www.elsevier.com / locate / bres
Interactive report
Functional plasticity in extrastriate visual cortex following neonatal
1visual cortex damage and monocular enucleation
a ,
*
a b c dKurt R. Illig
, Yuri P. Danilov , Aneeq Ahmad , Charlene B.Y. Kim , Peter D. Spear
a
Department of Anatomy and Centre for Neuroscience, University of Wisconsin Medical School, 1300 University Avenue, Madison, WI 53706, USA
b
Laboratory of Membrane Biochemistry and Biophysics, National Institute on Alcohol Abuse and Alcoholism, Rockville, MD 20852, USA
c
Department of Ophthalmology and Visual Sciences, University of Wisconsin, Madison, WI 53792, USA
d
Department of Psychology, University of Colorado, Boulder, CO 80309-0275, USA
Accepted 10 September 2000
Abstract
Neonatal lesions of primary visual cortex (areas 17, 18 and 19; VC) in cats lead to significant changes in the organization of visual pathways, including severe retrograde degeneration of retinal ganglion cells of the X /bclass. Cells in posteromedial lateral suprasylvian (PMLS) cortex display plasticity in that they develop normal receptive-field properties despite these changes, but they do not acquire the response properties of striate neurons that were damaged (e.g., high spatial-frequency tuning, low contrast threshold). One possibility is that the loss of X-pathway information, which is thought to underlie striate cortical properties in normal animals, precludes the acquisition of these responses by cells in remaining brain areas following neonatal VC damage. Previously, we have shown that monocular enucleation at the time of VC lesion prevents the X- /b-cell loss in the remaining eye. The purpose of the present study was to determine whether this sparing of retinal X-cells leads to the development of striate-like response properties in PMLS cortex. We recorded the responses of PMLS neurons to visual stimuli to assess spatial-frequency tuning, spatial resolution, and contrast threshold. Results indicated that some PMLS cells in animals with a neonatal VC lesion and monocular enucleation displayed a preference for higher spatial frequencies, had higher spatial resolution, and had lower contrast thresholds than PMLS cells in cats with VC lesion alone. Taken together, these results suggest that preserving X-pathway input during this critical period leads to the addition of some X-like properties to PMLS visual responses. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Visual cortex: extrastriate
Keywords: Posteromedial lateral suprasylvian; Plasticity; Visual cortex damage; X-pathway
1. Introduction suggests that each class of cell is responsible for carrying a
particular type of information into the visual system. In the mammalian eye, at least three types of retinal Further, the physiological properties of cells in different ganglion cells (RGCs) have been identified on the basis of cortical areas tend to reflect the input they receive. anatomical and physiological studies [2,5,6,9,17,19,20,32, Receptive-field characteristics of most area 17 cortical 33,38]. Termed X-, Y-, and W-cells, each of these primary cells have properties like X-cells in that they respond to RGC types projects to corresponding cells in the dorsal high spatial frequencies, have high spatial resolution, and lateral geniculate nucleus (dLGN), which in turn project to have low contrast thresholds. Responses of cells in areas primary visual cortex. The functional segregation and 18 and 19 are more like those of Y- and W-cells in that parallel organization of these three major input pathways they respond preferentially to low spatial frequencies, have low spatial resolution, and have high contrast thresholds [3,8,12,22,23,26,36,37].
1
Published on the World Wide Web on 25 September 2000.
Removal of primary visual cortex (areas 17, 18 and 19;
*Corresponding author. Tel.: 11-608-262-1607; fax: 1
1-608-262-VC) in the neonatal cat leads to significant changes in
7306.
E-mail address: [email protected] (K.R. Illig). remaining visual structures, including retrograde
tion of the dLGN and transneuronal retrograde degenera- to examine the physiological response properties of PMLS tion of RGCs [4,25,27,40]. The loss of dLGN cells is cortical cells in animals that received a VC lesion and severe; in adult cats that received a VC lesion on the day of monocular enucleation on the day of birth. Specifically, we birth, the volume of the dLGN is only 14% of that in recorded PMLS cell responses to visual stimuli in VC-normal adult cats [15]. Cells that remain have an anomal- lesion animals that had one eye removed at the time of the ous projection to postero-medial lateral suprasylvian lesion to investigate whether X-like response properties (PMLS) extrastriate cortex [7,15,16,24,31]. would develop in PMLS cortex, thereby making PMLS The degeneration of cells in the retina also is severe cortex more like striate cortex. We hypothesized that following neonatal VC damage, but it appears to be sparing retinal X-cells after a neonatal VC lesion would restricted to a single class of RGCs. Following a VC lesion lead to a novel extrastriate X-pathway input along the on the day of birth, nearly 80% of physiologically iden- enhanced geniculo-PMLS pathway and that this novel tified retinal X-cells are lost, whereas there is little or no projection would lead to changes in response properties in loss of Y- or W-cells [27,39,40]. Correspondingly, a loss of PMLS cortical cells that would reflect this novel input, up to 80% of cells with medium-sized somata has been including higher spatial-frequency tuning, higher spatial observed following a VC lesion within 1 week of birth resolution, and higher contrast sensitivity compared to
[14,25]. normal PMLS cells.
Physiological studies of extrastriate cortical neurons provide evidence that some functional reorganization accompanies the changes observed in the retina and dLGN
following a neonatal VC lesion [31]. Most cells in normal 2. Materials and methods
adult PMLS cortex are direction selective, respond poorly
to flashed stimuli, and can be driven by both eyes. After a 2.1. Subjects VC lesion in adulthood, abnormal response properties are
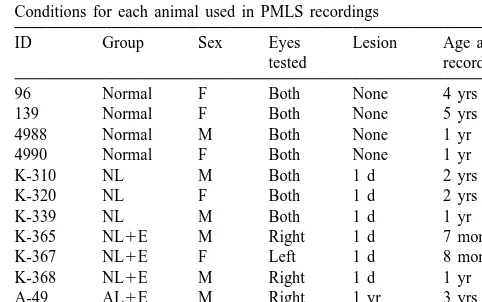
observed: there is a reduction in the percentage of direc- All procedures used in these experiments were per-tion-selective cells, an increase in the percentage of cells formed in accordance with NIH guidelines for animal use that respond to flashed stimuli, and a decrease in the and authorized by protocols approved by the University of percentage of cells that respond to the ipsilateral eye. This Wisconsin Research Animal Resource Center. Twelve is true even though there is little or no RGC loss associated animals were used for electrophysiological recording of with VC damage in adulthood. Despite the large reduction PMLS cortex (see Table 1): four normal animals, three of the dLGN and extensive RGC loss following neonatal animals that received a unilateral VC lesion within 24 h of VC damage, cells in PMLS develop normal receptive-field birth (neonatal lesion, NL), three animals that received a characteristics [27,39]. This represents a functional com- unilateral VC lesion and monocular enucleation within 24 pensation in that normal response properties develop in h of birth (neonatal lesion with enucleation, NL1E), and PMLS cortex despite the markedly different organization two animals that received a unilateral VC lesion in of its afferent visual pathways. adulthood and a monocular enucleation on the first day of A question that arises is why PMLS cells do not take on recording (adult lesion with enucleation, AL1E). All the properties of damaged neurons (e.g., in striate cortex) animals were born in the laboratory breeding colony, and following a neonatal VC lesion. One possibility is that animals that received VC lesions survived for at least 6 many properties of striate cortex, such as tuning for high months after VC lesion prior to electrophysiological re-spatial frequencies and high contrast sensitivity, are sub- cording.
served by the X-pathway [12,13,18,37]. Therefore, perhaps
development of striate cortical properties in PMLS cortex Table 1
following an early VC lesion does not occur because of the Conditions for each animal used in PMLS recordings
substantial retinal X-cell loss in these animals. ID Group Sex Eyes Lesion Age at
We were interested to find out whether sparing retinal tested recording
X-cells during a period of anatomical and physiological 96 Normal F Both None 4 yrs
plasticity could lead to incorporation of X-like response 139 Normal F Both None 5 yrs
properties by PMLS cells. We previously have demon- 4988 Normal M Both None 1 yr
4990 Normal F Both None 1 yr
strated that monocular enucleation prevents the
trans-K-310 NL M Both 1 d 2 yrs
neuronal retrograde degeneration of medium-sized RGCs
K-320 NL F Both 1 d 2 yrs
(i.e., X- /b-cells) following a neonatal VC lesion, possibly
K-339 NL M Both 1 d 1 yr
by eliminating binocular competition for survival factors in K-365 NL1E M Right 1 d 7 months
the dLGN [14]. This RGC sparing might allow X-pathway K-367 NL1E F Left 1 d 8 months
K-368 NL1E M Right 1 d 1 yr
information to be incorporated into remaining cortical
A-49 AL1E M Right 1 yr 3 yrs
areas, perhaps via the enhanced retino-geniculo-PMLS
A-50 AL1E F Left 3 yrs 6 yrs
2.2. VC lesions 2.4. Visual stimulation
All neonatal and adult lesions were performed under For initial evaluation of neuronal responses, visual sterile conditions as previously described [28–31,39]. stimuli were presented on the tangent screen with a hand-Briefly, cats were anaesthetized with 1.5–3.0% halothane held projector. Spots and bars of light were used to in air, and brain tissue corresponding to areas 17, 18 and determine receptive field location and to map the borders 19 [41] was removed from the left hemisphere by aspira- of the excitatory receptive-field center on the tangent tion. One eye was removed immediately following the screen. Once the receptive field was mapped, the non-brain lesion in neonatal-lesion animals, and immediately dominant eye (in non-enucleate animals) was covered and prior to recording for adult-lesion animals. All animals the tangent screen was replaced with a display monitor received a subcutaneous injection of antibiotic solution with a 208diameter circular aperture, centered on the cell’s every 48 h for 1 week following the lesion procedure. receptive-field center. Only cells with receptive-fields smaller than the 208aperture were included in quantitative
2.3. Recording tests. Bars and gratings were produced by an Innisfree
Image Generator controlled by the computer via a CED Cats were anesthetized with 4% halothane in air during 1708 Picasso controller.
the initial surgical preparation and maintained with 0.75– Directional selectivity was assessed for each cell to 1.0% halothane in air throughout the experiment. A allow proper configuration of stimuli in subsequent tests. paralytic solution (1.27 g gallamine triethiodide in 200 ml This was accomplished by moving a sine-wave grating 0.9% saline with 5% dextrose) was administered intraven- (78% peak-to-trough contrast; 0.30 cycles / degree (c / d) ously throughout the recording session at a rate that spatial frequency; 3.33 Hz temporal frequency) across the maintained paralysis and provided urinary output (typically receptive-field in random order ten times for each of 16 4–8 ml / h). The cat was placed in a stereotaxic device different directions (22.58 apart). Blank screens also were facing a white screen that was 26 cm away and tangent to included as a measure of background firing. The direction the nodal point of the eyes. The scalp and skull overlying of movement that produced the greatest response was PMLS cortex were opened to allow placement of the considered the preferred direction for the cell and was used recording electrode. The pupils were dilated and accom- in all subsequent tests.
modation was blocked pharmacologically. The corneas Once the preferred direction was determined, spatial-were protected with zero-power contact lenses that in- frequency tuning was assessed by drifting a series of cluded a 3 mm diameter artificial pupil. Retinal landmarks, sine-wave gratings (78% peak-to-trough contrast) of nine including the area centralis, were reflected and plotted on a different spatial frequencies (0.15–1.35 c / d in 0.15 c / d tangent screen using a fiber-optic light source. The eyes increments) in random order across the receptive field in were focused on the tangent screen by sequentially placing the preferred direction. Blank screens also were randomly spectacle lenses of increasing power in front of the eyes presented for a measure of background activity.
until the fundus projection on the tangent screen was sharp. Following completion of the spatial frequency test, sine-The position of retinal landmarks was checked periodically wave gratings of the preferred spatial frequency (the throughout the recording session. spatial frequency that elicited the greatest response from Extracellular single-cell recordings were made with the cell) were drifted in the preferred direction at each of tungsten-in-glass microelectrodes [21] in PMLS cortex seven levels of contrast (1.5, 3, 6, 12, 24, 48, and 78%). ipsilateral to the VC lesion. Despite some shifting of the Again, blank screens were randomly interleaved.
brain and distortion of the hemisphere as a result of the
long-term VC lesion, the middle suprasylvian cortex was 2.5. Data analysis easily recognized in all animals by its shape and position
with respect to the ectosylvian gyrus and the posterior For each test, only cells for which a complete set of data ectosylvian sulcus. Electrode penetrations were made was collected (i.e., ten complete trials for every stimulus) down the medial bank of the middle suprasylvian sulcus were included in the analyses. Unit discharges during (roughly parallel to the sulcus) within 1–5 mm of the individual stimulus or blank trials were collected in posterior bend of the sulcus, to correspond to the re- peristimulus time histograms (PSTHs) with a bin width of tinotopic location of the cortical lesion and of retinal 10 ms. Responses to grating stimuli were Fourier trans-degeneration following neonatal VC lesion. Neural activity formed, and the mean61 standard error (S.E.) of the was amplified and monitored with an audio monitor and a average neural discharge (F0) and the discharge at the storage oscilloscope. For quantitative analysis, action fundamental frequency of the drifting grating (F1) were potentials were led through a window discriminator to a determined for the ten trials presented in each test. The computer. Following recording, the electrode was ad- response to the initial cycle of the drifting grating was vanced at least 100 mm before attempting to record from excluded from the analyses to eliminate the effects of any
tested with grating stimuli responded with an increase in 2.7. Retinal ganglion-cell measurement both the F0 and F1 components; two cells in AL1E
animals and one cell in an NL animal displayed only an F0 Sampling in all animals was restricted to a portion of the response. Otherwise, F0 and F1 responses were highly hemiretina that undergoes transneuronal retrograde degen-correlated, as described in previous studies [10,11]. Be- eration in neonatal-lesion animals. Accordingly, 400 mm cause results were similar for the two components, only square sampling boxes were centered 2.4 mm dorsal to the those obtained for F0 responses are reported. horizontal meridian and 1.2 mm on either side of the For subsequent analyses of receptive-field size, spatial- vertical meridian in both hemiretinae, corresponding to frequency tuning, and contrast sensitivity, data were 2108 elevation and 58 azimuth. The visual-field repre-pooled across animals within each group. Justification for sentation of this region was removed from areas 17, 18 and pooling comes from two observations that strongly suggest 19 in all neonatal-lesion cats as evidenced by the pattern of that responses of individual PMLS cells were independent degeneration in the dLGN and MIN. Cell measurements of each other. First, these cellular response properties were were made using methods described previously [14]. independent of cortical position; measures of
receptive-field size, spatial frequency tuning, and contrast sensitivity
showed no correlation with electrode position. Second, no 3. Results
clustering of these response properties was observed.
Indeed, there were only a few instances where two 3.1. Lesion verification adjacent cells displayed similar results on any one of these
tests, and there were no cases where neighboring cells For all VC-lesion animals (NL, NL1E, and AL1E displayed similar results for all tests. In contrast, the angle groups), the appearance of the cortical surface and the of preferred line orientation or direction of movement was pattern of retrograde degeneration in the dLGN and MIN often observed to progress systematically during electrode indicated that area 17 was completely removed. In rare advancement through the cortex, as reported previously instances, a small representation of the far peripheral visual
[1,45]. field in area 18 was spared. The most common area of
Since all recordings were from the area in the left PMLS cortical sparing was in the representation of the upper corresponding to the VC lesion, every cell’s receptive field visual field (.58 elevation) and the far periphery (.258 was in the lower contralateral visual field. In the cat, inputs azimuth) of area 19 in the inferior portion of the posterior from the left and right eye each encompass a full repre- suprasylvian gyrus [42]. We attempted to place the record-sentation of this visual field. Thus, as far as retinal input is ing electrode so that we would sample neurons in PMLS considered, there should be no difference between animals that had receptive-fields within the retinotopic representa-with a right- or left-sided enucleation. To confirm this, we tion of damaged cortex for all three areas. However, three counter-balanced enucleations in each group, and found no PMLS cells had receptive-fields within the retinotopic overall differences between animals within the enucleated representation of spared cortex for at least one area. The
groups. data for these cells, all of which were from AL1E
animals, were excluded from further analyses. 2.6. Histological preparation and verification of VC
lesion and recording electrode path 3.2. Retinal ganglion-cell measurement
Following each recording session, the animal was Previously, we showed that a monocular enucleation euthanized with an intravenous injection of a lethal dose of accompanying a VC lesion at 1 week of age prevented sodium pentobarbital (.60 mg / kg). Immediately follow- RGC loss in the remaining eye [14]. To determine whether ing euthanasia, the animal was transcardially perfused with a monocular enucleation also alleviates the RGC loss 4% paraformaldehyde. The eyes were removed and the associated with a cortical lesion received on the day of retinae were whole-mounted and Nissl-stained [34]. The birth, we compared RGC distributions in the two brain was removed and immersion-fixed in 4% paraformal- hemiretinae of eyes from each group. Fig. 1 shows the dehyde for at least 1 h. Coronal sections at 40mm were results of RGC comparisons for one representative eye mounted and stained with cresyl violet. The pattern of from each group. Cell sizes are expressed as a percent of retrograde degeneration in the dLGN and medial interlami- the average of the 10 smallest cells to correct for overall nar nucleus (MIN) ipsilateral to the cortical lesion was size differences between nasal and temporal hemiretinae analyzed to determine the regions of visual-field repre- [14,35]. Each panel represents the difference between the sentation that were damaged in areas 17, 18 and 19 two hemiretinae in the proportion of cells at each size. An [29,30]. Surface analyses and examination of serial sec- upward deflection in the curve corresponds to a loss of tions aided this process. The path of the recording elec- RGCs in the hemiretinae projecting to the damaged trode through PMLS cortex was confirmed by examining hemisphere for animals with a VC lesion.
there was a severe transneuronal retrograde degeneration of medium-sized RGCs (about 180–350% of the size of
2
the smallest cells, or about 120–250 mm ) in the hemiretina projecting to the damaged hemisphere, (Fig. 1B). However, this loss was prevented by monocular enucleation in NL1E animals, and the cell size distribu-tion in the remaining eye resembled that for normal animals (Fig. 1C). These results extend the results of an earlier study [14], and suggest that retinal X-cells that normally degenerate following a neonatal VC lesion are spared in NL1E animals. In agreement with previous studies, a VC lesion in adulthood did not produce a loss of medium-sized RGCs (Fig. 1D).
3.3. Visual field sampling and receptive-field size
Receptive field centers from 350 neurons were hand-mapped on the tangent screen (Normal n5106; NL n578; NL1E n599; AL1E n567). So that our stimulus would activate the entire receptive-field center, we only included cells with a center smaller than the size of the display screen (i.e.,,208diameter) in our analyses. Fig. 2 shows a plot of PMLS cell receptive-field center size vs. eccentrici-ty for these cells. Although the sensitivieccentrici-ty of handmapping is not sufficient to yield information about the size or fine structure of the receptive fields, this plot illustrates that visual-field sampling was similar in all groups. In addition, no significant differences in receptive-field size among groups were observed (ANOVA F( 3, 346 )51.90; P.0.10). Thus, sparing medium-sized RGCs after a neonatal VC lesion did not increase the incidence of PMLS cells with very small, striate-like receptive-field centers.
Fig. 1. Results of RGC comparisons in a representative animal from each group. (A) Comparison in normal animals, showing very little difference between the nasal and temporal hemiretinae. (B) In NL animals, there was a substantial loss of medium-sized RGCs (180–300% of the average of the size of the 10 smallest RGCs). (C) By contrast, RGC comparison in NL1E animals revealed no significant lesion-induced loss of RGCs. (D) Comparison in AL1E animals indicated no significant loss of RGCs following a lesion in adulthood.
Fig. 2. Receptive-field center size by eccentricity for PMLS cells tested
and temporal hemiretinae in normal animals, a difference in each group. Note that only cells with receptive-field centers less than of up to 10% in the proportion of cells of any given size the size of the stimulus display (i.e.,,208diameter) were included in this
3.4. Spatial-frequency tuning
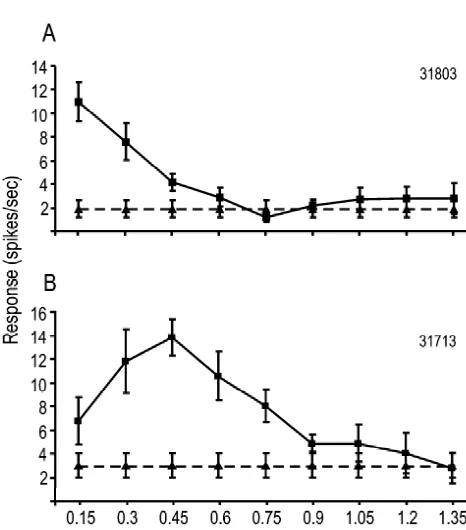
Two measures of spatial-frequency tuning were evalu-ated in this study. First, the spatial frequency to which each neuron responded with the greatest firing rate was considered to be the cell’s preferred spatial frequency. We hypothesized that if X-cells were spared in the remaining eye of NL1E animals, then a greater-than-normal propor-tion of PMLS cells in these animals would display a preference for higher spatial frequencies. Fig. 3 illustrates two typical spatial-frequency tuning curves observed in the present study. The distribution of cells in each group (Fig. 4A) appeared to indicate a shift in preference toward higher spatial frequencies in the NL1E group. To visual-ize this shift more clearly, the proportion of cells in each group that exhibited a preferred response to the lowest third (0.15–0.45 c / d) middle third (0.6–0.9 c / d) or highest third (1.05–1.35 c / d) of the tested spatial fre-quencies is shown in Fig. 4B. Approximately 30% of all cells in the NL1E group exhibited a preference for the highest spatial frequencies tested ($1.05c / d), which is
2
significantly greater than in other groups (x 543.92, P, 0.001).
As a second test of spatial frequency tuning, we
Fig. 4. Spatial frequency responses in each group. (A) Distribution of cells in each group with the indicated preferred spatial frequency. (B) Summary graph depicting the percentage of cells in each group exhibiting a preferred response for the spatial frequencies indicated. *P,0.001 with
2
x.
determined each cell’s spatial resolution (the highest spatial frequency to which the neuron responded with a firing rate significantly higher than baseline). As before, a comparison of the distribution of cells in each group (Fig. 5A) suggested a shift in resolution in the NL1E group toward higher spatial frequency stimuli. The proportion of
Fig. 3. Examples of spatial frequency response curves recorded from
PMLS cells. (A) A cell from a normal animal, illustrating a typical cells in each group that exhibited low (0.15–0.45 c / d),
‘low-pass’ spatial frequency-dependent response, with a preferred spatial medium (0.6–0.9 c / d), or high ($1.05 c / d) spatial frequency of 0.15 c / d and a spatial resolution of 0.45 c / d. (B) A cell resolution is shown in Fig. 5B. Nearly 40% of cells in the from a NL1E animal, showing a typical ‘band-pass’ response. This cell
NL1E group responded to the highest frequencies tested
had a preferred spatial frequency of 0.45 c / d and a spatial resolution of
($1.05 c / d) which is significantly greater than in any of
0.75 c / d. Symbols denote mean response (6S.E.) for 10 trials. j5
2
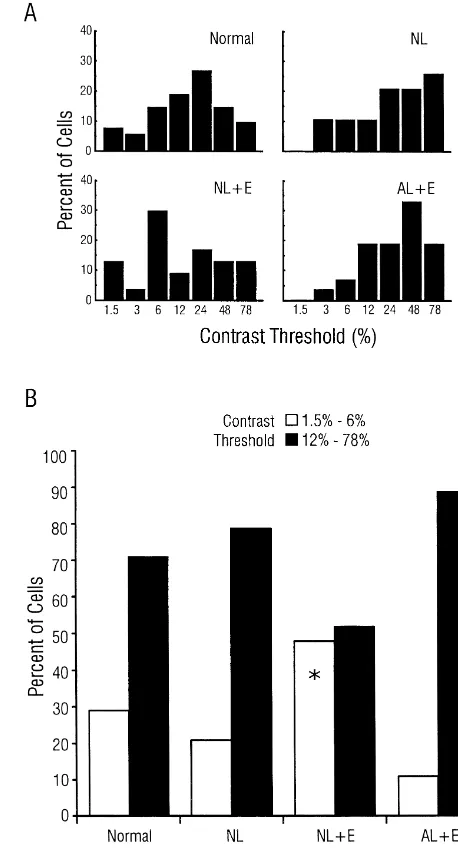
Fig. 6. Contrast sensitivity in each group. (A) Distribution of cells in each group with a significant response at the indicated contrast. (B) Summary Fig. 5. Spatial resolution by group. (A) Distribution of cells in each group
graph illustrating the percentage of cells in each group displaying low or with the indicated spatial resolution. (B) Summary graph illustrating the
2
high contrast threshold. *P,0.001 withx. percentage of cells in each group exibiting the spatial resolutions
2
indicated. *P,0.001 withx .
4. Discussion
3.5. Contrast sensitivity
4.1. Retinal ganglion-cell survival Contrast threshold, defined as the lowest contrast that
elicited a response significantly greater than background In a previous study we demonstrated that a VC lesion at activity, was determined for each cell. The distribution of 1 week of age leads to transneuronal RGC loss (observed 4 cells in each group (Fig. 6A) indicated that threshold weeks later), and that this RGC loss was ameliorated by a values tended to be lower in the NL1E group, and Fig. 6B monocular enucleation performed at the same time as the shows the proportion of cells in each group that responded VC lesion [14]. The results of the present study extend to low (1.5–6% peak-to-trough contrast) or high (10–78% these findings by demonstrating that removal of one eye contrast) contrast visual stimuli. A significantly higher immediately after VC lesion attenuated the associated RGC percentage of cells in NL1E animals responded to stimuli degeneration in the remaining eye as early as the day of with low contrast compared to normal, NL, and AL1E birth. We further extend the results of our previous study
2
after the VC lesion. A full discussion of issues surrounding Indeed, recent work has demonstrated that dendritic fields this observation (effects on RGC size, etc.) has been are approximately 80% larger in the b-class RGCs that
published previously [14]. remain following a neonatal VC lesion [46]. However, this
We have hypothesized that during development after an increase is observed in retinae that have a dramatic early VC lesion, inputs from the two eyes compete for reduction in RGC numbers. Whether such dendritic field survival factors in the dLGN. Removing half of these enlargement occurs in X- /b-cells following a neonatal VC projections via monocular enucleation may increase the lesion with monocular enucleation (i.e., in a retina without amount of target-derived trophic support available to an overall RGC loss) remains to be tested.
projections from the remaining eye [14]. This hypothesis
awaits direct experimental test. Likewise, the question of 4.3. Results for adult-lesion animals with acute where these spared cells project remains to be studied. We monocular enucleation
hypothesize that the spared X- /b-cells make connections
with remaining cells in the degenerated dLGN that have To verify that the results observed in NL1E animals been shown to display an enhanced projection to PMLS (i.e., high spatial frequency tuning, low contrast threshold)
cortex [15]. were a result of sparing RGCs during the critical period for
plasticity in PMLS cortex rather than a feature of PMLS
4.2. Receptive-field size cortex lacking input from VC and one eye, we included a
group of animals that received a VC lesion in adulthood Sparing X- /b-cells in the retina might be expected to and a monocular enucleation immediately prior to the increase the percentage of cortical cells with very small recording session. These animals resemble NL1E animals receptive field centers. However, we found that the re- in two ways. First, both groups of animals were tested ceptive-field center size of PMLS neurons did not change following a VC lesion with long-term survival and an dramatically following a neonatal VC lesion or following a enucleation. Second, the retinal X- /b-cells are intact in neonatal or adult lesion with monocular enucleation. There both groups of animals: in NL1E animals by virtue of the are a number of possible reasons why this effect was not enucleation following the VC lesion, and in AL1E animals observed in PMLS cells of NL1E animals. One possibility because RGC loss does not occur following an adult VC is that hand-mapping is not a sufficiently sensitive measure lesion. The timing of the enucleation differed between the of receptive-field size, so small differences may have been two groups; the NL1E group had a long-term monocular missed with our analysis. Even so, it is clear that PMLS enucleation, while the AL1E group had an acute enuclea-receptive-field sizes in NL1E animals were not substan- tion. This difference arose because we added the adult-tially smaller than normal PMLS and did not resemble lesion group later in our experiments to rule out effects of those found in normal striate cortex. binocular interactions on the results we observed in NL1E
A second possibility is that large receptive-fields are an animals.
intrinsic property of PMLS cells. The mechanism that Our finding that PMLS cortex in AL1E animals did not would allow such intrinsic properties may be related to the display an increase in high spatial tuning and contrast number and type of inputs a single cortical cell receives. sensitivity characteristics suggests that the appearance of For instance, if inputs onto a single PMLS cell converge these properties in NL1E animals was not due to the from a relatively large retinal area, then a large receptive- combined effect of having removed inputs from VC and field would be expected to follow even if the individual one eye. Instead, these results suggest that sparing retinal inputs have small receptive-fields (e.g., from X-cells). If X- /b-cells during a period of cortical response plasticity is this is the case, our finding that there were no differences important for the changes observed in PMLS cortex. among groups suggests that such organization does not Further, the results with spatial frequency and contrast change following a neonatal VC lesion despite the en- measures for AL1E animals were like those previously hanced geniculo-PMLS projection. observed in PMLS cortex of animals with an adult lesion A third possibility is that the influence of Y- and [11,29], suggesting that these properties of PMLS cortex W-pathway receptive fields that continue to project to following an adult lesion are not sensitive to acute PMLS cortex overshadows any new X-pathway influence. monocular enucleation.
For instance, a single PMLS cell may have a number of
excitatory inputs with many receptive field sizes (i.e., X-, 4.4. Spatial-frequency tuning and contrast sensitivity in Y-, and W-pathway input). If some of these inputs respond NL1E animals
to a large portion of the visual field, then the PMLS cell
than PMLS cells in normal animals. Further, more PMLS References
cells in NL1E animals had high contrast sensitivity than in
normal animals (Fig. 6). [1] C. Blakemore, T.J. Zumbroich, Stimulus selectivity and functional organization in the lateral suprasylvian visual cortex of the cat, J.
Taken together, these results suggest that sparing
X-Physiol. (Lond.) 389 (1987) 569–603.
class RGCs after a neonatal VC lesion in cats leads to
¨
[2] B.B. Boycott, H. Wassle, The morphological types of ganglion cells
incorporation of some X-pathway response properties by
of the domestic cat’s retina, J. Physiol. (Lond.) 240 (1974) 397–
some PMLS cortical cells (i.e., tuning for high spatial 419.
frequencies, high spatial resolution, high contrast sensitivi- [3] J. Bullier, G.H. Henry, Neural path taken by afferent streams in
ty). This incorporation may occur via a novel X-pathway striate cortex of the cat, J. Neurophysiol. 42 (1979) 1264–1270. [4] E.C. Callahan, L. Tong, P.D. Spear, Critical period for the marked
projection through the enhanced retino-geniculo-PMLS
loss of retinal X-cells following visual cortex damage in cats, Brain
pathway that has been observed following a neonatal VC
Res. 323 (1984) 302–306.
lesion [15,16,27]. [5] B.G. Cleland, W.R. Levick, Brisk and sluggish concentrically
A possible limitation of the present study is that all cells, organized ganglion cells in the cat’s retina, J. Physiol. (Lond.) 240 regardless of receptive field center size, were tested with (1974) 421–456.
[6] B.G. Cleland, W.R. Levick, Properties of rarely encountered types of
stimuli that covered the entire 208monitor aperture. Thus,
ganglion cells in the cat’s retina and an overall classification, J.
the stimuli fell on varying fractions of the surround portion
Physiol. (Lond.) 240 (1974) 457–492.
of the receptive fields. Receptive-field properties of cells in
[7] P. Cornwell, W. Overman, C. Ross, Extent of recovery from neonatal
primary visual cortex are often tested with full-field stimuli damage to the cortical visual system in cats, J. Comp. Phys. Psych. that include surround activation, but it has been demon- 92 (1978) 255–270.
strated previously that activity of PMLS cells can be [8] B. Dreher, A.G. Leventhal, P.T. Hale, Geniculate input to cat visual cortex: a comparison of area 19 with areas 17 and 18, J.
Neuro-modulated by stimuli outside of the receptive field center.
physiol. 44 (1980) 804–826.
The effect of stimulating the surround with a stimulus that
[9] Y. Fukuda, J. Stone, Retinal distribution and central projections of
is in phase with the stimulus presented to the receptive
Y-, X-, and W-cells of the cat’s retina, J. Neurophysiol. 37 (1974)
field center is to suppress the response [43,44]. Such 749–772.
suppression may have occurred for cells in our study as a [10] W. Guido, P.D. Spear, L. Tong, How complete is physiological
result of the large visual stimuli used, though the degree to compensation in extrastriate cortex after visual cortex damage in kittens?, Exp. Brain Res. 91 (1992) 455–466.
which such suppression may have been influenced by other
[11] W. Guido, L. Tong, P.D. Spear, Afferent bases of spatial- and
manipulations (e.g., monocular enucleation) cannot be
temporal-frequency processing by neurons in the cat’s posteromedial
determined. Even with this limitation, however, we found lateral supraylvian cortex: Effects of removing areas 17, 18 and 19, significant changes in the receptive field properties of J. Neurophysiol. 64 (1990) 1636–1651.
PMLS cells following a neonatal VC lesion and monocular [12] K.-P. Hoffmann, J. Stone, Conduction velocity of afferents to cat visual cortex: A correlation with cortical receptive field properties,
enucleation that suggest a novel X-pathway influence.
Brain Res. 32 (1971) 460–466.
Further experiments are necessary to examine the details of
[13] K.-P. Hoffmann, J. Stone, S.M. Sherman, Relay of receptive-field
the receptive-field structure in these cells. properties in the dorsal lateral geniculate nucleus of the cat, J. Does sparing retinal X-cells after VC lesion allow PMLS Neurophysiol. 36 (1972) 409–424.
cells to function like the damaged striate cortex? Although [14] K.R. Illig, V.R. King, P.D. Spear, Monocular enucleation prevents retinal ganglion cell loss following neonatal visual cortex damage in
the visual responses of many PMLS cells in NL1E
cats, Visual Neurosci. 15 (1998) 1097–1105.
animals clearly displayed a shift toward higher spatial
[15] R.E. Kalil, Removal of visual cortex in the cat: Effects on the
frequency and / or lower contrast visual stimuli, most cells morphological development of the retino-geniculo-cortical pathway, displayed properties of normal PMLS cells; they had in: J. Stone, B. Dreher, D.H. Rapaport (Eds.), Development of
relatively large receptive fields, low contrast sensitivity, Visual Pathways in Mammals, A.R. Liss, New York, 1984, pp. 257–274.
and low spatial resolution. Further, the values for cells
[16] R.E. Kalil, L. Tong, P.D. Spear, Thalamic projections to the lateral
with high spatial frequency tuning and high contrast
suprasylvian area in cats with neonatal or adult visual cortex
sensitivity were within the range of normal PMLS cells, damage, J. Comp. Neurol. 314 (1991) 512–525.
although cells with these properties are encountered only [17] H. Kolb, R. Nelson, A. Mariani, Amacrine cells, bipolar cells and
rarely in normal PMLS cortex. Moreover, even those cells ganglion cells of the cat retina: A golgi study, Vision Res. 21 (1981) 1081–1114.
that displayed X-like properties did not display all the
[18] P. Lennie, Parallel visual pathways: A review, Vision Res. 20 (1980)
characteristics of striate cells. For example, they lacked the
561–594. 2
characteristically small (,3 deg ) receptive fields, and [19] A.G. Leventhal, Morphology and distribution of retinal ganglion they displayed either high spatial frequency tuning or high cells projecting to different layers of the dorsal lateral geniculate
contrast sensitivity, but not both. Thus, the putative X- nucleus in normal and siamese cats, J. Neurosci. 2 (1982) 1024– 1042.
projection along the enhanced retino-geniculo-PMLS
path-[20] A.G. Leventhal, J. Keens, I. Tork, The afferent ganglion cells and
way does not appear to turn PMLS into striate cortex;
cortical projections of the retinal recipient zone (RRZ) of the cat’s
rather, it seems that some PMLS cells incorporate X-like pulvinar complex, J. Comp. Neurol. 194 (1980) 535–554. response properties in addition to their normal response [21] E.G. Merrill, A. Ainsworth, Glass-coated platinum-plated tungsten
[22] J.A. Movshon, The velocity tuning of single units in cat striate morphology in cat retinal ganglion cells: Evidence of further cortex, J. Physiol. (Lond.) 249 (1975) 445–468. variation in the gamma-cell class, J. Comp. Neurol. 192 (1980) [23] J.A. Movshon, I.D. Thompson, D.J. Tolhurst, Spatial and temporal 211–217.
contrast sensitivity of neurones in areas 17 and 18 of the cat’s visual [36] J. Stone, B. Dreher, Projection of X- and Y-cells of the cat’s lateral cortex, J. Physiol. (Lond.) 283 (1978) 101–120. geniculate nucleus to areas 17 and 18 of visual cortex, J. Neuro-[24] E.H. Murphy, R.E. Kalil, Functional organization of lateral genicu- physiol. 36 (1973) 551–567.
late cells following removal of visual cortex in the newborn kitten, [37] J. Stone, B. Dreher, A.G. Leventhal, Hierarchical and parallel Science 206 (1979) 712–716. mechanisms in the organization of visual cortex, Brain Res. Rev. 1 [25] H.E. Pearson, D.R. Labar, B.R. Payne, P. Cornwell, N. Aggarwal, (1979) 345–394.
Transneuronal retrograde degeneration in the cat retina following [38] J. Stone, Y. Fukuda, Properties of cat retinal ganglion cells: A neonatal ablation of visual cortex, Brain Res. 212 (1981) 470–475. comparison of W-cells with X- and Y-cells, J. Neurophysiol. 37 [26] W. Singer, F. Tretter, M. Cynader, Organization of cat striate cortex: (1974) 722–748.
A correlation of receptive field properties with afferent and efferent [39] L. Tong, R.E. Kalil, P.D. Spear, Critical periods for functional and connections, J. Neurophysiol. 38 (1975) 1080–1098. anatomical compensation in lateral suprasylvian visual area follow-[27] P.D. Spear, Neural mechanisms of compensation following neonatal ing removal of visual cortex in cats, J. Neurophysiol. 52 (1984)
cortex damage, in: W.M. Cowan (Ed.), Synaptic Plasticity and 941–960.
Remodeling, Guilliford Press, New York, 1985, pp. 111–167. [40] L. Tong, P.D. Spear, R.E. Kalil, E.C. Callahan, Loss of retinal [28] P.D. Spear, T.P. Baumann, Receptive-field characteristics of single X-cells in cats with neonatal or adult visual cortex damage, Science
neurons in lateral suprasylvian visual area of the cat, J. Neuro- 217 (1982) 72–75.
physiol. 38 (1975) 1403–1420. [41] R.J. Tusa, L.A. Palmer, A.C. Rosenquist, The retinotopic organiza-[29] P.D. Spear, T.P. Baumann, Effects of visual cortex removal on tion of area 17 (striate cortex) in the cat, J. Comp. Neurol. 177
receptive-field properties of neurons in lateral suprasylvian visual (1978) 213–236.
area of the cat, J. Neurophysiol. 42 (1979) 31–56. [42] R.J. Tusa, A.C. Rosenquist, L.A. Palmer, Retinotopic organization [30] P.D. Spear, T.P. Baumann, Neurophysiological mechanisms of of areas 18 and 19 in the cat, J. Comp. Neurol. 185 (1979) 657–678.
¨
recovery from visual cortex damage in cats: Properties of lateral [43] M.W. von Grunau, B.J. Frost, Double-opponent process mechanism suprasylvian visual area neurons following behavioral recovery, underlying RF-structure of directionally specific cells of cat lateral Exp. Brain Res. 35 (1980) 177–192. suprasylvian visual area, Exp. Brain Res. 49 (1983) 84–92.
¨
[31] P.D. Spear, R.E. Kalil, L. Tong, Functional compensation in lateral [44] M.W. von Grunau, T.J. Zumbroich, C. Poulin, Visual receptive field suprasylvian visual area following neonatal visual cortex removal in properties in the posterior suprasylvian cortex of the cat: A cats, J. Neurophysiol. 43 (1980) 851–869. comparison between the areas PMLS and PLLS, Vision Res. 27 [32] L.R. Stanford, X-cells in the cat retina: Relationships between the (1987) 343–356.
morphology and physiology of a class of cat retinal ganglion cells, J. [45] Y. Wang, L. Wang, B. Li, Y.C. Diao, How is direction selectivity Neurophysiol. 58 (1987) 940–964. organized in the extrastriate visual area PMLS of the cat?, Neuro-[33] L.R. Stanford, S.M. Sherman, Structure / function relationships of Report 6 (1995) 1969–1974.
retinal ganglion cells in the cat, Brain Res. 297 (1984) 381–386. [46] A.J. Weber, R.E. Kalil, L.R. Stanford, Dendritic field development [34] J. Stone, The Wholemount Handbook: A Guide To the Preparation of retinal ganglion cells in the cat following neonatal damage to and Analysis of Retinal Wholemounts, Maitland Publications, visual cortex: Evidence for cell class specific interactions, J. Comp.
Sydney, 1981. Neurol. 390 (1998) 470–480.