sea urchin grazing in regulating subtropical seagrass meadows: evidence from field manipulations in the northern Gulf of Mexico. J. Exp. Mar. Biol. Ecol. 154, 215–230. Valentine, J.F., Heck, K.L., 1993. Mussels in seagrass meadows: their influence on macroinvertebrate abundance and secondary production in the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 96, 63–74. Virnstein, R.W., Carbonara, P.A., 1985. Seasonal abundance and distribution of drift algae and seagrasses in the
mid-Indian River Lagoon, Florida. Aquat. Bot. 23, 67–82.
Virnstein, R.W., Howard, R.K., 1987. Motile epifauna of marine macrophytes in the Indian River Lagoon, Florida. II. Comparisons between drift algae and three species of seagrasses. Bull. Mar. Sci. 41, 13–26. Virnstein, R.W., Mikkelson, P.S., Cairns, K.D., Capone, M.A., 1983. Seagrass beds versus sand bottoms: the
trophic importance of their associated benthic invertebrates. Fla. Sci. 46, 363–381.
Zieman, J.C., 1982. The ecology of the seagrasses of south Florida: a community profile, US Fish and Wildlife Service, Biol. Serv. Program FWS / OBS-82 / 25.