PULMONARY CIRCULATION DEMONSTRATION
USING AN ISOLATED RAT LUNG MODEL
Thomas C. Resta,1 Mark R. Eichinger,2 Roy D. Russ,3 and Benjimen R. Walker1
Depa rtm ents of1Cell Biology a nd Physiology a nd 2Anesthesiology,
University of New Mexico Hea lth Sciences Center, Albuquerque, New Mexico 87131; a nd 3Division of Ba sic Medica l Sciences a nd Depa rtm ent of Pedia trics,
Mercer University School of Medicine, Ma con, Georgia 31207
W
e have developed a pulmonary circulation laboratory exercise that effectively illustrates basic concepts typically taught in a graduate physiology curricu-lum. The demonstration uses an isolated, perfused rat lung model to delineate the mechanisms by which pulmonary vascular resistance can be altered either passively or in an active manner by contraction or relaxation of vascular smooth muscle. The exercise further offers an opportunity to closely observe an experimental prepara-tion commonly used to study the pulmonary circulaprepara-tion and allows students the opportunity to interpret the resulting physiological data. Student evaluations indicate that the demonstration was received with enthusiasm and provides an effective teaching tool for reinforcing concepts in pulmonary vascular physiology.AM. J. PHYSIOL. 275 (ADV. PHYSIOL. EDUC. 20): S85–S95, 1998.
Key words: pulmonary vascular resistance; distension; recruitment; endothelium-dependent vasodilation; hydrostatic zones of lung
The pulmonary circulation differs from the systemic circulation in many aspects. For example, the entire cardiac output passes through the pulmonary circuit, making it an ideal location for activation or inactiva-tion of biologically active compounds. However, the lung is primarily a gas-exchange organ, and thus the pulmonary circulation serves to maximize exposure of blood to alveolar gas at the capillary level. This anatomic arrangement of capillaries surrounded by air-filled chambers is unique and has mechanical consequences affecting hemodynamics. In addition, the pulmonary circuit is normally a low-pressure, low-resistance circulation that responds passively to a number of physical factors to alter vascular resistance. Pulmonary vascular resistance may also be altered in an active manner by contraction or relaxation of vascular smooth muscle. The laboratory demonstra-tion described here is designed to illustrate many of these properties of this unique vascular bed.
We have conducted this demonstration each of the last eight years as part of a physiology course that is required for all first-year MS and PhD students in biomedical sciences. This laboratory exercise has proven to solidify the students’ understanding of basic concepts and relationships regarding the pulmonary circulation, to provide an opportunity to examine methods by which physiological data are generated, and to allow students to interpret the resulting data. A student group size of four to six is optimal for this demonstration, because it allows students to closely examine the preparation and the various signals dis-played on the chart recorder and/or computer moni-tor and is conducive to questions and discussion. Handouts describing the various experimental proto-cols outlined here and a brief description of methodol-ogy should be provided to the students with sufficient time to carefully study the protocols and predict the results before the laboratory exercise. The
demonstra-I N N O V A T I O N S A N D I D E A S
1043 - 4046 / 98 – $5.00 – COPYRIGHTr1998 THEAMERICAN PHYSIOLOGICAL SOCIETY
VOLUME20 : NUMBER 1 – AD VAN CES IN PH YSIO LO GY ED U CATIO N – DECEMBER 1998
tion is most effective when performed after treatment of related topics on the pulmonary circulation in lecture. These topics include1) passive factors affect-ing vascular resistance within the pulmonary circula-tion, such as effects of changes in arterial, venous, and airway pressure and perfusate viscosity (18);2) active factors affecting pulmonary vascular resistance, includ-ing receptor-mediated vasoconstrictors and endothe-lium-dependent vasodilators (Ref. 2; reviewed in Refs. 8, 10, and 13); 3) Poiseuille’s equation; and 4) hydrostatic zones of the lung (18).
ISOLATED LUNG PREPARATION
All protocols and surgical procedures used in this study were reviewed and approved by the Institu-tional Animal Care and Use Committee of the Univer-sity of New Mexico School of Medicine. The proce-dure for lung isolation was described previously (6). Male Sprague-Dawley rats (200–350 g; Harlan Indus-tries) are anesthetized with pentobarbital sodium (25 mg ip). After the trachea is cannulated with a 17-gauge needle stub, the lungs are ventilated using a Harvard positive-pressure rodent ventilator (model 683) at a frequency of 55 breaths/min and a tidal volume of 2.5 ml with a gas mixture containing 6% CO2 in room air. This gas mixture is humidified and warmed by bub-bling through deionized water contained within a water-jacketed Plexiglas chamber maintained at 38°C by a recirculating water bath. Peak inspiratory pres-sure is set at 9 cmH2O, and positive end-expiratory pressure is maintained at 2 cmH2O. After a median sternotomy, heparin (100 U in 0.1 ml) is injected directly into the right ventricle. A 13-gauge needle stub is advanced into the pulmonary artery via an incision in the right ventricle and secured into posi-tion with 2-0 silk suture. The needle stub is covered with a 2- to 3-mm segment of heat-shrink tubing at its tip, which helps to secure the cannula inside the pulmonary artery. The preparation is immediately perfused at 0.8 ml/min by a Masterflex microproces-sor pump drive (model 7524–10) with a physiological saline solution (PSS) containing (in mM) 129.8 NaCl, 5.4 KCl, 0.83 MgSO4, 19 NaHCO3, 1.8 CaCl2, and 5.5 glucose with 4% albumin (wt/vol) (all from Sigma). The left ventricle is cannulated with a plastic tube (4-mm OD) via an incision at the apex and tied in place with a 2-0 silk suture. A 3-mm segment of Tygon
tubing is placed on the end of the ventricular cannula, allowing the cannula to be secured in position. The heart and lungs are removed en bloc and suspended directly above a water-jacketed Plexiglas chamber containing PSS maintained at 38°C. A plastic cover is placed around the lung preparation to provide a warmed and humidified chamber. The perfusion rate is gradually increased to 30 ml.min21.kg body wt 21 and maintained at this rate unless otherwise stated. Twenty milliliters of perfusate are washed through the lungs and discarded before recirculation is initiated with the remaining forty milliliters of PSS. The dead space of this recirculating perfusion system is,20 ml.
The remaining 20 ml of perfusate are contained within a reservoir (50-ml centrifuge tube), which is sus-pended from a Grass model FT03 force-displacement transducer for continuous measurement of reservoir weight. Because the perfusion system is a closed circuit, monitoring reservoir weight (and thus vol-ume) provides a means of assessing changes in vascu-lar fluid volume (6). The venous line-perfusate reser-voir-force transducer assembly is attached with clamps to a vertical rod to provide easy vertical adjustment for those protocols requiring changes in pulmonary ve-nous pressure. Perfusate is pumped through a water-jacketed Radnoti bubble trap maintained at 38°C before entering the pulmonary circulation. Lungs are allowed 30 min to equilibrate before protocols are begun. Pulmonary arterial pressure (Pa), venous pres-sure (Pv), and airway pressure (PA) are measured via side ports in the arterial, venous, and airway lines, respectively, with Spectramed model P23 XL pressure transducers and recorded on a Gould RS 3400 chart recorder. Output signals from the Pa, Pv, PA, and reservoir weight channels of the chart recorder are continuously displayed on a computer screen using a data acquisition and analysis system (AT-CODAS, Dataq Instruments).
EXPERIMENTAL PROTOCOLS
Pr otocol 1: Effect of Incr eased Flow
single isolated lung used for one of our demonstra-tions. Because the venous reservoir is set below the lung, Pvis,0. Thus the signal for Pvis not apparent in Fig. 1. Figures 2–7 include data obtained from this same lung.
Question 1: Is the lung inzone 1,2, or3condition?
Answer: The lung is inzone 2, because Pa.PA.Pv.
Question 2: From Poiseuille’s equation, and assuming the pulmonary circulation behaves like a series of rigid tubes with laminar flow, what effect would you predict that doubling flow would have on the ob-served arterial-venous pressure gradient?
Answer: Poiseuille’s equation states that
R5DP
Q 5
8hl
pr4 (1)
where R is resistance to flow, DP is the pressure gradient across the pulmonary vasculature (i.e., Pa2 Pv), Q is flow,his perfusate viscosity,lis vessel length, andris vessel radius.
Rearranged, this gives
DP58hl
pr43Q (2)
FIG. 1.
Effect of incr eased flow (protocol 1). Repr esentative traces for pulmonary arterial pr essur e (Pa), airway pr essur e (PA),
pul-monary venous pr essur e (Pv), and r eservoir weight ex
-pr essed as a change in volume (DVol) fr om an individual isolated lung. Vertical line attim e 0indicates point at which flow was doubled. Venous r eservoir was set below lung for this pr otocol, r esulting in Pv , 0 mmHg. Doubling flow
decr eases r eservoir volume and r esults in a less than twofold incr ease in Pabecause of r ecruitment.
I N N O V A T I O N S A N D I D E A S
VOLUME 20 : NUMBER 1 – AD VAN CES IN PH YSIO LO GY ED U CATIO N – DECEMBER 1998
Therefore, becauseDP is directly proportional to Q, a doubling of Q would produce a twofold increase inDP (i.e., the arterial-venous pressure gradient).
Question 3: However, the pulmonary circulation behaves quite differently than this simple model. What is your prediction for the arterial pressure response to a doubling of flow? Why?
Answer: A doubling of flow will decrease pulmonary vascular resistance and result in a less than twofold increase in Pa for two reasons: 1) the resulting recruitment, and possibly some distension as well, will increase the radius term (r) in Poiseuille’s equa-tion for those newly perfused and distended vessels, thereby decreasing pulmonary vascular resistance;
and2) resistances in parallel add as reciprocals
Rtotal
21 5R 1
211R 2
211R 3
21. . . (3)
Therefore, because recruitment increases the number of parallel resistances at the microvascular level, resistance will decrease in that segment, thereby attenuating the increase in Pa.
Question 4: Would you expect vascular fluid volume (as detected by a change in reservoir weight) to change in response to this stimulus? Why?
Answer: Reservoir weight will decrease in response to a doubling of flow, thus indicating an increase in lung fluid volume. This increased vascular volume occurs secondary to recruitment because a greater vascular FIG. 2.
Effect of incr eased PA in zone 2 conditions (protocol 2).
Repr esentative traces for Pa, PA, Pv, and r eservoir volume
(DVol) fr om an individual isolated lung. PAwas incr eased at tim e 0. Venous r eservoir was set below lung for this pr otocol, r esulting in Pv,0 mmHg. Incr eased PAleads to an incr ease
surface area is being perfused. An additional contribu-tion to the decrease in reservoir weight is increased fluid flux as a result of greater capillary hydrostatic pressure.
Pr otocol 2: Effect of Incr eased Airway Pr essur e inZone 2Conditions
Flow is returned to 30 ml.min21.kg body wt21, and PA is increased by elevating positive end-expiratory pres-sure (Fig. 2).
Question 5: What do you predict will be the arterial pressure response to this stimulus?
Answer: Because the lungs remain inzone 2(i.e., Pa. PA.Pv), an increase in PAwill compress the pulmo-nary capillaries, thereby decreasing the radius term in
Poiseuille’s equation for those vessels being com-pressed, leading to an increase in pulmonary vascular resistance. Because
DP5R3Q (4)
and because Q is constant, an increase inRwill result in an increase in DP. Because Pvis held constant, an increase in Pa is observed. Note the corresponding decrease in reservoir weight associated with recruit-ment (Fig. 2).
Pr otocol 3: Effect of Incr eased Venous Pr essur e inZone 3Conditions
On restoration of PA to control levels (i.e., 1–3 mmHg), the venous reservoir is elevated to achieve a Pvof 5 mmHg (Fig. 3).
FIG. 3.
Effect of incr eased Pv in zone 3 conditions (protocol 3).
Repr esentative traces for Pa, PA, Pv, and r eservoir volume
(DVol) fr om an individual isolated lung. Pvwas incr eased at tim e 0. Incr eased Pvr esults in a decr ease in r eservoir volume
and incr eased Painzone 3conditions.
I N N O V A T I O N S A N D I D E A S
VOLUME 20 : NUMBER 1 – AD VAN CES IN PH YSIO LO GY ED U CATIO N – DECEMBER 1998
Question 6: What will happen to Pa and reservoir weight? Why?
Answer: Because the elevation in Pvplaces the lungs inzone 3 conditions (i.e., Pa.Pv.PA), the increase in Pvwill be transmitted across the pulmonary vascula-ture, thus increasing Pa. Reservoir weight will de-crease because of inde-creased lung fluid volume associ-ated primarily with distension of pulmonary veins. Increased fluid flux may additionally contribute to this fall in reservoir weight.
Pr otocol 4: Effect of Incr eased Airway Pr essur e inZone 3Conditions
With Pvmaintained at ,5 mmHg, PAis increased to ,4 mmHg (Fig. 4).
Question 7: What will be the effect of this maneuver on pulmonary vascular resistance and Pa? Why?
Answer: The lungs will remain in zone 3 conditions (Pa.Pv.PA) despite the elevation of PA. Therefore, because microvascular pressure exceeds the surround-ing alveolar pressure, the increase in alveolar pressure will not compress the microvasculature and pulmo-nary vascular resistance and Pawill be unaltered.
Pr otocol 5: Effect of Incr eased Per fusate Viscosity
With PA and Pv returned to control conditions, the saline perfusate is replaced with heparinized whole blood (Fig. 5). This is achieved by emptying the effluent into a waste container until all saline has been drained from the perfusate reservoir, followed by immediate addition of whole blood to the reservoir. Any small bubbles carried from the reservoir will be removed by the bubble trap. A three-way stopcock in the venous line is useful for diverting flow away from the perfusate reservoir and into the waste container. FIG. 4.
Effect of incr eased PA in zone 3 conditions (protocol 4).
Repr esentative traces for Pa, PA, Pv, and r eservoir volume
The effluent is allowed to continue to drain into the waste container until most of the saline has been removed from the system. Finally, flow is diverted back towards the perfusate reservoir using the three-way stopcock in the venous line, thus restoring the system to a closed, recirculating circuit. Whole blood is drawn into heparinized syringes by direct cardiac puncture of donor rats anesthetized with pentobarbi-tal sodium (25 mg ip) as well as from the lung donor immediately before lung isolation.
Question 8: What will be the effect of perfusion with whole blood on pulmonary vascular resistance? Ex-plain.
Answer: Because blood is more viscous than saline, perfusion with blood will increase the viscosity param-eter (h) in Poiseuille’s equation, thereby increasing pulmonary vascular resistance. This increase in
resis-tance is detected by an increase in Pa. Note the patches of red on the lung surface that may develop as blood enters the lung. Within several seconds, the surface of the lung exhibits a more homogeneous color as a consequence of recruitment secondary to the increase in Pa. Reservoir weight decreases because of recruitment and increased lung fluid flux.
Pr otocol 6: Response to Pulmonary Vasoconstrictor U-46619
The synthetic thromboxane analog U-46619 (9,11-dideoxy-9a,11a-methanoepoxy prostaglandin F2a; Cay-man) is added to the perfusate reservoir in cumulative doses until a stable arterial pressor response of,10
mmHg is achieved (Fig. 6). This agent stimulates thromboxane A2 receptors on pulmonary vascular smooth muscle cells, resulting in an increase in the concentration of intracellular calcium ([Ca21]
i) (3). FIG. 5.
Effect of incr eased per fusate viscosity (protocol 5). Repr esen-tative traces for Pa, PA, Pv, and r eservoir volume (DVol) fr om
an individual isolated lung. Per fusion with whole blood was initiated at tim e 0. Incr eased per fusate viscosity caused by per fusion with blood incr eases Pa and decr eases r eservoir
volume.
I N N O V A T I O N S A N D I D E A S
VOLUME 20 : NUMBER 1 – AD VAN CES IN PH YSIO LO GY ED U CATIO N – DECEMBER 1998
U-46619 is prepared in 95% ethanol at a stock concen-tration of 10 µg/ml and stored at280°C. A recirculat-ing concentration of,100–200 nM is usually sufficient
to produce a stable 10-mmHg pressor response. Because U-46619 constricts both arterial and venous segments of the rat pulmonary vasculature (5, 12), larger constrictions will elevate microvascular pres-sure resulting in edema. In the demonstration de-picted by Fig. 6, 143 nM U-46619 was required to achieve a stable pressor response of 11 mmHg. This was achieved by adding two successive doses of 1 µg of U-46619 to the perfusate reservoir. Note the artifac-tual increase in reservoir weight with each dose.
Question 9: What will be the effect of U-46619 on Pa and reservoir weight? Explain.
Answer: The increase in [Ca21]
icauses contraction of pulmonary vascular smooth muscle, thus constricting
the muscular pulmonary vasculature. The resultant decrease in vessel radius will increase resistance as demonstrated by Poiseuille’s equation, leading to an increase in Pa. The increased vascular fluid volume and flux that occurs with increased Pais evidenced by a greater rate of fluid loss from the pefusate reservoir.
Pr otocol 7: Response to
Endothelium-Dependent Pulmonary Vasodilator Ar ginine Vasopr essin
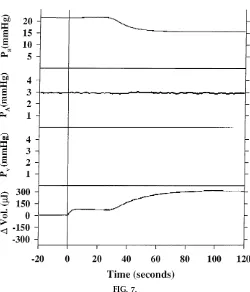
At the plateau of the pressor response to U-46619, the hormone arginine vasopressin (AVP) is added to the perfusate reservoir to achieve a circulating concentra-tion of 2.5 nM (Fig. 7). In the pulmonary circulaconcentra-tion, receptors for AVP appear to be localized to the vascular endothelium (not on vascular smooth muscle), which likely elicit an increase in [Ca21]
ion activation (14, 15).
FIG. 6.
Response to pulmonary vasoconstrictor U-46619 (protocol 6). Repr esentative traces for Pa, PA, Pv, and r eservoir weight
Question 10: What do you predict will be the re-sponse to AVP? What is the likely mechanism of this response?
Answer: An increase in endothelial [Ca21]iwill
stimu-late the enzyme endothelial nitric oxide (NO) syn-thase (eNOS), thus increasing NO synthesis (reviewed in Ref. 8). This NO diffuses to the underlying vascular smooth muscle where it elicits relaxation. The result-ing vasodilatory response leads to a decrease in vascular resistance according to Poiseuille’s equation. The decrease in Pa in response to AVP will initiate translocation of fluid from the vascular compartment to the perfusate reservoir as a consequence of dere-cruitment, thus increasing reservoir weight. The admin-istration of AVP to the perfusate reservoir is respon-sible for the artifactual increase in reservoir weight at tim e 0 in Fig. 7. Although an increase in endothelial [Ca21]i in response to AVP may be predicted to
stimulate the production of prostacyclin and endothe-lium-derived hyperpolarizing factors (EDHF) as well, previous studies from our laboratory do not support a role for either prostaglandins or EDHF in mediating the pulmonary vasodilatory response to AVP (4, 16). Although we have demonstrated AVP to be an ex-tremely potent and efficacious vasodilator in the isolated perfused rat lung (5), large vasodilatory re-sponses to AVP are not always observed at this point in the demonstration, probably as a result of de-creased endothelial viability after a lengthy series of experimental protocols.
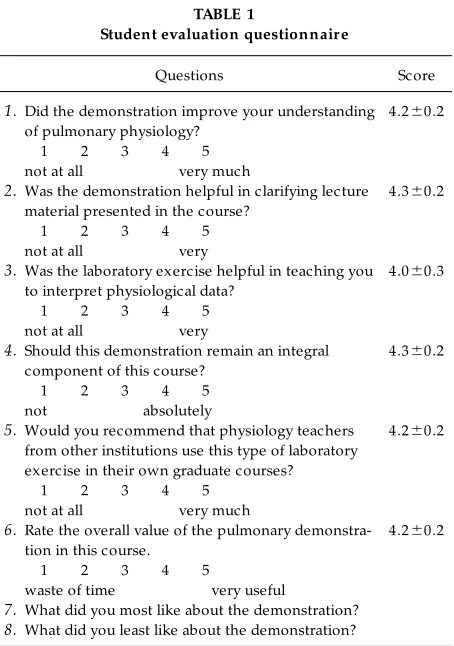
STUDENT EVALUATIONS
Students were asked to anonymously fill out a question-naire at the end of the course that was designed to assess the perceived value of this exercise (Table 1). Our findings indicate that the demonstration was FIG. 7.
Response to endothelium-dependent pulmonary vasodilator ar ginine vasopr essin (protocol 7). Repr esentative traces for Pa, PA, Pv, and r eservoir weight (DVol) fr om an individual
isolated lung. Ar ginine vasopr essin (2.5 nM) was added to per fusate r eservoir at tim e 0to demonstrate endothelium-dependent pulmonary vasodilation.
I N N O V A T I O N S A N D I D E A S
VOLUME 20 : NUMBER 1 – AD VAN CES IN PH YSIO LO GY ED U CATIO N – DECEMBER 1998
generally well received by the students. The over-whelming response toquestion 7was an appreciation for observing methods by which physiological data are generated, as opposed to only reading about general results and conclusions in a textbook. Further-more, the students felt that reviewing concepts and equations previously covered in class in the context of an experimental situation was extremely valuable in reinforcing those topics. The major criticism (ques-tion 8) of this exercise was that it required too much time (,1.5 h) to complete, although other students
suggested that more time be allotted for this exercise. Another criticism was the lack of hands-on participa-tion by students, although such participaparticipa-tion would be very difficult to incorporate into the demonstra-tion.
DISCUSSION
Additional topics in pulmonary physiology could be included in this demonstration depending on the
areas covered in preceding lectures. For example, our students have usually received lectures in cellular mechanisms of vascular smooth muscle contraction and relaxation as well as lectures on endothelium-dependent responses before the demonstration, pro-viding us with the opportunity to review in detail signal transduction mechanisms associated with recep-tor-mediated pulmonary vasoconstrictors such as the thromboxane mimetic U-46619 and with endothelium-derived NO (EDNO)-dependent pulmonary vasodila-tors such as AVP. The signal transduction pathways associated with vascular smooth muscle thromboxane-receptor activation are similar to other thromboxane- receptor-mediated vasoconstrictors and have been described previously (3). EDNO-dependent pulmonary vasodila-tors stimulate eNOS by increasing endothelial [Ca21]i
as described in protocol 7. The NO produced elicits relaxation of vascular smooth muscle through various mechanisms, which include stimulation of soluble guanylyl cyclase and direct activation of potassium channels on vascular smooth muscle. The signal transduction pathways associated with these re-sponses have also been previously described (2, 10, 13).
Other pulmonary vasoconstrictor stimuli or endothe-lium-dependent dilators may alternatively be used for this demonstration. For example, the lung may be ventilated with a hypoxic gas mixture to demonstrate hypoxic vasoconstriction (11), allowing review of the current evidence regarding the cellular mechanisms associated with this response (7; reviewed in Ref. 17). Furthermore, many other receptor-mediated endothe-lium-dependent vasodilators may be used in this preparation, including serotonin, endothelin-1, and histamine (9, 12).
A further concept that could be incorporated into the present demonstration is that of vascular permeability and the Starling equation (18). Measurement of reser-voir weight not only provides an index of acute changes in vascular fluid volume but is useful in determining lung fluid flux under steady-state condi-tions (6). For example, the doubling of flow and associated increase in Painprotocol 1results in a rapid drop in reservoir weight as recruitment causes lung fluid volume to increase. However, the rate of change in reservoir weight under steady-state conditions is greater after the pressure increase than before. This TABLE 1
Student evaluation questionnair e
Questions Score
1.Did the demonstration improve your understanding of pulmonary physiology?
1 2 3 4 5 not at all very much
4.260.2
2.Was the demonstration helpful in clarifying lecture material presented in the course?
1 2 3 4 5 not at all very
4.360.2
3.Was the laboratory exercise helpful in teaching you to interpret physiological data?
1 2 3 4 5 not at all very
4.060.3
4.Should this demonstration remain an integral component of this course?
1 2 3 4 5 not absolutely
4.360.2
5.Would you recommend that physiology teachers from other institutions use this type of laboratory exercise in their own graduate courses?
1 2 3 4 5 not at all very much
4.260.2
6.Rate the overall value of the pulmonary demonstra-tion in this course.
1 2 3 4 5
waste of time very useful
4.260.2
7.What did you most like about the demonstration?
increased lung fluid flux can be attributed to greater capillary hydrostatic pressure as described by the Starling equation. The rationale for adding albumin to the perfusion buffer to maintain proper oncotic pres-sure can also be explained by this relationship. Finally, simple protocols may be incorporated to demonstrate methods for calculating the capillary filtration coeffi-cient in the isolated lung as previously described (1)
In summary, we have described a pulmonary circula-tion laboratory demonstracircula-tion that reinforces con-cepts in pulmonary physiology typically taught in a first-year graduate curriculum. This demonstration allows students to observe methods by which physi-ological data are generated using an isolated, perfused rat lung model, a preparation frequently used in studies of the pulmonary circulation, and provides students an opportunity to interpret the results. This preparation uses a commonly used laboratory animal and can be set up with standard equipment found in most cardiovascular physiology laboratories. Student evaluations revealed that this laboratory exercise was generally perceived by the students as an effective and enjoyable learning experience.
Address for reprint requests: T. C. Resta, Vascular Physiology Group, Dept. of Cell Biology and Physiology, Univ. of New Mexico Health Sciences Ctr., 915 Camino de Salud NE, Albuquerque, NM 87131-5218.
Received 6 May 1998; accepted in final form 24 August 1998.
Refer ences
1. Adkins, W. K., and A. E. Taylor.Role of xanthine oxidase and neutrophils in ischemia-reperfusion injury in rabbit lung. J. Appl. Physiol. 69: 2012–2018, 1990.
2. Bolotina, V. M., S. Najibi, J. J. Palacino, P. J. Pagano, and R. A. Cohen.Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Na ture 368: 850–853, 1994.
3. Dor n, G. W. II, and M. W. Becker.Thromboxane A2
stimu-lated signal transduction in vascular smooth muscle.J. Pha rm a -col. Exp. Ther. 265: 447–456, 1993.
4. Eichinger, M. R., R. D. Russ, and B. R. Walker.Potassium channels are not involved in vasopressin-induced vasodilation
in the rat lung.Am . J. Physiol. 266 (Hea rt Circ. Physiol. 35): H491–H495, 1994.
5. Eichinger, M. R., and B. R. Walker. Enhanced pulmonary arterial dilation to arginine vasopressin in chronically hypoxic rats. Am . J. Physiol. 267 (Hea rt Circ. Physiol. 36): H2413– H2419, 1994.
6. Eichinger, M. R., and B. R. Walker.Nitric oxide and cGMP do not affect fluid flux in isolated rat lungs. J. Appl. Physiol. 80: 69–76, 1996.
7. Gelband, C. H., and H. Gelband.Ca21release from
intracellu-lar stores is an initial step in hypoxic pulmonary vasoconstric-tion of rat pulmonary artery resistance vessels.Circula tion96: 3647–3654, 1997.
8. Griffith, O. W., and D. J. Stuehr. Nitric oxide synthases: properties and catalytic mechanism. Annu. Rev. Physiol. 57: 707–736, 1995.
9. Isaacson, T. C., V. Hampl, E. K. Weir, D. P. Nelson, and S. L. Ar cher. Increased endothelium-derived NO in hypertensive pulmonary circulation of chronically hypoxic rats. J. Appl. Physiol. 76: 933–940, 1994.
10. Lincoln, T. M., P. Komalavilas, and T. L. Cor nwell. Pleiotro-pic regulation of vascular smooth muscle tone by cyclic GMP-dependent protein kinase.Hypertension23: 1141–1147, 1994.
11. McMurtry, I. F., A. B. Davidson, J. T. Reeves, and R. F. Gr over.Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs.Circ. Res. 38: 99–104, 1976.
12. Resta, T. C., and B. R. Walker.Chronic hypoxia selectively augments endothelium-dependent pulmonary arterial vasodila-tion.Am . J. Physiol. 270 (Hea rt Circ. Physiol. 39): H888–H896, 1996.
13. Robertson, B. E., R. Schubert, J. Hescheler, and M. T. Nelson.cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am . J. Physiol. 265 (Cell Physiol. 34): C299–C303, 1993.
14. Russ, R. D., T. C. Resta, and B. R. Walker. Pulmonary vasodilatory response to neurohypophyseal peptides in the rat.
J. Appl. Physiol. 73: 473–478, 1992.
15. Russ, R. D., and B. R. Walker. Role of nitric oxide in vasopressinergic pulmonary vasodilatation.Am . J. Physiol. 262 (Hea rt Circ. Physiol. 31): H743–H747, 1992.
16. Russ, R. D., and B. R. Walker. Maintained endothelium-dependent pulmonary vasodilation following chronic hypoxia in the rat.J. Appl. Physiol. 74: 339–344, 1993.
17. Weir, E. K., and S. L. Ar cher. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels.
FASEB J. 9: 183–189, 1995.
18. West, J. B.Respira tory Physiology, the Essentia ls(5th ed.). Baltimore, MD: Williams and Wilkins, 1995.
I N N O V A T I O N S A N D I D E A S
VOLUME 20 : NUMBER 1 – AD VAN CES IN PH YSIO LO GY ED U CATIO N – DECEMBER 1998