Leaf variations in
Elaeagnus angustifolia
related to
environmental heterogeneity
Marı´a Guadalupe Klich *
Departamento de Agronomı´a,Centro de Recursos Naturales Reno6ables de la Zona Semia´rida(CERZOS),
Uni6ersidad Nacional del Sur,C.C. 738,8000-Bahı´a Blanca,Argentina
Received 3 August 1999; received in revised form 6 April 2000; accepted 10 April 2000
Abstract
Elaeagnus angustifolia(Russian olive) is a Eurasian tree that has become naturalized and has invaded zones along watercourses in many arid and semiarid regions of the world. These habitats are characterized by vertical environmental gradients, thus trees must develop some plasticity to adapt to the wide range of site conditions. This study was undertaken to test the hypothesis that variations in leaf anatomy and morphology ofE.angustifoliareflect their adaptability to the differences in the microclimate that occur within the canopy of single trees. Foliar architecture, blade and petiole epidermal and internal anatomy were examined in leaves at different canopy positions and related to environmental conditions. Upper sun-leaves are exposed to higher solar irradiance and lower air humidity and are smaller, more slender and thicker than the lower, half-exposed and shade-leaves. Color varies between the leaves at different levels, from silvery grey-green in the upper strata, to dark green in the lower one. Bicolor is more evident in half-exposed and shaded leaves. When compared with the lower half-exposed and shade-leaves, the upper leaves ofE. angustifolia have a greater areole density, a higher mesophyll proportion and stomatal density. Trichomes are multicellular, pedestalled, stellate-branched or peltate and their form and density can be associated with leaf color and appearance. The slender petioles of the upper leaves have proportionally more epidermis, collenchyma and phloem and less parenchyma and xylem than those of lower leaves, when observed in transverse sections. Foliar morphological and anatomical variability inE.angustifoliamay be considered an adaptive advantage that enables leaves to develop and function in habitats marked by strong variations of solar radiation, air temperature and humidity. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Leaf heterogeneity; Leaf architecture; Phenotypic plasticity; Water stress; Xeromorphy; Canopy microclimate
www.elsevier.com/locate/envexpbot
1. Introduction
Developmental responses to small-scale envi-ronmental heterogeneity can be important for
plant adaptation (Novoplansky, 1996). Leaves are the plant organs most exposed to aerial conditions and the changes in their characters have been interpreted as adaptations to specific environ-ments (Fahn and Cutler, 1992). Variations in the morphological and anatomical features of leaves developed at different levels in the plants have
* Corresponding author. Fax: +54-291-4541224.
E-mail address:[email protected] (M.G. Klich).
M.G.Klich/En6ironmental and Experimental Botany44 (2000) 171 – 183 172
been reported for many species and related espe-cially to the amount of sun exposure or water availability. (Kaufmann and Troendle, 1981; Niinemets and Kull, 1994; Smith et al., 1997).
Elaeagnus angustifolia L. (Russian olive) is a Eurasian tree that has become naturalized, form-ing monotypic stands along the watercourses in the Rı´o Negro valleys of Argentina. This species is known for its capacity to grow over a wide range of environmental conditions. For example, seedlings are tolerant of shade and mature trees
can live exposed to high light intensities
(Shafroth et al., 1995; Lucchesini and Mensuali-Sodi, 1996). E. angustifolia can displace native woody species and has been so successful in colonising disturbed areas and old fields, that its use is prohibited in some areas (Dawson, 1990). The ability of E. angustifolia to establish, grow and invade new areas has led to investigations of the conditions that might favor its spread (Shafroth et al., 1995).
Visiting the invaded zone, I noticed that within clustered individuals of E.angustifolia there were variations in form and color between leaves growing at different levels in a tree. I evaluated
the environmental heterogeneity within the
canopy of trees growing along the Rio Negro watercourse in this dry-region. Anatomical and morphological studies were performed in order to prove if the externally observed leaf differ-ences were correlated with internal adaptations. I tested the hypothesis that variations in develop-mental responses of the E. angustifolia leaves to spatial heterogeneity are related to the ecological strategies of this invasive species.
2. Material and methods
The study was conducted during three growth periods (1995 – 1998), using leaf samples origi-nated from a stand of E. angustifolia growing at the margin of the Rı´o Negro, Argentina (39°30% S, 65°30% W) in an area where the expansion of this species has been notable in the last 20 years. The climate is temperate semiarid to cold arid. The average temperature during the coldest month (July) is 6.83°C and during the hottest
month (January) is 23.02°C. The average annual precipitation is 300 mm, most falling during the spring and autumn. Average relative air humidity ranges from 48% (January) to 70% (June). The
average annual evapotranspiration is \800 mm,
with a negative water balance throughout the year. The former regional climatic data were ob-tained from the Meteorological Station at Fray Luis Beltra´n, which is 30 km away from the study site. Soils are alluvial and occasionally sub-jected to flooding. During the study, the local data of ambient air temperature and humidity were recorded with an hygrothermograph and those of solar radiation and rain with an auto-matic data recorder (KADEC-U, Kona System, Sapporo, Japan). Soil water content was deter-mined monthly by gravimetry.
Undamaged leaves of ten well-developed trees (8 – 9 m height) were collected from the upper sun-exposed crown (from 5 m up), the medium half sun-exposed branches (between 1 and 3 m
height) and the lower shaded crown (B1 m
height).
Leaf water content (LWC) during the growing
period was determined by collecting :100 g of
leaf material at each level of the ten trees. The fresh weight was determined in recently cut leaves and the dry weight after heating them at 60°C for 48 h. Sampling was made in October after the beginning of the growing period, in December, in February and finally in April, just before the initiation of the cold latency period. Leaf blade size was determined with a portable area meter (LI-COR, LI 3000A) from ten leaves of each level of the ten trees.
specially cut thin glasses. The mounted leaves were photographed and the photographs en-hanced so that the determination of the
vena-tion’s pattern could be easily performed.
Drawings of the smaller veins, veinlets and are-oles were made using a Wild M 5 stereoscopic microscope and a Wild M 20 binocular micro-scope, both with drawing tubes. Microphoto-graphs were taken with a Zeiss Photomicroscope II. Drawings of each epidermis were made after removing the other epidermis and most of the mesophyllic tissue. Means of measurements of anatomical features were based on five measures per leaf in five leaves of each of the three levels of the ten trees.
The estimation of leaf volume, mesophyll vol-ume, proportion of mesophyll in the leaf and proportion of spongy and palisade parenchyma were made with data obtained, by stereological
procedures, from drawings of photographed
transverse sections of paraffin-embedded mate-rial, stained in safranin-fast green and mounted in balsam. The sampling of tissue blocks to be used for stereological measurements of leaf anatomy and the estimation of the mentioned characters was made following the recommenda-tions of Kubı´nova´ (1993). Data were obtained from five transverse sections per leaf in five leaves of each of the three levels of the ten trees.
Leaf material for scanning electron microscopy (SEM) was fixed in FAA. Samples were dehy-drated in a series of alcohol of increasing strength to absolute alcohol and in a series of alcohol – acetone to pure acetone, treated in a Polaroid critical point dryer and coated with a film of gold with a sputter coater Pelco 91000. Both epidermis of each sample were examined with a JEOL 35 SEM operated at 7 KV.
The relation between the various tissues of the petioles at the different level in the plants was determined by drawings of the cross-section at the basal and distal end of the petioles, using UTHSCSA Image Tool (University of Texas Health Science Center, San Antonio, TX). Free hand sections were made on five petioles of each of the three levels of the ten trees.
The evaluation of the data was carried out by means of ANOVA and the mean values of the treatments were compared using the Student – Newman – Keuls’ test.
3. Results
Table 1 shows the data of the environmental conditions registered during the growth period 1997 – 1998 in the sampling zone. Air tempera-ture outside the shaded understorey reached higher values, up to a maximum of 42°C in Jan-uary 1998. Air humidity in the lower protected strata reached a minimum of 36% and attained 100% every night, but in the upper sun and wind exposed zone, values ranged from 18% up to a maximum of 84%.
Fig. 1 represents the average monthly mea-sures of daily solar radiation as well as the maxi-mum hourly records in the upper canopy of the E. angustifolia trees. During spring and summer time, solar radiation is very high and the values
climb up to 927 calorie cm2/day in December.
Light attenuation at the lower level reaches 90% and at the medium level ranges between 60 and 80%.
Leaf water content decreased through the grow-ing period at all levels in the plants, but while in spring the higher values were those of
half-ex-Fig. 1. Mean daily solar radiation (cal. cm2/day) and maxi-mum hourly solar radiation (cal. cm2/h) registered during the period April 1997 to March 1998 in the upper canopy of theE.
M
.
G
.
Klich
/
En
6
ironmental
and
Experimental
Botany
44
(2000)
171
–
183
174
Table 1
Environmental conditions of the sampling area during the growth period (1997–1998) ofE.angustifoliaa
% AH (lower height)
AT (°C) (lower height) % AH (upper height)
% SWC (20, 40 and 60 cm Rain (mm)
(min, max)
(max, min, mean) (min, max)
depth)
30 81 4.5
33.6 27.8 9.3
August 13.3 1.5 7.4 53 100
September 26.3 16.5 15.9 14.3 1.7 8.0 51 100 30 73 17.0
28.0
17.6 14.1 19.7 17.3 4.8 11.1 41 100 30 69
October
28 63 16.0
20.6 8.1 14.3
November 33.8 30.9 29.0 37 100
52 100 25 61 30.0
23.9 10.1 17.3
December 16.1 15.9 13.7
3.5
7.0 11.3 11.8 25.4 11.4 18.4 36 100 18 60
January
21 69 47.5
11.4 14.0 11.0
February 22.3 10.5 16.4 56 100
35 80 22.5
21.6 6.9 14.2
March 10.0 14.3 15.3 48 100
34 84
48 100
18.1 6.5 11.9 19.5
April 13.9 17.4 18.3
Table 2
Comparison of leaf water content (LWC%) ofE.angustifolia
leaves developed at different heights in a treea
ML
LL UL
74.11b
October 75.90b 70.20a
December 74.54b 73.27b 68.99a
57.90a 57.54a February 62.07b
51.10a 55.81b 51.13a
April
aLL, lower shade leaves (B1 m height); ML, medium leaves
( 1–3 m height); UL, upper sun leaves (\5 m height). Values
are the mean for 30 measurements. For each row, values which have the same letter are not significantly different at the 0.05 level, as determined by Student–Newman–Keuls’ test.
the tree level increases but its course is generally straight. The angles of divergence of secondary veins were found to be always acute, although they are wider in the upper leaves. The relative thickness of secondary veins is moderate and their course is mainly curved. The intersecondary veins are simple and the intercostal areas are irregular. The pattern of tertiary veins is randomly reticu-late. The higher order of venation is quaternary and a looped marginal ultimate venation is ob-served. The areoles are imperfect, pentagonal to polygonal and randomly arranged. In the upper leaves, the density of areoles is higher (17 areoles/ mm2) than in the medium and lower ones (15 and 14 areoles/mm2, respectively). Veinlets are mainly branched. One to three veinlets generally enter each areole, but areoles lacking terminal veinlets are also common.
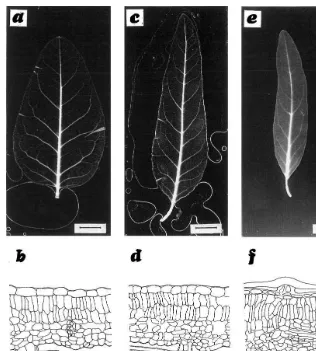
The thickness of the foliar blades increases with the height and the degree of sun exposure, while their area decreases, so that the mean volume of the leaves at the evaluated levels has no significant differences (Table 4).
Leaves are bifacial, with a biseriate palisade in the two inferior levels, but a third poorly orga-nized stratum is observed in the upper leaves (Fig. 2b,d,f). Spongy mesophyll cells are round or elon-gate, not lobed and randomly oriented. The pro-portion of mesophyll tissue is higher in the upper leaves (81.86%) than in the medium (74.93%) and lower (73.97%) leaves, but the ratio between pal-isade and spongy parenchyma remains constant (average 60 and 40%, respectively) at all leaf levels.
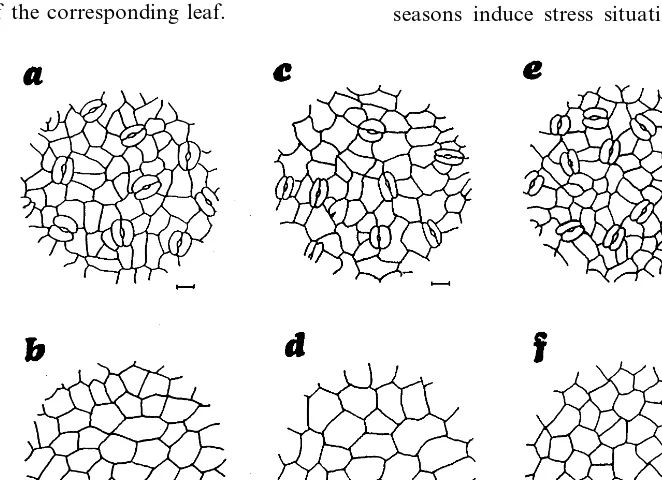
Leaves are hypostomatous, with anomocytic stomata (Fig. 3). Stomata are at the level of the neighboring ordinary epidermal cells. The pentag-onal or hexagpentag-onal epidermal cells are smaller in the lower than upper epidermis. Adaxial epider-mis is thinner in the upper leaves but the outer tangential walls are thicker than in the lower leveled leaves. Stomata density is higher in the upper leaves, but there are no significant differ-ences in their length and width between the three analyzed leaf levels (Table 3). The abaxial surface of the leaves is always more pubescent than the
adaxial one. Trichomes are multicellular,
pedestalled, stellate-branched or peltate, and their posed and shade-leaves, in April these were the
M.G.Klich/En6ironmental and Experimental Botany44 (2000) 171 – 183 176
form and density can be associated with leaf level,
color and appearance. Lower leaves have
branched hairs and present a woolly appearance abaxially (Fig. 4a,b). The abaxial epidermis of the
Table 3
Comparison of morphological and anatomical features ofE.angustifolialeaves developed at different heights in a treea Medium leaves
Lower shade leaves Upper sun leaves
(1–3 m height)
(B1 m height) (\5 m height)
Microphyll from 7.5 to 18.7
Lamina area (one side) cm2 Microphyll-mesophyll from Microphyll from 4.2 to 10.5 4.6 to 23.7
Lamina length Upto 10 cm. Upto 10 cm. Upto 10 cm.
Symmetrical Symmetrical
Shape: whole lamina Symmetrical
Symmetrical Generally symmetrical
Shape: base only Symmetrical
Oblong: narrow-oblong Ovate: ovate (l/w: 1.5:1) Ovate: lanceolate (l/w:3:1)
Form: whole lamina (l/w:
(l/w:3:1) Elliptic: length/width) narrow-ovate (l/w: 2:1)
narrow-elliptic (l/w:3:1) lanceolate (l/w: 3:1)
Acute normal Acute cuneate rounded to Acute cuneate obtuse
Form: base only
decurrent cordate
Form: apex only Acute, obtuse or obtuse Acute or obtuse Acute mucronate
Entire Entire
Margin Entire
Texture Chartaceous Chartaceous Chartaceous
Silvery grey–green (bicolor) Appearance Adaxial dark green (notable Adaxial light green (notable
bicolor) bicolor)
Attachment (petiole) Normal Normal Normal
Pinnate eucamptodromous
Type of venation Pinnate eucamptodromous Pinnate eucamptodromous
Moderate (1.25–2%) to stout Stout (2–4%) to massive Size of primary vein % vein Stout (2–4%)
(2–4%) (\4%)
width/leaf width
Course of primary vein Straight (some slightly Straight (some slightly curved) Straight curved)
Acute: narrow (B45°) to
Angle of divergence of Acute: narrow (B45°) to Acute: moderate (45–65°) to wide (65–80°)
moderate (45–65°) secondary veins moderate (45–65°)
Lower and upper secondary Uniform
Variation in angle of Lower and upper secondary
veins more obtuse than middle divergence of secondary veins more obtuse than
veins middle sets sets
Moderate
Course of secondary veins Curved Straight to curved
Pattern of tertiary veins Random reticulate Random reticulate Random reticulate
42.90b 29.37a
43.16b Adaxial epidermal thickness
(mm)
Adaxial epidermal outer wall 5.36a 5.02a 9.09b
thickness (mm)
18.18a
Abaxial epidermal thickness 20.31a 16.99a
(mm)
2.49a
2.72a 3.01a
Abaxial epidermal outer wall thickness (mm)
312c 247a
Stomata/mm2 270b
Guard cell length (mm) 16.96a 16.94a 17.02a
Guard cell width (mm) 10.10a 10.07a 10.11a
327b
Abaxial indumentum height 341b 257a
(mm)
Fig. 2. Cleared leaves ofE.angustifoliaand drawings of transverse sections. (a,b) Lower shade leaves; (c,d) medium half sun-exposed leaves; (e,f) upper sun-leaves. (For a,c,e, bar: 1 cm; for b,d,f, bar: 50mm).
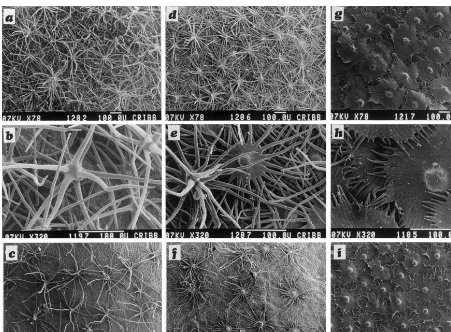
light green leaves from half exposed branches have several hair layers, the external ones com-posed of hairs totally branched or stellate with cells arranged radially and joined near the tri-chome stalk and the internal layer formed by peltate hairs with the cells of the shield partially joined. The rays of adjacent trichomes interlock and form a dense cover over the abaxial leaf surface (Fig. 4d,e). The silvery highly sun exposed leaves from the upper branches have only peltate hairs that, in the abaxial leaf surface, are arranged to form two or three layers of their flattened multicellular shields (Fig. 4g,h). Abaxial indumen-tum height is greater in the lower leaves (Table 3). All abaxial epidermal cells remain covered by the
indumentum. The same type of hairs are found in the respective adaxial epidermis of each type of analyzed leaf, but they are too sparse and, al-though their rays have a greater length and spread, they do not form a canopy and many epidermal cells remain exposed, especially in the two lower levels of leaves (Fig. 4c,f,i).
M.G.Klich/En6ironmental and Experimental Botany44 (2000) 171 – 183 178
Table 4
Comparison of leaf dimensions and tissue compositions ofE.
angustifolialeaves developed at different heights in a treea
ML UL
LL
0.326a 0.382b
Thickness (mm) 0.474c
(without hairs)
1132.00a,b
Area (mm2) 1250.08b 955.91a Volume (mm3) 407.53a 432.42a 450.82a % Mesophyll 73.97a 74.93a 81.86b
58.40a
60.23a 62.62a
% Palisade
41.60a
% Spongy 39.77a 37.38a
aLL, lower shade leaves (B1 m height); ML, medium leaves
(1–3 m height); UL, upper sun leaves (\5 m height). Values
are the mean for 250 measurements. For each row, values which have the same letter are not significantly different at the 0.05 level as determined by Student–Newman–Keuls’ test.
4. Discussion
The environmental measurements made during the study demonstrate the great variation in the habitat conditions to which the leaves at different levels are exposed. As the area is included in a semiarid to arid region, summer temperature out-side the shaded understorey reaches much higher values than under the canopy. Differences are also found in air humidity; while in the lower strata it reaches saturation every night, at the upper ex-posed canopy the values are always lower. The high solar radiation is greatly attenuated by the foliage in such a way that the lower leaves can really be considered as growing in the shade. As there is an exponential relationship between light absorption and cumulative foliage area, the light gradient across the canopy should be greater for higher values of incident irradiance (Niinemets, 1996b).
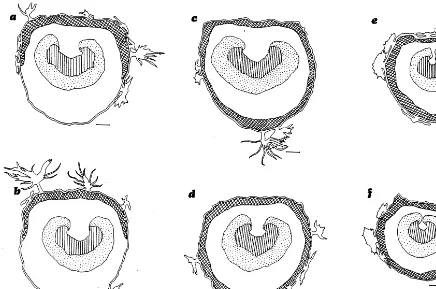
If the climatic data are considered in a general regional sense, it can be assumed that the scarcity of soil water resources and/or the high evaporative demand of the atmosphere during the warmer seasons induce stress situations. The survival of an open vascular arc and it becomes incurved to
the distal end. Though the vascular strand nearly forms a cylinder in the petioles of the upper leaves, the vascular arc remains slightly open (Fig. 5). Trichomes on petiole epidermis are in accord with those of the corresponding leaf.
Fig. 4. Scanning electron micrographs of the epidermis of E. angustifolia. (a,b,c) Lower shade leaves; (d,e,f) medium half sun-exposed leaves; (g,h,i) upper sun-leaves. (a,b,d,e,g,h) Abaxial epidermis; (c,f,i) adaxial epidermis.
the plants to adverse conditions requires long-and short-term plasticity responses long-and trees may develop stress avoidance mechanisms by an ade-quate canopy architecture (Save´ et al., 1995), root development (Ferna´ndez et al., 1988) or leaf het-erogeneity (Niinemets, 1996a). When light is the limiting factor, since trees have the potential to reach unshaded overstorey, the adaptability of crown architecture may be an important determi-nant of competitive strength than foliar plasticity (Niinemets, 1996b). However, when trees develop at different strata, the canopy of the same plant is exposed to diverse natural environments. Thus, the leaf morphoanatomical plasticity with respect to the combined conditions of incident irradiance, air temperature and humidity may be one of the
Table 5
Comparison of petiole tissue composition (%) ofE.angustifo
-lialeaves developed at different heights in a treea
ML UL
LL
10.04a 16.97b 23.90c Collenchyma
18.75b 16.44a
Phloem 16.30a
54.14c 48.58b 35.90a Parenchyma
7.20a 8.82b
Xylem 9.42c
9.94a 9.32a
Epidermis 14.30b
aLL, lower shade leaves (B1 m height); ML, medium leaves (1–3 m height); UL, upper sun leaves (\5 m height). Values
M.G.Klich/En6ironmental and Experimental Botany44 (2000) 171 – 183 180
Fig. 5. Transverse sections through the basal (a,c,e) and distal (b,d,f) end of the petioles ofE.angustifolia. (a,b) Lower shade leaves; (c,d) medium half sun-exposed leaves; (e,f) upper sun-leaves. (External white: epidermis; squared: collenchyma; internal white: parenchyma; dashed: xylem; dotted: phloem). (Bar: 150mm).
features that confers the species success in their permanence and even in their competition with other species.
The changes in the dimensions — especially the width — of the leaves of E. angustifolia, can be considered to have a functional significance re-lated to the different natural conditions in which they developed. The shaded as well as the half-ex-posed branches, with wider ovate leaves, develop in an environment of low irradiance, relatively high air humidity content and are protected from the wind. The upper narrow oblong or elliptic leaves are exposed to high light, low air humidity and dry winds. The reduction of leaf dimension generates an increase in convective heat dissipa-tion, that is important to counteract the negative effects of overheating and high transpiration rates (Gates, 1980) such as those to which the upper leaves ofE. angustifolia are exposed.
under storey ofE.angustifoliaclusters is neverthe-less greatly attenuated.
Bicolor is a common phenomena among species that occupy shaded habitats; the leaf side that faces away from the sun is lighter in color than the leaf surface facing towards the sun. The lighter surface may act as a reflective surface and enhance the participation of the spongy mesophyll in leaf pho-tosynthesis. Smith et al. (1997) suggest that the presence in shaded environments of thin, horizon-tal, bicolor laminar leaves that have stomata only in the abaxial epidermis may have evolved as a result of a selective pressure to enhance light capture while avoiding the detrimental effect of exposing the stomata directly to sunlight and minimizing transpirational water-loss. InE. angus-tifolia the difference in color between both leaf surfaces is especially evident in the two lower levels, but it also occurs in the upper level. As sun leaves have a silvery grey – green adaxial face and a brilliant silver colored abaxial one and as they upfold through the medium vein under the higher solar radiation conditions, they mainly expose this highly reflective surface.
Though there are some variations in the gross
morphology of the leaves of E. angustifolia at
different levels in a tree, the pattern of major venation is the same in all of them, which is logical, as the latter is a character considered of systematic value (Dilcher, 1974). Increased vein density is positively correlated with water stress in the habi-tat (Pyykko¨, 1966) and the higher areole density in
the upper leaves of E. angustifolia may be a
response to the dryer habitat to which they are submitted. The proportionally greater primary vein size of upper leaves means an additional mechanical advantage under those stress environ-mental conditions.
The use of only the size and morphology of the leaf as indicators for xeromorphy or plasticity is insufficient and anatomical features, such as the volume and organization of the mesophyll tissue, should be taken into consideration (Fahn and Cutler, 1992). The greater thickness of the upper sun E. angustifolia leaves is related to a higher proportion of mesophyllic tissue. These character-istics are mentioned as structural mechanisms that increase photosynthesis per unit leaf area and
enable a greater water-use efficiency. Leaves in the upper part of the canopy or sun leaves have higher rates of carbon assimilation and water loss and are thus, physiologically more active (Boardman, 1977). Leaves exposed to high irradiation condi-tions or sun leaves generally develop a well-defined palisade parenchyma and those that grow under low irradiation condition or shade leaves, are thinner and present a less defined palisade layer than the former (Vogelmann, 1993). The tissue distribution in the mesophyll remains constant among the studied E. angustifolialeaves, but the average diameter and length of palisade cells are smaller in the upper ones in which an incomplete third palisade stratum is a common feature. A higher number of palisade cells in the mesophyll volume may imply an increase in photosynthetic efficiency. The columnar cells of the palisade tis-sue, beside their contribution in the CO2exchange, may have also an optical function (Vogelman, 1989). The directionality of light can affect light gradients within leaves. Vogelman and Martin (1993) stated that palisade appears to facilitate the penetration of directional but not diffuse light. This is particularly important in leaves exposed to direct sunlight, where the irradiance is highly colli-mated.
The epidermis and cuticle strengthen the leaf. The epidermal thickness of the upper, more-ex-posed leaves of E. angustifolia is smaller when compared with the other leaves, but has a propor-tionally greater outer tangential wall (measured as wall plus cuticle) and this feature has been related to increased aridity and/or insolation (Pyykko¨, 1966; Fahn and Cutler, 1992). No differences were found in the size of stomata but their density increase from the lower to the upper leaves of E. angustifolia. Size and density of stomata have been widely studied and related to many environmental factors (Salibury, 1927; Shields, 1950; Klich et al., 1996a,b). Small stomata in large numbers seemed
to be characteristics of xeromorphic leaves
M.G.Klich/En6ironmental and Experimental Botany44 (2000) 171 – 183 182
Pubescence is another characteristic that con-fers stress resistance (Ehleringer, 1980; Klich et al., 1996b) and the presence of hairs on the aerial parts of the plants is regarded as an adaptation to arid conditions (Fahn, 1986). Hairs may affect transpiration by influencing the water diffusion boundary as an indumentum decreases air move-ments on the leaf surface, creates a zone of still air and reduces diffusion of water vapor from the leaf interior to the atmosphere. An indirect influence of trichomes on plant water economy is through temperature regulation (Ehleringer, 1980). The microroughness caused by anatomical characteris-tics, as epidermal cell surface contours and dense trichome layers, substantially increases leaf reflec-tance and reduces radiation absorption which re-sults in the reduction of leaf temperature and
consequently of leaf transpiration rates
(Premachandra et al., 1991, McWhorter, 1993; Karabourniotis et al., 1995; Klich et al., 1997).
The abaxial epidermis of all the leaves of E.
angustifolia is densely covered by hairs and al-though the indumentum height of the lower leaves is greater than that of the upper leaves, the tri-chomes of the latter, with their overlapping flat-tened shields, may constitute a stronger barrier to gas diffusion.
The lower leaves ofE.angustifoliaare the most sensitive to environmental changes and when soil water content decreases or when these leaves be-come exposed because of the removal of neigh-boring plants, the trees shed the leaves of the inferior branches (personal observations, unpub-lished data). Lower strata are also the first to shed their leaves at the end of the growth period, initiated by a decrease in their water content.
The higher proportion of epidermis and col-lenchyma of the upper leaf petioles is not unex-pected, as Pyykko¨ (1966) already confirmed a correlation between strong development of me-chanical tissue and habitat aridity.
Leaf variability inE.angustifoliacan be consid-ered an adaptive advantage of both upper and lower leaves in habitats marked by strong varia-tions of sun radiation, air humidity and tempera-ture and wind exposition. Upper leaves show many xeromorphic characters that enables the trees to maintain their canopy foliage even under
the unfavorable conditions — high solar radia-tion, high temperature, low humidity — during the summer. Lower leaves show many traits of shaded leaves and allow the plant to compete for space also in the understorey.
These data confirm the hypothesis that varia-tions in developmental responses ofE.angustifolia leaves to spatial heterogeneity are related to its ecological strategies. This invasive species relies, at least partially, on its foliar plasticity to over-come the environmental gradient between the lower and upper parts of a developed tree and even to compete against other species when it grows in places where spatial environment hetero-geneity can easily manifest, as in a river valley of a semiarid region.
Acknowledgements
I would like to thank Marı´a Elena Garcı´a and Lucrecia Gallego for their technical assistance and their help with the drawings; Federico and Luis Fresser for their help with field temperature and humidity measurements and the technical assis-tance of the Electron Microscopy Laboratory of the Centro Regional de Investigaciones Ba´sicas y Aplicadas de Bahı´a Blanca (CRIBABB), Ar-gentina. This research was supported by a grant from the Universidad Nacional del Sur, Bahı´a Blanca, Argentina
References
Boardman, N.K., 1977. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Phys. 28, 355 – 377. Dawson, J.O., 1990. Interactions among actinorhizal and
asso-ciated plant species. In: Schwintzer, C.R., Tjepkema, J.D. (Eds.), The Biology of Frankia and Actinorhizal Plants. Academic Press, New York, pp. 299 – 316.
Dilcher, D.L., 1974. Approaches to the identification of an-giosperm leaf remains. Bot. Rev. 40, 1 – 157.
Ehleringer, J., 1980. Leaf morphology and reflectance in rela-tion to water and temperature stress. In: Turner, N.C., Kramer, P.J. (Eds.), Adaptations of plants to water and high temperature stress. Wiley, New York, NY, pp. 295 – 308.
Fahn, A., Cutler, D.F., 1992. Xerophytes. Gebru¨der Born-traeger, Berlin. 180 pp.
Ferna´ndez, O.A., Montani, T., Distel, R.A., 1988. El sistema radical de especies de zonas a´ridas y semia´ridas. Algunas estrategias de supervivencia. Interciencia 13 (1), 25 – 30. Gates, D.M., 1980. Biophysical Ecology. Springer – Verlag,
New York, NY.
Hickey, L.J., 1974. Clasificacio´n de la arquitectura de las hojas de dicotiledo´neas. Bol. Soc. Arg. Bot. 16, 1 – 26.
Karabourniotis, G., Kotsabassidis, D., Manetas, Y., 1995. Trichome density and its protective potential against ultra-violet-B radiation damage during leaf development. Can. J. Bot. 73, 376 – 383.
Kaufmann, M.R., Troendle, C.A., 1981. The relationship of leaf area and foliage biomass to sapwood conducting area in four subalpine forest tree species. Forest Sci. 27, 477 – 482.
Klich, M.G., Didone´, N.G., Ferna´ndez, O.A., Mu´jica, M.B., 1996a. Response ofSpirodela intermedia W. Koch to os-motically induced water shortage. Aquat. Bot. 55, 107 – 114.
Klich, M.G., Mu´jica, M.B., Brevedan, R.E., 1996b. Effect of soil water deficit on the epidermal characters of some forage grasses. Biocell 20, 67 – 76.
Klich, M.G., Brevedan, R.E., Villamil, S.C., 1997. Leaf anatomy and ultrastructure of Poa ligularisafter defolia-tion and water stress. Proceedings of the 18th Internadefolia-tional Grassland Congress, Canada 1, 37 – 38.
Kubı´nova´, L., 1993. Recent stereological methods for the measurement of leaf anatomical characteristics: estimation of volume density, volume and surface area. J. Exp. Bot. 44, 165 – 173.
Lucchesini, M., Mensuali-Sodi, A., 1996. In vitro regeneration from different types of explants from Oleaster (Elaeagnus angustifoliaL.) seedlings. Gartenbauwissenschaft 61, 276 – 280.
McWhorter, C.G., 1993. Epicuticular wax on Johnsongrass (Sorghum halepense) leaves. Weed Sci. 41, 475 – 482. Meidner, H., Mamsfield, T.A., 1968. Physiology of stomata.
McGraw-Hill, UK. 179 pp.
Niinemets, U8., 1996a. Plant growth-form alters the relation-ship between foliar morphology and species shade-toler-ance ranking in temperate woody taxa. Vegetation 124, 145 – 153.
Niinemets, U8., 1996b. Changes in foliage distribution with relative irradiance and tree size: differences between the sapling ofAcer platanoidesandQuercus robur. Ecol. Res. 11, 269 – 281.
Niinemets, U8., Kull, K., 1994. Leaf weight per area and leaf size of 85 Estonian woody species in relation to shade tolerance and light availability. Forest Ecol. Manage. 70, 1 – 10.
Novoplansky, A., 1996. Developmental responses of individ-ualOnobrychisplants to spatial heterogeneity. Vegetation 127, 31 – 39.
Premachandra, G.S., Saneoka, H., Hanaya, M., Ogata, S., 1991. Cell membrane stability and leaf surface wax content as affected by increasing water deficit in Maize. J. Exp. Bot. 12, 167 – 171.
Pyykko¨, M., 1966. The leaf anatomy of east Patagonian xeromorphic plants. Ann. Bot. Fenn. 3, 453 – 622. Salibury, E.J., 1927. On the causes and ecological significance
of stomatal frequency with special reference to the wood-land flora. Roy. Soc. (Lond.) Phil. Trans. B. 216, 1 – 65. Save´, R., Biel, C., Domingo, R., Ruiz-Sa´nchez, M.C.,
Tor-recillas, A., 1995. Some physiological and morphological characteristics of citrus plants to drought resistance. Plant Sci. 11, 167 – 172.
Shafroth, P.B., Auble, G.T., Scott, M.L., 1995. Germination and establishment of native plains cottonwood (Populus deltoidesMarshall subsp. monilifera) and the exotic Rus-sian-olive (Elaeagnus angustifolia L.). Conserv. Biol. 9, 1169 – 1175.
Shields, L.M., 1950. Leaf xeromorphy as related to physiolog-ical and structural influences. Bot. Rev. 16, 399 – 447. Smith, W.K., Vogelman, T.C., DeLucia, E.H., Bell, D.T.,
Shepherd, K.A., 1997. Leaf form and photosynthesis. Bio-Science 47, 785 – 793.
Vogelman, T.C., 1989. Penetration of light into plants. Pho-tochem. Photobiol. 50, 895 – 902.
Vogelmann, T.C., 1993. Plant tissue optics. Annu. Rev. Plant Phys. 44, 231 – 251.
Vogelman, T.C., Martin, G., 1993. The functional significance of palisade tissue: penetration of directional versus diffuse light. Plant Cell Environ. 16, 65 – 72.
Yamada, T., Suzuki, E., 1996. Ontogenic change in leaf shape and crown form of a tropical tree,Scaphium macropodum
(Sterculiaceae) in Borneo. J. Plant Res. 109, 211 – 217.