www.elsevier.com / locate / bres
Research report
Influence of estrous cycle on vaginocervical sensitivity: a
fos-immunohistochemical study of lumbosacral spinal cord
a a ,
*
b bA. Ghanima , M. Bennis
, O. Rampin , J.P. Rousseau
a
´ ´
Laboratoire de Neuroscience du Comportement, Faculte des Sciences Semlalia, Departement de Biologie, Bd. Prince My Abdallah, 40 000, Marrakech, Maroc
b
´ ´
Laboratoire de Neurobiologie des Fonctions Vegetatives, INRA, Jouy-en-Josas, France
Accepted 2 August 2000
Abstract
Expression of c-fos in L –S spinal segments in response to mechanical vaginocervical stimulation was investigated in both cycling5 1 and ovariectomized females. The aim of this paper was to verify the influence of estrous cycle on females genital tract sensitivity using immunodetection of a neural activity endogenous marker. The results indicate that lumbosacral spinal Fos-labeling was highly increased in vaginocervical stimulated rats relative to control, and labeled neurons were present more intensively in the dorsal horn in comparison to other spinal areas. Significant differences in Fos-labeling were observed according to the estrous cycle stage at which the stimulation was applied. In estrous females, the response was greater than that obtained at diestrous and much greater than the response of proestrous females. The spinal Fos-labeling of ovariectomized females is equivalent to that of diestrous females. These results give evidence that the vaginocervical induced expression of c-fos is modulated by cyclic changes in circulating sex hormones, whereas results observed in ovariectomized females indicate the likely involvement of other mechanisms independent of ovarian hormones. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory system
Topic: Spinal cord
Keywords: Female rat; Vaginocervical stimulation; Fos; Lumbosacral spinal cord; Estrous cycle
1. Introduction show tail-flick responses to radiant heat [20].
Vaginocervi-cal afferent activity enters the spinal cord via the pelvic Vaginocervical stimulation (VCS) elicits a variety of and hypogastric nerves, two nerves which innervate struc-neuroendocrine, behavioral and neural changes in female tures of the female reproductive tract, including the vagina, rats [32,45]. VCS potentiates both the lordosis reflex, and cervix and the uterine [3,19,20,46], as shown by elec-proceptive pacing behaviors [13,27,45]. It regulates neuro- tophysiological recording [5] and horseradish peroxydase endocrine reflexes associated with luteinizing hormone neural tracings [5,36,37,48]. The input of the pelvic nerve, release [47] and induces prolactin surges during the first which occurs at lumbosacral spinal cord, is critical for days of pseudo-pregnancy or early pregnancy [14,21]. It is VCS-induced changes, since transection of the pelvic involved in sperm transport, and fertility [1,33] and nerve blocks or attenuates the behavioral, autonomic and pregnancy [2,15]. In addition, VCS attenuates neural and neuroendocrine effects of VCS [14,25].
behavioral responses to noxious stimulation [13,26,40]. Recent studies have demonstrated that the expression of The analgesic effect of VCS has been measured for immediate-early genes such as c-fos can be used as example, by the increased vocalization thresholds in endogenous markers of neural activity in response to response to tail shock [13,20] or increased latencies to sensory stimulation [24]. Populations of neurons that respond to VCS were successively identified in several brain areas [42,49] using immunodetection of Fos, the
*Corresponding author. Tel.: 1212-4-4346-4649; fax: 1
212-4-437-protein product of c-fos gene. Nevertheless, most of the
412.
E-mail address: [email protected] (M. Bennis). available data have been developed in castrated or
used in this study. Rats were housed in animal facilities ered significant for P,0.05.
under a 12:12 h Light / Dark cycle (light on at 08:00 h) To determine the estrous stage of each female, vaginal with free access to food and water. smears were colored with 10% Giemsa and analyzed under VCS was performed in an experimental group of a light microscope. We have observed that the smears females, lightly anaesthetized with an intra-peritoneal performed using the balloon or by a conventional tech-injection of 25 mg of ketamine chlorhydrate / kg bw, nique (gentle swap) gived similar results. In the
ex-ˆ ´
(IMALGENE 500, Rhone Merieux, Lyon, France). Anes- perimental group, cycling females were assigned to one of thesia prevented animal motility and agitation during the three groups: estrous, diestrous and proestrous. The num-stimulation which obliged the operator to apply unwanted ber of rats at each stage was 5. Statistical analysis of Fos skin stimulation to maintain the animal. A latex balloon data in cycling, non-stimulated control rats, revealed no was inserted through the vagina at the vaginocervical level differences due to estrous cycle. Five rats were then and then inflated via a catheter with 0.5 ml of water (20 selected randomly to form the control group of cycling mmHg) to distend the wall for 10 min. To record vag- females.
inocervical contractions, the catheter was connected to a In order to verify the effect of ovariectomy, ten females pressure transducer (Telco M52). Pressure signal was sent were ovariectomized bilaterally under Ketamine to a paper chart recorder (CS 600). At the end of the chlorhydrate anesthesia (100 mg / kg) via lumbar incisions. stimulation, the balloon was deflated and used to perform a After a recovery of 4 weeks, 5 females received vag-vaginal smear. inocervical stimulation and the 5 others were used as
Control rats were lightly anaesthetized and handled as ovariectomized, non-stimulated controls. long as the experimental group. No VCS was applied. The
vaginal smear was performed just before perfusion.
After a surviving period of 60 min (after the end of the 3. Results
VCS), rats were killed by an overdose of anaesthetic
(Nembutal) and perfused intracardially with 250 ml of 3.1. Vaginal and cervix activity during VCS phosphate buffered saline (PBS, 0.1 M, pH 7.4), followed
by 500 ml of 4% paraformaldehyde in PBS. The spinal In anaesthetized female rats, vaginocervical contractions cord was exposed and the L , L and S segments were5 6 1 were recorded during VCS. Fig. 1 represents a typical removed and post-fixed overnight at 48C in the same recording of vaginocervical activity which showed periodic fixative. Transverse sections (40 mm thick) were cut on a contractions followed by a period of rest, corresponding to vibratome, processed as free-floating sections and Fos the activity of smooth muscle of vaginocervical area. The immunostained according to the avidin–biotin–peroxidase contractions lasting 36.5067.26 s and reaching method [23]. Sections were pre-incubated for 1 h in normal 12.4361.39 mmHg. The mean duration of the rest period goat serum (2% in PBS) and 0.3% Triton X-100, followed was 21.8067.82 s.
by incubation for 48 h at 48C in the primary antibody,
raised against Fos protein, diluted at 1:1000 (Oncogene 3.2. Fos-labeling in control and experimental cycling Science Paris, France). Sections were then rinsed in PBS, females
incubated in biotinyled goat anti-rabbit antiserum (Vector
Laboratories, Burlingame, CA) at 1:200 for 4 h, washed in A preliminary study had shown that the most meaning-PBS and then incubated for 1 h 30 at room temperature in ful c-fos expression in response to VCS was observed in avidin–biotin–peroxidase complex (Vectastain Elite ABC L –S5 1 segments in comparison with the other lumbar Kit, Vector Laboratories, Burlingame, CA). The peroxidase segments. In the three spinal segments L , L and S , c-fos5 6 1
Fig. 1. Typical recording of intravaginal pressure recorded during vaginocervical stimulation of ovariectomized female showing periodic contractions of nearly equal amplitude and duration.
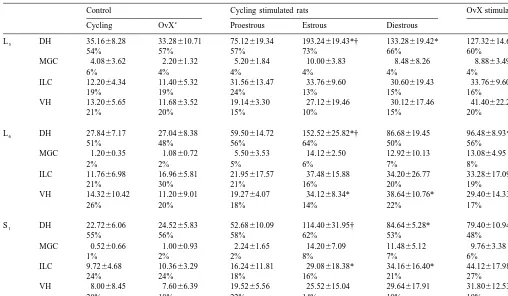
horn (DH), (ii) the intermediolateral cell column (ILC), Fos-labeled neurons than diestrous and proestrous (P,0.05 (iii) the dorsal gray commissure (MGC) and (iv) the for each).
ventral horn (VH) (Fig. 2). The DH contained the greatest In L and L , estrous, diestrous and proestrous females5 6
number of Fos-positive neurons (6067% of the total Fos- displayed significantly more Fos-positive neurons in re-labeled nuclei), whereas the MGC, the VH and the ILC sponse to VCS, relative to cycling non stimulated controls
24
contained respectively 1864%, 1764% and 562% (Table (respectively F(3,16)523.6, P,1.10 and F(3,16)521.2,
24
1). P,1.10 ). In S , only estrous and diestrous females1
One way ANOVA revealed significant differences in the significantly displayed a greater number of Fos-immuno-total number of Fos-labeled neurons over the L –S spinal5 1 reactive neurons relative to control (F(3,16)513.6, P,
24
portion between the four groups (estrous, diestrous, 1.10 ). Cycling females showed different quantities of proestrous and cycling non-stimulated controls) (F(3,16)5 Fos-labeled neurons in response to VCS. Estrous females
24
31.7, P,1.10 ). Student–Newman–Keuls test further had a greater number of Fos-labeled neurons than proestr-revealed that stimulated rats displayed significantly more ous in L5 (264.1620.0 and 131.0613.5, respectively,
23
Fos-labeled neurons than control rats (Fig. 3) and among F(2,12)510.7, P52.2.10 ), in L6 (238.2618.4 and
23
stimulated females, estrous rats had a greater number of 106.2613.4, respectively, F(2,12)511.9, P51.4.10 ) and in S1 (183.2628.7 and 90.7610.1, respectively,
22
F(2,12)55.7, P51.82.10 ) and than in diestrous in L6
(238.2618.4 and 172.4624.2 respectively, F(2,12)511.9,
23
P51.4.10 ). The number of Fos-positive neurons in the diestrous group was constantly greater than that of proestr-ous in L5 (202.5625.7 and 131.0613.5, respectively,
23
F(2,12)510.7, P52.2.10 ), in L6 (172.4624.15 and
23
106.1613.7, respectively, F(2,12)511.9, P51.4.10 ) and in S1 (159.9617.2 and 90.7610.1, respectively,
22 F(2,12)55.7, P51.82 10 ).
Data analysis of the different spinal segment areas revealed that the DH of estrous females contained sig-nificantly more Fos-positive neurons than proestrous and diestrous in L , L and S (P5 6 1 ,0.05 for each). In diestrous females, the DH displayed more Fos-labeled neurons than proestrous in L5 and S1 (P,0.05 for each). The ILC presented significant differences only in the S1 segment
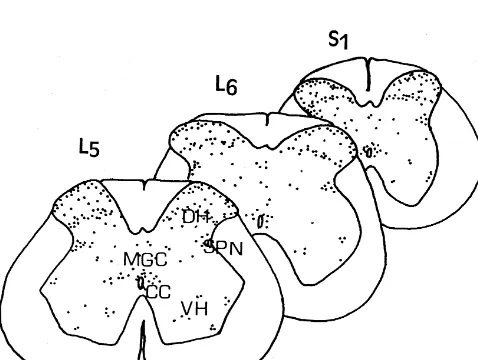
Fig. 2. Examples of transverse sections of the L , L and S spinal cord5 6 1 between estrous and diestrous females vs proestrous (P,
segments of an estrous female showing distribution of Fos-labeled 0.05 for each). In contrast, there was no difference in the neurons in the dorsal horn (DH), intermediolateral cell column (ILC),
number of Fos-positive neurons in the MGC between the
dorsal grey commissure (MGC) and the ventral horn (VH) after
vag-different estrous cycle stages. The number of Fos-labeled
inocervical stimulation. CC: central canal. Each dot represents a
MGC 1.2060.35 1.0860.72 5.5063.53 14.1262.50 12.92610.13 13.0864.95
Significantly higher than proestrous. P,0.05.
†
Significantly higher than diestrous. P,0.05.
c
OvX: ovariectomized.
between estrous and proestrous (P,0.05) and between three spinal segments. The total number of Fos-positive neurons over the L –S spinal segments observed in diestrous and proestrous (P,0.05) (Fig. 4). 5 1
ovariectomized females, in response to VCS represents 80% of that of estrous females, 102% of diestrous females 3.3. Fos-labeling in ovariectomized females
and 167% of proestrous females.
Data of different spinal segment areas indicate that In ovariectomized, female rats, the VCS induced a
Fos-differences between stimulated females of both ovariec-labeling distribution similar to that described in cycling
tomized and cycling groups, were restricted to differences females in the three spinal segments. The number of
in Fos-labeling in the DH. No difference occurred in the Fos-positive neurons was significantly higher in
ovariec-other spinal areas between groups. The number of Fos-tomized vaginocervical stimulated rats relative to
ovariec-labeled neurons in DH was greater in the estrous group and tomized non-stimulated controls in L5 (212.4615.2 and
24
smaller in the proestrous group, relative to the ovariectom-58.664.7, respectively, F(1,8)592.3, P,1.10 ), in L6
ized vaginocervical-stimulated group in L (F(3,16)534.8, (172.2612.5 and 56.366.4, respectively, F(1,8)567.9, 5
24 24 24
P,1.10 ) and in S (165.11 619.2 and 43.164.3, respec- P,1.10 ), L6 (F(3,16)522.8, P,1.10 ) and S1
24 24
tively, F(1,8)537.9, P,1.10 ). (F(3,16)510.1, P56.10 ). Concerning other spinal areas, There was no difference in the total number of Fos- the ILC of S in proestrous females contained a smaller1
labeled neurons between control groups of both cycling number of Fos-positive neurons relative to ovariectomized and ovariectomized rats at the three spinal segments. In vaginocervical-stimulated rats.
contrast, the number of Fos labeled neurons in responses to VCS was significantly higher in ovariectomized females
than that of proestrous but lower than that of estrous, 4. Discussion
24
constantly in L5 (F(5,24)527.9, P,1.10 ), in L6 24
(F(5,24)523.1, P,1.10 ) and in S (F(5,24)1 514.9, P, 4.1. Fos-labeling in the spinal cord
24
1.10 ). No difference was observed between
an overlap between Fos-positive neurons activated by electrical stimulation of the pelvic nerve [7] and those activated by VCS. In addition, responsive neurons to cervix stimulation have been detected by electophysiological recording throughout the DH of L –S [4]. Such results6 2
indicate that the spinal levels described above are the principal spinal regions where vaginocervical sensitivity is conveyed directly or through interneurons as shown by Fos immunohistochemistry [24].
In the present study, VCS is shown to increase the number of Fos-positive neurons in the superficial layers and the medial part of the DH, where afferent projections to the thalamus, hypothalamus and lower brainstem origi-nate. Almost differences in Fos labeling between stimu-lated females of both cycling and ovariectomized groups relative to their respective controls or between the different estrous cycles stages are restricted to differences in DH Fos-labeling. This result underlined the important role of this area as a major part of the spinal segment that receives vaginocervical sensitivity and suggested that VCS likely activates spinothalamic and spinohypothalamic [8] path-ways probably used in the neuroendocrine (e.g. LH, prolactin and / or oxytocin release [21]) and analgesic responses to VCS (e.g. vocalization thresholds in response to tail shock [13]). Indeed, the VCS can stimulate anti-nociceptive but also anti-nociceptive systems and the balance between the two opposing systems can determine the nature of the response to VCS. The use of low dose of Ketamine (a non-competitive blocker of N-methyl-D
-aspar-tate channel) [43] as anesthetic, if not totally antagonizes the nociceptive component of the VCS, probably reduces it and the response obtained will correspond in large part to non-nociceptive sensations [9].
Fos-labeled neurons are also found in the MGC, which
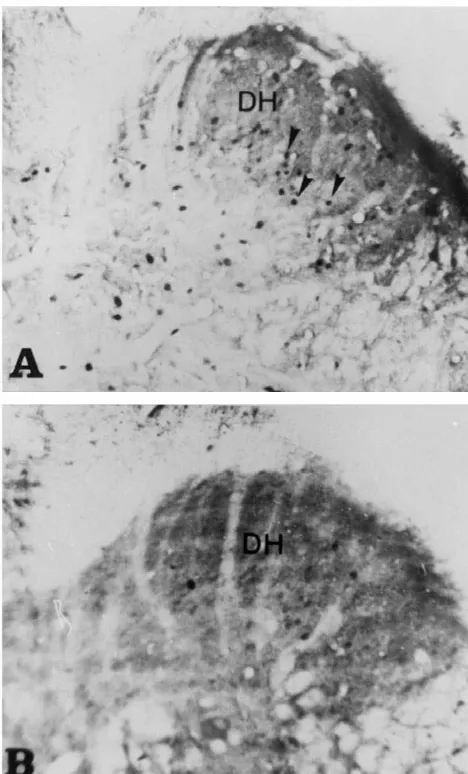
Fig. 3. Photomicrographs of a transverse section of the S spinal cord1
showing Fos-positive neurons in the dorsal horn area, (arrows). A: is reported to receive a part of pelvic nerve inputs [36]. vaginocervical stimulation in estrous female; B: control estrous female. Significant differences are also observed between stimu-Note the difference in the number of Fos-immunoreactive neurons
lated and control groups in this area. In addition,
Fos-between A and B mainly in the superficial layers of the dorsal horn. Bar:
labeling is observed in the ILC, which contains cell bodies
17.7mm.
of preganglionic neurons whose axons run in the pelvic nerve [36], and neurons that project to the medial hypo-reporting that VCS induces a net activation of the immedi- thalamus [8]. MGC and ILC neurons could be implicated ate-early gene expression in the lumbosacral spinal cord. in visceral reflexes such as lubrication of the vaginal canal, Both cycling and ovariectomized groups displayed a motor control of the vaginocervical area and endocrine greater number of Fos-positive neurons in response to VCS reflexes. Indeed, a sequence of regular contractions re-relative to their respective controls, in the three examined corded in response to VCS and probably induced reflexive-spinal segments. We assumed that the Fos-labeling ob- ly, may explain the labeling of motoneurons in the ILC. In served in the control group is due to the lightly cutaneous contrast, the VH contains very few Fos-positive neurons somatic stimulation, which is applied during rats handling and shows no differences in Fos-labeling between all [31]. groups at the L –S segments and this indicates that the5 1
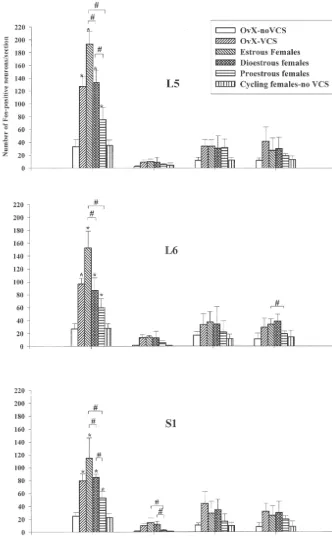
Fig. 4. Histograms displaying the number of Fos-labeled neurons in the dorsal horn (DH), intermediolateral cell column (ILC), dorsal grey commissure (MGC) and ventral horn (VH) of the L , L and S spinal cord in vaginocervical stimulated females of both cycling (Estrous, Diestrous, Proestrous) and5 6 1
ovariectomized rats (VCS-OvX) relative to their respective control (Normal female control, OvX-Control). Note that the control group presents significantly less Fos-labeling than the experimental groups. The DH contained more Fos-positive neurons in comparison to the ILC, the MGC and the VH. At the estrous stage, the number of Fos-positive neurons in the DH is greater than in the diestrous stage and much more than proestrous. The response of ovariectomized rats was comparable to that of diestrous. *: P,0.05 relative to control.[:P,0.05 for comparison between stimulated groups.
of neurons normally activated by cutaneous somatic inputs by immunohistochemical and in-situ hybridization tech-received from male mounts and this suggests that VCS niques [38]. Furthermore, neural conductivity and endo-may be capable of initiating spinal and / or brain mecha- crine surges of certain hormones are also affected by the nisms of analgesia. The study of Jonson and Komisaruk estrous cycle. It had been effectively established that both [17] provides evidence that VCS attenuates nociceptive receptive field and response threshold of nerves could activity in the dorsal horn. Number of events mediated by change according to the hormonal environment and that VCS are very closely related to the integrity of the pelvic the same stimulation could recruit more or less fibers nerve. With respect to afferent fibers in the pelvic nerve, depending on the day of estrous producing a variation in their wide-ranging sensitivity to vaginal and cervix stimu- the number of influx carried by the nerves [28,30]. In lation and their variegated and specific responses to both addition, endocrine changes characteristic of pseudo-preg-mechanical and chemical stimuli [6], suggest their impor- nancy in response to VCS are affected by the stage of the tance for sensations, hormonal events, and behaviors cycle at which the stimulation was performed [10] and this associated with mating and with the passage of fetus confirms the importance of hormonal status in the modula-through the cervix and vagina during parturition. Be- tion of female genital tract sensitivity, more specially the havioral studies conducted in rats and goats have shown vaginocervical part. All these data support the hypothesis that pelvic neurectomies interfere with sensory-dependent that sex hormones may promote or alter c-fos expression in reproductive events such as the induction of pseudo-preg- the central nervous system and produce changes of both nancy and lordosis [25] and fetus-expulsion, or Ferguson endocrine functions and nerve proprieties.
reflex [22,39], which occur when the cervix is stimulated. However, the response of ovariectomized females to VCS, is equivalent to that of diestrous females and is not 4.2. Estrous cycle and c-fos expression in the spinal similar to that of low sex-hormone level stage (estrous) as
cord expected. This suggests the contribution of factors differ-ent from ovarian hormones in the modulation of vag-The result of our study reported that VCS produced a inocervical sensitivity. Ovariectomy is followed by an conspicuous variation of c-fos expression in the lum- activation of the hypothalamic LH–RH release [47] that it bosacral spinal cord according to the estrous cycle. The is suspected to be implicated in the response of ovariec-most important spinal Fos expression occurred at the tomized females. The involvement of this factor in the estrous stage and the less important response is obtained at neuroendocrine and behavioral responses induced by VCS the proestrous. On the basis of the reviewed analysis of the was in fact, previously suspected in other works which ovarian cycle of the rat [16], which report dramatic suggested that the effect on central nervous system should changes in circulating sex steroids during the estrous cycle, not only be exerted on neurons themselves [10,11,35,41]. we can establish a relationship between hormonal status The site of action of the released hypothalamic neuro-and c-fos expression neuro-and / or vaginocervical sensitivity. The peptide may also be a peripheral tissue (genital tract) and estrous, period of strong expression of c-fos, correspond to the modification of neural activity as evidenced by c-fos the low circulating estradiol phase. At diestrous, where the expression, may only be an indirect effect.
blood concentration of estradiol began to rise significantly, In conclusion, numerous behavioral and endocrine the c-fos expression started to decrease and reached its changes, such as prolongation of lordosis, sexual receptivi-lowest intensity at proestrous, period of maximal estradiol ty [45] and hormonal changes [18] reported to be induced blood concentration. From these relationships, we carried by VCS may probably involve spinal neurons evidenced in out that the higher concentration of estrogen seemed to our study. Most of the differences that we observed alter the early gene c-fos expression in lumbosacral spinal between experimental groups are effectively restricted to cord. This might be explained by the fact that during this differences in Fos-labeling occurring in the DH. But, we period of high receptiveness to the male, the elevated rate are not able at the present time, to confirm that the VCS of estradiol would have the effect to reduce vaginocervical induced expression of c-fos in the lumbosacral spinal cord sensitivity so that the intromission is not discerned as a is due to a direct projection of vaginocervical sensitivity or painful stimulus. indirect reafferentation activated by vaginal contraction. In Sex steroids are believed to regulate sexual behavior in fact, the extra-spinal pathways from the female genital part by altering gene expression and neural excitability tract to the brainstem as demonstrated by spinal transection within special regions of the brain [12]. The differences experiments are also very important pathways of the observed in the present study, might be due in large part to vaginocervical sensitivity [29]. The variation in the c-fos the hormonal environment, which probably influence the expression according to the estrous cycle underlined the female genital tract sensitivity and / or spinal gene expres- important role of sex-hormone.
44–61.
References
[22] T. Higuchi, K. Uchide, K. Honda, H. Negoro, Pelvic neurectomy abolished the fetus-expulsion reflex and induces dystocia in the rat, [1] N.T. Adler d, J.P. Toner, The effects of copulatory behavior on Exp. Neurol. 96 (1989) 443–455.
sperm transport and fertility in rats, in: B.R. Komisaruk (Ed.), [23] S.M. Hsu, L. Raine, H. Fanger, Use of avidin–biotin peroxidase Reproduction: A Behavioral and Neuroendocrine Perspective, New complex (ABC) in immunoperoxidase techniques: a comparison York Academy of Sciences, New York, 1986, pp. 21–32. between ABC and unlabeled antibody (PAP) procedures, J. Histoch-[2] N.T. Adler, J.A. Resko, R.W. Goy, The effects of copulatory em. Cytochem. 29 (1981) 577–580.
behavior on hormonal change in the female rat prior to implantation, [24] S.P. Hunt, G. Pini, G. Evan, Induction of c-fos like protein in spinal Physiol. Behav. 5 (1970) 1003–1007. cord neurons following sensory stimulation, Nature 328 (1987) [3] K.J. Berkley, H. Hotta, A. Robbins, Y. Sato, Functional properties of 632–634.
afferent fibers supplying reproductive and other pelvic organs in [25] E.J. Kollar, Reproduction in the female rat after pelvic nerve pelvic nerve of female rat, J. Neurophysiol. 63 (1990) 256–272. neurectomy, Anat. Rec. 115 (1953) 641–658.
[4] K.J. Berkley, C.H. Hubscher, P.D. Wall, Neural responses to [26] B.K. Komisaruk, J. Wallman, Antinociceptive effects of vaginal stimulation of the cervix, uterus, colon, colon and skin in the rat stimulation in rats: neurophysiological and behavioral studies, Brain spinal cord, J. Neurophysiol. 69 (1993) 545–556. Res. 137 (1977) 85–107.
[5] K.J. Berkley, A. Robbins, Y. Sato, Afferent fibers supplying the [27] B.R. Komisaruk, C. Diakow, Lordosis reflex intensity in rats in uterus in the rat, J. Neurophysiol. 59 (1988) 142–163. relation to estrous cycle, ovariectomy estrogen administration and [6] K.J. Berkley, A. Robbins, Y. Sato, Functional differences between mating behavior, Endocrinology 93 (1973) 548–557.
afferent fibers in the hypogastric and pelvic nerves innervating [28] B.R. Komisaruk, N.T. Adler, J. Hutchison, Genital sensory field: female reproductive organs in the rat, J. Neurophysiol. 69 (1993) enlargement by estrogen treatment in female rats, Science 178
533–544. (1972) 1295–1298.
[7] L.A. Birder, J.R. Roppolo, M.J. Ladarola, W.C. de Groat, Electrical [29] B.R. Komisaruk, R. Bianca, G. Sansone, L.E. Gomez, R. Cueva-stimulation of visceral afferents pathways in the pelvic nerve Ralon, C. Beyer, B. Whipple, Brain-mediated responses to vag-increases c-fos in lumbosacral spinal cord, Neurosci. Lett. 129 inocervical stimulation in spinal cord-transected rats: role of the
(1991) 193–196. vagus nerves, Brain Res. 708 (1996) 128–134.
[8] R. Burstein, K.D. Cliffer, G.J. Giesler, Cells of origin of the [30] L.M. Kow, D.W. Pfaff, Effect of estrogen on size of receptive field spinohypothalamic tract in the rat, J. Comp. Neurol. 291 (1990) and response threshold of pudendal nerve in female rat,
Neuroen-329–344. docrinology 13 (1973) 299–313.
[9] M. Caba, B.R. Komizaruk, C. Beyer, Analgesic synergism between [31] J.W. Lee, M.S. Erskine, Vaginocervical stimulation suppresses the AP5 (an NMDA receptor antagonist) and vaginocervical stimulation expression of c-fos induced by mating in thoracic, lumbar and sacral in the rat, Pharmacol. Biochim. Behav. 61 (1) (1988) 45–48. segments of the female rat, Neuroscience 74 (1997) 237–349. [10] A. Castro-Vasquez, A.H. Luque, N.B. Carreno, Modulation of [32] M. Martinez-Gomez, R. Chrino, C. Beyer, B.R. Komisaruk, P.
sensitivity to cervicovaginal stimulation during the estrous cycle: Pacheco, Visceral and postural reflexes evoked by genital stimula-Evidence of an extrapituitary action of LH–RH, Brain Res. 305 tion in urethane-anesthetized female rats, Brain Res. 575 (1992)
(1984) 231–273. 279–284.
[11] E. Cattaneo, A. Maggi, c-fos induction by oestrogen in specific rat [33] M. Matthews, N.T. Adler, Facilitative and inhibitory influences of brain areas, Eur. J. Pharmacol. Mol. Sec. 118 (1990) 153–159. reproductive behavior on sperm transport in rats, J. Comp. Physiol. [12] S. Chinapen, J.M. Swann, J.L. Steinman, B.R. Komisaruk, Expres- Psychol. 91 (1977) 727–741.
sion of c-fos protein in lumbosacral spinal cord in response to [34] D. Menetrey, A. Gannon, J.D. Levine, A.I. Basbaum, Expression of vaginocervical stimulation in rats, Neurosci. Lett. 145 (1992) 93– c-fos protein in interneurons and projection neurons of the rat spinal
96. cord in response to noxious somatic, articular and visceral
stimula-[13] W.R. Crowley, R. Jacobs, J. Volpe, J.F. Rodriguez-Sierra, B.R. tion, J. Comp. Neurol. 285 (1989) 177–195.
Komisaruk, Analgesic effect of vaginal stimulation in rats: modula- [35] R.L. Moss, S.M. McCann, Induction of mating behavior in rats by tion by graded stimulus intensity and hormones, Physiol. Behav. 16 luteinizing hormone-release factor, Science 181 (1973) 177–179. (1976) 483–488. [36] I. Nadelhaft, A.M. Booth, The location and morphology of pre-[14] M.S. Erskine, Prolactin release following mating and genitosensory ganglionic neurons and the distribution of visceral afferents from the stimulation in females, Endocrinol. Res. Rev. 16 (1995) 508–528. rat pelvic nerve: a horseradish peroxidase study, J. Comp. Neurol. [15] J.W. Everett, Provoked ovulation of long-delayed pseudopregnancy 226 (1984) 238–245.
and immunocytochemical studies, Brain Res. Bull. 21 (1988) 701– [44] A. Robbins, Y. Sato, H. Hotta, K.J. Berkley, Responses of
hypo-709. gastric nerve afferent fibers to uterine distension in oestrus and
[38] R.E. Nappi, M.J. Bonneau, S. Rivest, Influence of the estrous cycle metestrous rats, Neurosci. Lett. 110 (1990) 82–85.
on c-fos and CRH gene transcription in the brain of endotoxine- [45] J.F. Rodriguez-Sierra, W.R. Crowley, B.R. Komisaruk, Vaginal challenged rats, Neuroendocrinology 65 (1997) 29–46. stimulation in rats induces prolonged alordosis responsiveness and [39] G. Peeters, N. De Vos, A. Houvenaghel, Elimination of the Ferguson sexual receptivity, J. Comp. Physiol. 89 (1975) 79–85.
reflex by section of the pelvic nerves in the lactating goat, J. [46] S. Sato, R.H. Hayashi, R.E. Garfield, Mechanical responses of the Endocrinol. 49 (1971) 125–130. rat uterus, cervix and bladder to stimulation of hypogastric and [40] D.W. Pfaff, Estrogens and Brain Function, Springer, New York, pelvic nerves in vivo, Biol. Reprod. 40 (1989) 209–219.
1980. [47] H.G. Spies, G.D. Niswender, Levels of prolactin, LH and FSH in the [41] D.W. Pfaff, Luteinizing hormone-releasing factor (LRF) potentiates serum of intact and pelvic-neurectomized rats, Endocrinology 88
lordosis behavior in hypophysectomized ovariectomized female rats, (1971) 937–940.
Science 182 (1973) 1148–1149. [48] J.L. Steinman, S.M. Carlton, W.D. Willis, The segmental distribution [42] E.K. Polston, M.S. Erskine, Patterns of induction of the immediate- of afferent fibers from the vaginal cervix and hypogastric nerves in
early genes c-fos and erg-1 in female rat brain following differential rats, Brain Res. 575 (1992) 25–31.
amounts of mating stimulation, Neuroendocrinology 62 (1995) 370– [49] M.J. Tetel, M.J. Getsinger, G.F. Gebhart, Fos expression in the rat
384. brain following vaginocervical stimulation by mating or manual
[43] J.-P. Rivot, A. Sousa, J. Montagne-Clavel, J.-M. Besson, Nitric probing, J. Neuroendocrinol. 5 (1993) 397–404. Oxyd (NO) release by glutamate and NMDA in the the dorsal horn