Cornelius Gross, Luca Santarelli, Dani Brunner, Xiaoxi Zhuang, and Rene´ Hen
The study of genetically altered mice has been used successfully to determine the influence of different neuro-transmitter receptors on fear and anxiety. Mice with a genetic deletion of the serotonin 1A receptor (5-HT1AR
knockout [KO]) have been shown to be more fearful in a number of behavioral conflict tests, confirming the impor-tant role of this receptor in modulating anxiety. Factor analysis of the behavior of WT and 5-HT1AR KO mice in
the open field test shows that locomotion and anxiety measures segregate independently, supporting the idea that the anxious behavior of the KO mice is not the result of altered locomotion. KO mice also show increased anxiety in the novelty-suppressed feeding task, which differs from the other conflict tests in the motivational drive of the animals. In response to a discrete aversive stimulus, foot shock, the KO mice show increased freezing and increased tachycardia. However, activation of the hypothalamic–pituitary–adrenal axis in response to stress appears to be slightly blunted in the KO animals. To-gether, these data support the idea that the 5-HT1AR
modulates an important fear circuit in the brain. The dual function of the 5-HT1AR as both a presynaptic
autorecep-tor, negatively regulating serotonin activity, and a postsynaptic heteroreceptor, inhibiting the activity of non-serotonergic neurons in forebrain structures, has compli-cated interpretation of the anxious phenotype of these KO mice. A more complete understanding of the function of the 5-HT1AR awaits further study of its role in behaving
animals using tissue-specific antagonists and novel trans-genic mice with tissue-specific expression of the receptor.
Biol Psychiatry 2000;48:1157–1163 © 2000 Society of
Biological Psychiatry
Key Words: 5-HT1A, anxiety, depression, knockout mice, stress response, tachycardia
Introduction
I
n 1998, three laboratories independently published the characterization of a knockout of the serotonin 1A receptor (5-HT1AR KO) in mice (Heisler et al 1998; Parkset al 1998; Ramboz et al 1998; for reviews, see Julius 1998; Lesch and Moessner 1999; unless otherwise indi-cated, data presented in this review were obtained using the KO made in our laboratory). The 5-HT1AR, one of at least 14 serotonin receptors, is found in two distinct populations in the brain. The first is expressed on seroto-nergic neurons in the raphe nuclei of the brainstem where it hyperpolarizes the membrane potential via Gi proteins and serves to negatively regulate serotonergic cell firing. The second population of 5-HT1AR is found on nonsero-tonergic neurons in forebrain structures, primarily the hippocampus, septum, and cortex. In these tissues the receptor also appears to be coupled to Giand hyperpolar-izes the membrane potential. The 5-HT1AR has been well characterized pharmacologically thanks to the availability of selective and potent agonists and antagonists. Both agonists and antagonists have been used to modulate anxietylike behaviors in animals (for a review, see Menard and Treit 1999), and more importantly, although it is not considered first-line treatment, the 5-HT1AR partial ago-nist, buspirone, has been used successfully to treat anxiety in humans (Davidson et al 1999). Given the strong evidence for the modulation of emotional states by this receptor, it was not unexpected that increased anxietylike behaviors were observed in all three strains of mutant mice.
Behavior in Conflict Tests
In each of the three laboratories, 5-HT1AR KO mice displayed increased avoidance of aversive stimuli in be-havioral conflict tests, suggesting that these animals have an enhanced fear response (Table 1). These mice spent less time in the aversive center of an open field and explored the exposed areas of an elevated maze less frequently. Using factor analysis, the mice’s behavior in an open field can be segregated into two distinct types of activities. The first factor reflects general locomotor and exploratory activity, including path length, rearing, and nose pokes, whereas the second factor reflects an anxiety component revealed in the fraction of total path length in the center (Table 2). The similar segregation of factors in WT and 5-HT1AR KO animals argues that in both genotypes the anxiety measures used in this test vary independently of From the Center for Neurobiology and Behavior, Columbia University, New York,
New York.
Address reprint requests to Rene´ Hen, Ph.D., Columbia University, Center for Neurobiology & Behavior, 722 West 168th Street, PI Annex 731, New York NY 10032.
Received April 28, 2000; revised August 11, 2000; accepted August 11, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
locomotor activity and are likely to be a true reflection of the animals’ fear of the aversive environment.
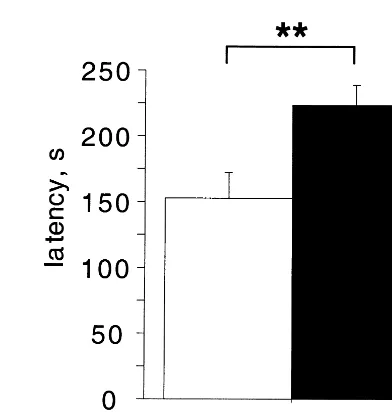
Further evidence that the KO mice are indeed more fearful of the exposed areas, and not simply less motivated to explore, is provided by data from the novelty-sup-pressed feeding task. This anxiety test is similar to the open field test except that the animal’s motivation to move to the center of the open field is enhanced by placing a food pellet in the center of the open field and depriving the animals of food 24 hours before testing. The latency to begin eating in this test is reduced by anxiolytic drugs (Bodnoff et al 1989). The KO mice take longer to begin eating, despite the fact that they eat equal amounts of food when placed back into the familiar surroundings of their home cage (Figure 1). An increase in latency to begin eating could result either from increased avoidance of the center of the open field or from an inability to suppress a
fearful behavioral response that is incompatible with feeding. Together, the data from these conflict tests argue that an increased fear of aversive environments lies behind the altered behavior of the 5-HT1AR KO mice.
Response to Inescapable Aversive Stimuli
A very different sort of behavior is seen when the 5-HT1AR KO mice are faced with an inescapable stressful situation. In the forced swim and tail suspension tests the KO mice make increased attempts to escape, a behavior seen after treatment with certain antidepressants and in-terpreted as reflecting decreased despair (Porsolt et al 1977). However, although these tests have good predictive validity, they lack construct validity, and thus are primar-ily useful in evaluating the antidepressant effects of novel drugs. Therefore, the increased activity of the KO mice in these tests cannot reliably be attributed to decreased despair.
Freezing in response to aversive stimuli is a well characterized and ethologically validated measure of fear in rodents (Bolles 1970). When exposed to a mild electric foot shock, rodents show increases in locomotion, heart rate, and respiration. In the minutes following these unconditioned responses, the animals begin to develop freezing behavior. Such freezing in response to foot shock is thought to reflect the formation of fear toward the
Figure 1. Serotonin 1A receptor knockout mice show increased latency to feed in the novelty-suppressed feeding task. Mice were deprived of food for 24 hours and then placed individually into an open field (40350 cm) with bedding, and the latency to begin eating a food pellet placed in the center of the field was recorded (adapted from Bodnoff et al 1989; N528 –32, **p,
.01 by t test). When the mice were transferred back to their home cage and their food consumption was monitored for 5 min, no differences between KO and WT were observed.
Table 1. Behavioral Phenotype of Serotonin 1A Receptor Knockout Mice
Behavior Test
Strain and reference
Increased fear/ anxiety
Open field SWa, 129b, C57c Elevated plus maze 129bSWd Elevated zero maze C57c Novel object exploration C57c Novelty-suppressed feeding 129e Freezing after foot shock 129e Increased mobility
during inescable stress
Forced swim test Tail suspension test
SWa, 129b C57c
Increased autonomic response
Heart rate after foot shock 129e
Strains: SW, 129/sv backcrossed to Swiss–Webster; 129, pure 129/sv; C57, 129/sv backcrossed to C57BL/6.
aParks et al (1998). bRamboz et al (1998). cHeisler et al (1998). dSibille et al (2000). eThis study.
Table 2. Factor Analysis Matrix for Wild-Type and Serotonin 1A Receptor Knockout Behavior in the Open Field Test
Behavioral measure Factor 1 Factor 2
Wild type
Path length .859 —
Rearing .720 .467
Nose poke .888 —
Center path/total path — .944 1A KO
Path length .903 —
Rearing .875 —
Nose poke .410 .546 Center path/total path — .915
testing chamber in which the shock has been delivered (Fanselow 1980). When tested in such a paradigm, KO mice show enhanced freezing (Figure 2). These data support the idea that these mice are either more fearful when faced with a stressor or possibly show enhanced learning of fearful stimuli.
Autonomic Response to Stress
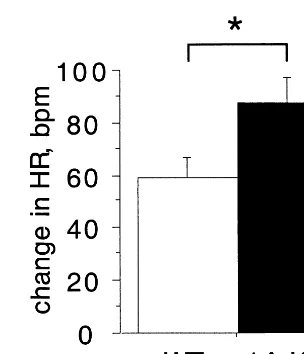
The fear circuitry of the brain has numerous outputs (for a review, see Charney and Deutsch 1996). One of these is the stimulation of autonomic responses, including heart rate and blood pressure. When heart rate is monitored during foot shock, KO animals show a greater heart rate increase than WT, suggesting that the circuits controlling autonomic response are hyperactive in these animals (Figure 3). Serotonin is known to increase sympathetic system function and systemic administration of the 5-HT1AR agonist, 8-OH-DPAT, causes decreased sympa-thetic nerve activity and bradycardia, presumably by reducing serotonergic firing via 5-HT1A autoreceptors (McCall et al 1987). The increased tachycardia seen in the 5-HT1AR KO mice is possibly a reflection of decreased serotonergic autoregulation in these animals.
HPA Axis Response to Stress
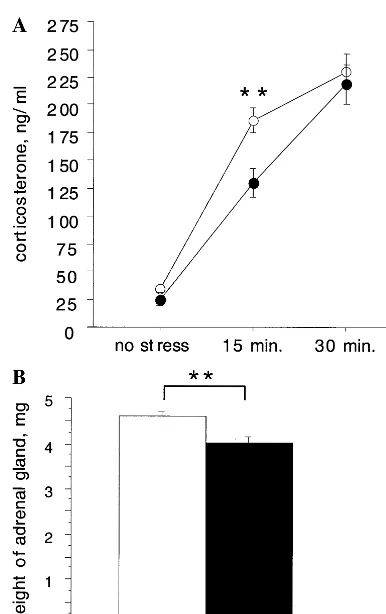
Another well-characterized output of the fear circuitry is the hypothalamic–pituitary–adrenal (HPA) axis. It is well known that the HPA axis becomes activated during stress-ful experience. The secretion of adrenocorticotropic hor-mone (ACTH) into the bloodstream by the pituitary and the subsequent release of corticosterone (cortisol in hu-mans) by the adrenal gland is known to help animals adapt to stress. Increased baseline cortisol and a decreased
ability to suppress cortisol when administered dexameth-asone has been observed in some depressed patients, whereas decreased baseline cortisol and enhanced nega-tive feedback have been documented in posttraumatic stress disorder, suggesting that HPA axis disregulation may be a feature of some affective disorders (Plotsky et al 1998; Yehuda et al 1996). The 5-HT1AR KO mice show a reduced HPA axis response. Although baseline corticoste-rone appears normal, the corticostecorticoste-rone response to be-havioral stress appears to be slightly blunted (Sibille et al 1998; similar results seen in our mice, Figure 4A). This reduced corticosterone response does not seem to be a developmental adaptation as it is already present in 12-day-old pups (data not shown). Furthermore, adrenal gland weight is reduced in the KO animals, consistent with their decreased corticosterone after stress and possibly reflect-ing long-term HPA axis hypoactivity (Figure 4B). Further endocrinologic studies will be necessary to locate the origin of the blunted HPA axis response in the KO animals, but the relatively minor changes seen do not suggest a strong link between anxiety and HPA axis activity. This conclusion is supported by data from female KO mice, which show increased anxiety like male mice, but show no changes in corticosterone or ACTH after stress and have normal adrenal weight (Ramboz et al 1998; data not shown).
Figure 2. Serotonin 1A receptor knockout mice freeze more in response to foot shock. Mice were placed into a novel environ-ment and allowed to habituate for 6 min, at which point a 0.5-mA, 2-sec shock was delivered via the grid floor. Freezing behavior was scored as complete immobility except for breathing [N 5 10 –12; genotype effect F(1,21) 5 10.71, p 5 .0036; genotype3time interaction F(17,357)52.086, p5.0072; post
Developmental Compensations in KO Mice
An important issue when studying KO animals is the existence of possible compensatory changes that have taken place in the animal over its lifetime in response to its altered genetic makeup. Compensations can both compli-cate the interpretation of changes seen in KO mice and at the same time reveal important links between different circuits in the brain. An increase in 5-HT1B autoreceptor function was the first compensation documented in the 5-HT1AR KO mice (Ramboz et al 1998). This receptor is found on serotonergic axon terminals, where it negatively regulates serotonin release. Increased activity of the 5-HT1B receptor may compensate for missing 5-HT1A autoreceptor in the KO mice.
After the observation of a reduced behavioral response to benzodiazepines in the 5-HT1AR KO mice, Sibille and colleagues have recently documented significant decreases ing-aminobutyric acid (GABA) binding in the
hippocam-pus and benzodiazepine binding in the central and baso-lateral nuclei of the amygdala (Sibille et al 2000). These observations are accompanied by a decrease in paired pulse inhibition in the hippocampus. Because the admin-istration of benzodiazepines directly into the hippocampus is anxiolytic in rodents (Menard and Treit 1999), such disinhibition could contribute to increased anxiety in these KO animals and suggests an as yet unknown intimate connection between the serotonin and GABA systems. At this point it is still not known whether such GABAA receptor deficits exist in the other KO strains. However, at least in the KO mice on the 129/sv background, no change in behavioral response to benzodiazepines as reported by Sibille and colleagues has been seen, suggesting that at least part of these compensations are strain specific (Pattij et al, in press; data not shown).
Taken together, these data suggest that the 5-HT1AR is a component of a critical fear circuit that mediates the normal motor and autonomic responses to stress. When the animal is confronted by an aversive stimulus this circuit is engaged and serves to promote behaviors that protect the animal from danger. Changes induced by removal of the 5-HT1AR from the fear circuit cause increased activity of this system and a resulting increased fear of aversive stimuli. Numerous structures have been suggested to form such a fear circuit in the brain, including the hippocampus, septum, prelimbic cortex, bed nucleus of the stria termi-nalis, periaqueductal gray, and, most prominently, the basolateral, lateral, and central nuclei of the amygdala (for reviews, see Davis and Shi 1999; LeDoux 1998). In the case of cued fear conditioning, as measured by both fear-potentiated startle and freezing, the precise circuits are well established and include the medial geniculate nucleus of the thalamus, which relays auditory information to the lateral nucleus of the amygdala where it is passed on to the basolateral and central nuclei and on to various output circuits. However, the 5-HT1AR is not expressed in the medial geniculate nucleus nor in the basolateral nuclei of the amygdala, and only at low levels in the central nucleus (data not shown). Systemic administration of 5-HT1A agonists does not appear to alter cued fear conditioning, at least when measured using startle (Davis et al 1988). In line with this data, cued fear conditioning as measured by freezing is normal in the KO animals (data not shown). In contrast, the circuits underlying conflict behaviors, such as the open field and elevated plus maze in which the KO mice show increased anxiety, are poorly defined. These behaviors are known to be affected by administration of anxiolytic compounds such as benzodi-azepines into many structures, including the amygdala, septum, hippocampus, hypothalamus, periaqueductal gray, and raphe nuclei of the brainstem (Menard and Treit 1999). Because the 5-HT1AR is expressed in many of these
Figure 4. Decreased hypothalamic–pituitary–adrenal axis activ-ity in serotonin 1A receptor knockout mice. (A) Serum cortico-sterone levels after open field exposure. Mice were either killed or placed into an open field and removed after 15 or 30 min for killing and collection of trunk blood (N520 –22; **p,.01 by
forebrain structures, as well as in serotonergic neurons of the raphe nuclei, it has so far been difficult to pinpoint which receptor population contributes to anxiety in the KO animals.
Presynaptic versus Postsynaptic Function of
the 5-HT
1AR
Strong expression of the 5-HT1AR is seen in the dorsal raphe and caudal linear nuclei and lower levels in the median raphe nucleus of the brainstem, where as an autoreceptor it inhibits the firing of serotonergic cells. Removal of the 5-HT1AR from serotonergic neurons should block the negative feedback of this system and lead to increased firing of these cells and increased release of serotonin. Indeed, a twofold increase in basal firing of serotonergic neurons in the dorsal raphe is observed in KO animals (Richer and Blier 2000). Surprisingly, however, basal extracellular levels of serotonin as measured by microdialysis are normal (He et al, in press). This may be due to the compensatory increase in function of the 5-HT1B autoreceptor located at serotonergic axon termi-nals, as discussed earlier (Ramboz et al 1998). However, under the stressful conditions of behavioral testing, levels of serotonin are known to change in certain structures (Hashimoto et al 1999; Kirby et al 1997; Vahabzadeh and Fillenz 1994), and under such conditions differences in extracellular serotonin may be revealed in the KO mice. Although microdialysis experiments have not yet been conducted in the KO animals during behavioral testing, dramatically increased extracellular serotonin levels have been seen in the KO animals in response to the serotonin reuptake inhibitor fluoxetine (He et al, in press). Thus, the 5-HT1AR KO mice may show increased serotonin levels under stressful circumstances such as behavioral testing. However, it is not yet clear what the effect of such increased serotonin activity would be on anxiety behavior, given the heterogeneous function of postsynaptic seroto-nin receptors.
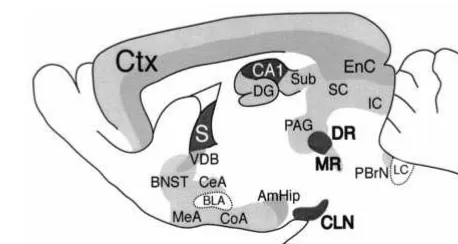
The most prominent expression of the 5-HT1AR in the forebrain is in the CA1 region of the hippocampus and the medial septum (Figure 5). Intermediate levels are found in the deep layers of the cortex, the ventral diagonal band, and the amygdala– hippocampus area. Lower levels of 5-HT1AR expression are found in the subiculum, dentate gyrus, CA2, and CA3 regions of the hippocampus; the more superficial layers of the cortex; the bed nucleus of the stria terminalis; the periaqueductal gray; the inferior and superior colliculi; and the parabrachial nucleus, and throughout the amygdala, with the notable exception of the lateral and basolateral nuclei. The effect of removing the receptor from these structures is not easy to predict. In the hippocampus the 5-HT1AR is expressed on the dendrites
and cell bodies of glutamatergic pyramidal neurons, where its function has been extensively studied (Andrade 1992). Surprisingly, it has been shown in both anesthetized and awake animals that 5-HT1AR antagonists do not alter the firing of pyramidal neurons in the hippocampus, suggest-ing that under basal conditions the 5-HT1AR is not activated (Haddjeri et al 1998; Tada et al 1999). Again, the situation may be different during the stressful conditions of behavioral testing, at which time serotonin levels may increase and the receptor may become activated. It is likely that postsynaptic 5-HT1AR activation is anxiolytic. In the elevated plus maze and open field tests of anxiety, administration of the selective 5-HT1AR agonist 8-OH-DPAT in the dorsal hippocampus is anxiolytic, whereas administration of the same compound in the amygdala, septum, or ventral hippocampus is ineffective, suggesting that the hippocampus may be a key site of 5-HT1AR action in anxiety (Menard and Treit 1999). Notably, there is one report showing that hippocampal administration of the nonselective 5-HT1AR antagonist NAN-190 is anxiogenic (Belcheva et al 1997). So it appears that both pre- and postsynaptic receptor deletion might contribute to anxiety in the 5-HT1AR KO.
Figure 5. Expression of the serotonin 1A receptor (5-HT1AR).
Levels of 5-HT1AR binding were determined in 129/sv mice
using MPP125
I. High levels of autoreceptor are found in the dorsal raphe (DR) and caudal linear nuclei (CLN), and lower levels in the median raphe nucleus (MR) of the brainstem. These presynaptic receptors respond to serotonin released locally and decrease firing of serotonergic neurons. The highest levels of postsynaptic receptor are found in the CA1 region of the hippocampus and in the septum (S), and intermediate levels in the entorhinal cortex (EnC) and deep layers of the cortex (Ctx), the ventral diagonal band (VDB), and the amygdala– hippocam-pus area (AmHip). Lower levels are found in the subiculum (Sub), dentate gyrus (DG), CA2, and CA3 regions of the hippocampus; the more superficial layers of the cortex; the bed nucleus of the stria terminalis (BNST); the periaqueductal gray (PAG); the inferior and superior colliculi (IC and SC); and the parabrachial nucleus (PBrN), and throughout the amygdala, with the notable exception of the lateral and basolateral nuclei (CeA, MeA, and CoA, but not BLA). Low levels of 5-HT1AR binding
Tissue-Specific Manipulation of the 5-HT
1AReceptor
The debate over pre- versus postsynaptic 5-HT1ARs high-lights the need for tissue-specific manipulation of this receptor in behaving animals. Such function of the recep-tor will be best revealed by a combination of tissue-specific application of the highly tissue-specific antagonist WAY100635 and the development of further transgenic mice expressing the receptor in specific tissues, a prospect feasible with current genetic technology. Tissue-specific expression of the receptor could be accomplished using a 5-HT1AR under control of the tetracycline transactivator protein (tTA) expressed in specific tissues (Zhuang et al 1999). The introduction of such a 5-HT1AR expressed in certain portions of its normal expression pattern onto the KO background would enable one to ask whether rescue of the receptor in specific structures is sufficient to restore a normal fear response. In this way a calmodulin kinase II promoter driving tTA could be used to rescue receptor in the hippocampus, septum, cortex, and amygdala. Alter-nately, tTA expressed in serotonergic brainstem cells by the 5-HT transporter promoter could confer presynaptic rescue. Moreover, temporal control of this system is possible using the drug doxycycline, which inhibits tTA and shuts off expression of the transgene. Such tissue-specific manipulations will help pinpoint structures critical for the increased anxiety of the KO, and eventually may help to identify the mechanism of serotonergic modulation of the fear circuitry.
Work included in this review was supported by Grant No. MH12364 (CG), a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (XZ), and Grants Nos. DA09862 and MH48125 and a NARSAD Independent Investigator Award (RH).
The authors thank Etienne Sibille and Pierre Blier for generously communicating results before publication, and Etienne Sibille for giving careful comments on the manuscript. They also thank Michael Myers and Harry Shair for help in performing and analyzing the heart rate measure-ments, and Gael Malleret for helping to initiate the freezing experiments. Aspects of this work were presented at the conference “Genetics and Brain Function: Implications for the Treatment of Anxiety,” March 22–23, 2000, Washington, DC. The conference was jointly sponsored by the Anxiety Disorders Association of America (ADAA), the ADAA Scientific Advisory Board, and the National Institute of Mental Health.
References
Andrade R (1992): Electrophysiology of 5-HT1Areceptors in the
rat hippocampus and cortex. Drug Dev Res 26:275–286. Belcheva I, Belcheva S, Petkov VV, Hadjiivanova C, Petkov VD
(1997): Behavioral responses to the 5-HT1A receptor antag-onist NAN190 injected into rat CA1 hippocampal area. Gen
Pharmacol 28:435– 441.
Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ (1989): A comparison of the effects of diazepam versus several
typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology 97:277–279.
Bolles RC (1970): Species-specific defense reactions and avoid-ance learning. Psychol Rev 77:32– 48.
Charney DS, Deutsch A (1996): A functional neuroanatomy of anxiety and fear: Implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol 10:419 – 446.
Davidson JR, DuPont RL, Hedges D, Haskins JT (1999): Efficacy, safety, and tolerability of venflaxine extended release and buspirone in outpatients with generalized anxiety disorder. J Clin Psychiatry 60:528 –535.
Davis M, Cassella JV, Kehne JH (1988): Serotonin does not mediate anxiolytic effects of buspirone in the fear-potentiated startle paradigm: Comparison with 8-OH-DPAT and ipsapi-rone. Psychopharmacology 94:8 –13.
Davis M, Shi C (1999): The extended amygdala: Are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann
N Y Acad Sci 877:281–291.
Fanselow MS (1980): Conditioned and unconditional compo-nents of post-shock freezing. Pavlov J Biol Sci 15:177–182. Haddjeri N, Blier P, de Montigny C (1998): Long-term antide-pressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci 18:10150 –10156.
Hashimoto S, Inoue T, Koyama T (1999): Effects of conditioned fear stress on serotonin neurotransmission and freezing be-havior in rats. Eur J Pharmacol 378:23–30.
He M, Sibille E, Benjamin D, Toth M, Shippenberg T (in press): Differential effects of 5-HT1A receptor deletion upon basal
and fluoxetine-evoked 5-HT levels as revealed by in vivo microdialysis. Brain Res.
Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH (1998): Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice.
Proc Natl Acad Sci U S A 95:15049 –15054.
Julius D (1998): Serotonin receptor knockouts: A moody subject.
Proc Natl Acad Sci U S A 95:15153–15154.
Kirby LG, Chou-Green JM, Davis K, Lucki I (1997): The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res 760:218 –230. LeDoux J (1998): Fear and the brain: Where have we been, and
where are we going? Biol Psychiatry 44:1229 –1238. Lesch K-P, Moessner R (1999): Knockout corner: 5-HT1A
receptor inactivation: Anxiety or depression as a murine experience. Int J Neuropsychopharmacol 2:327–331. McCall RB, Patel BN, Harris LT (1987): Effects of serotonin1
and serotonin2 receptor agonists and antagonists on blood
pressure, heart rate and sympathetic nerve activity. J
Phar-macol Exp Ther 242:1152–1159.
Menard J, Treit D (1999): Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci
Biobehav Rev 23:591– 613.
Parks CL, Robinson PS, Sibille E, Shenk T, Toth M (1998): Increased anxiety of mice lacking the serotonin1A receptor.
Proc Natl Acad Sci U S A 95:10734 –10739.
Pattij T, Hijzen TH, Oosting RS, Groenink L, Hen R, Olivier B (in press): Stress-induced hyperthermia in the 5-HT1A
recep-tor knockout is normal. Biol Psychiatry.
Plotsky P, Owens M, Nemeroff C (1998): Psychoneuroendocri-nology of depression. Hypothalamic-pituitary-adrenal axis.
Psychiatry Clin North Am 21:293–307.
Porsolt RD, Bertin A, Jalfre M (1977): Behavioral despair in mice: A primary screening test for antidepressants. Arch Int
Pharmacodyn Ther 229:327–336.
Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendel-sohn M, et al (1998): Serotonin receptor 1A knockout: An animal model of anxiety-related disorder. Proc Natl Acad Sci
U S A 95:14476 –14481.
Richer M, Hen R, Blier P (2000): Assessment of the function of presynaptic 5-HT and norepinephrine receptors in 5-HT1A knockout mice. Soc Neurosci Abstr 47.11.
Sibille E, Parks C, Robinson T, Sarnyai Z, Benjamin D, Baker H, et al (1998): Neurobiology of anxiety in serotonin 1A receptor knock out mice. Soc Neurosci Abstr 308.5.
Sibille E, Pavlides C, Benke D, Toth M (2000): Genetic inactivation of the 5-HT1Areceptor in mice results in
down-regulation of major GABAAreceptor a subunits, reduction of
GABAA receptor binding, and benzodiazepine-resistant anx-iety. J Neurosci 20:2758 –2765.
Tada K, Kasamo K, Ueda N, Suzuki T, Kojima T, Ishikawa K (1999): Anxiolytic 5-hydroxytryptamine1A agonists suppress firing activity of dorsal hippocampus CA1 pyramidal neurons through a postsynaptic mechanism: Single-unit study in unanesthetized, unrestrained rats. J Pharmacol Exp Ther 288:843– 848.
Vahabzadeh A, Fillenz M (1994): Comparison of stress-induced changes in noradrenergic and serotonergic neurons in the rat hippocampus using microdialysis. Eur J Neurosci 6:1205– 1212.
Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ (1996): Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biol
Psychiatry 40:79 – 88.