www.elsevier.com / locate / bres
Research report
Anatomical evidence for two spinal ‘afferent–interneuron–efferent’
reflex pathways involved in micturition in the rat: a ‘pelvic nerve’
reflex pathway and a ‘sacrolumbar intersegmental’ reflex pathway
a,b ,
*
a,b,cPedro L. Vera
, Irving Nadelhaft
a
Veteran’s Administration Medical Center, R&D Service (151), Bay Pines, FL 33744, USA
b
Department of Surgery, Urology Division, University of South Florida, Tampa, FL, USA
c
Department of Pharmacology, University of South Florida, Tampa, FL, USA
Accepted 18 July 2000
Abstract
We labeled interneurons in the L1–L2 and L6–S1 spinal cord segments of the rat that are involved in bladder innervation using transneuronal retrograde transport of pseudorabies virus (PRV) in normal animals and in animals with selected nerve transections. Preganglionic neurons were identified using antisera against choline acetyltransferase (ChAT). In some experiments we labelled parasympathetic preganglionic neurons (PPNs) in the L6–S1 spinal cord by retrograde transport of Fluorogold from the major pelvic ganglion. We identified bladder afferent terminals using the transganglionic transport of the anterograde tracer cholera toxin subunit b. We present anatomical evidence for two spinal pathways involved in innervation of the bladder. First, in the intact rat, afferent information from the bladder connects, via interneurons in L6–S1, to the PPNs that provide the efferent innervation of the bladder. The afferent terminals were located mainly in close apposition to interneurons located dorsal to the retrogradely labeled PPNs. Second, using L6–S1 ganglionectomies or L6–S1 ventral root rhizotomies we limited viral transport to the sympathetic pathways innervating the bladder. This procedure also labelled interneurons (but not PPNs) with PRV in the L6–S1 spinal cord in a location very similar to those described in the intact rat. These interneurons also receive bladder afferent terminals but we propose that they project to sympathetic preganglionic neurons, most of which are in the L1–L2 spinal segments. Based on this anatomical evidence, we propose the existence of two spinal reflex pathways involved in micturition: a pathway limited to a reflex arc in the pelvic nerve (presumably excitatory to the detrusor muscle); and a pathway involving the pelvic nerve and sympathetic nerve fibers, some of which may travel in the hypogastric (presumably inhibitory to the detrusor muscle). 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Somatic and visceral afferents
Keywords: Pseudorabies virus; Cholera toxin; Fluorogold; Acetylcholine transferase; Spinal interneuron; Bladder afferent
1. Introduction fibers within the pelvic nerve to the lumbosacral spinal cord. These fibers may synapse on interneurons that are Micturition is a complex behavior requiring the coordi- part of spinal and supraspinal pathways to relay this nated activity of the autonomic and somatic nervous information to spinal and higher brain centers leading to systems, both at the central and peripheral levels. In- awareness and appropriate voiding by activating descend-formation concerning bladder fullness travels via afferent ing parasympathetic pathways. The spinal organization of neurons involved in the micturition reflex has been studied extensively in the cat and the rat (for a recent review, see
*Corresponding author. Tel.:11-727-398-6661, ext. 4010; fax: 1
1-de Groat [6]). Anatomical studies have examined the
727-398-9457.
E-mail address: [email protected] (P.L. Vera). central termination pattern of afferent fibers traveling in
the pelvic nerve in the rat [17] and, more specifically, the sympathetic chain or the major pelvic ganglia to provide spinal distribution afferent fibers that innervate the bladder sympathetic control of the detrusor activity.
of the rat [22]. The locations of lumbosacral parasympa-thetic preganglionic neurons (PPNs) with axons in the
pelvic nerve and the sympathetic preganglionic neurons 2. Materials and methods (SPNs) with axons in the hypogastric nerve have been well
described [17,18,21]. Finally, previous experiments in the 2.1. Experiment I. Labeling of spinal cord neurons with rat using transneuronal labeling from PRV injections into PRV injections into the bladder in animals with L6 /S1 the bladder combined with retrograde labeling using a ganglionectomies
fluorescent tracer [19] showed a distinct group of
inter-neuronal cells in the L6 and S1 segments of the spinal Sprague–Dawley female rats (n58; Harlan, In-cord. These interneuronal cells were located close to the dianapolis, IN, average weight: 228 g) were anesthetized PPNs in the intermediolateral cell column (IML). The (sodium pentobarbital;40 mg / kg; i.p.) and a dorsal interneurons differed from the PPNs in three respects: (1) laminectomy exposed the L6–S1 dorsal root ganglia. they were usually located dorsal to the PPNs, (2) they These were crushed and transected to ensure their com-were smaller than the PPNs and (3) they com-were not plete disconnection from the spinal cord. The muscle was
cholinergic. closed (4-0 silk), the skin incision was closed with wound
The location of these interneurons is interesting since clips and a topical antibiotic ointment (Bacitracin zinc and several lines of anatomical and electrophysiological evi- Polymyxin B sulfate; Fougera) was applied to the skin dence point to the possible involvement of spinal inter- incision. The animal was then laid supine and an abdomi-neurons in the micturition process. Afferent fiber terminals nal incision was made to expose the urinary bladder. Two from the pelvic nerve of the rat [11] appear to surround a injections, 2ml each, of pseudorabies virus (Bartha strain,
8
similarly located group of interneurons. Furthermore, 2.6310 pfu / ml) were made, at different points, into the bladder afferent terminal fibers in the rat can be seen in the detrusor muscle. This amount of PRV injected was chosen vicinity of the IML [17,22]. Therefore, previous ana- according to the results of previous experiments that tomical investigations suggest that these interneurons may showed effective infections from such injections [19]. The receive bladder afferent terminals. In addition, electro- incision was closed with sutures and clips and the animal physiological studies of micturition reflexes in the rat returned to its cage. A heating pad at low heat was placed reported a spinal reflex present in about 15% of the intact beneath the cage to keep the animal warm until it regained rats [16] but in 100% of the spinally transected rats, which consciousness. The bladders were expressed manually was of considerably shorter duration than the spinal– twice daily until the animals were sacrificed.
supraspinal micturition reflex. Finally, studies in the cat Animals were killed at 3 (n52), 4 (n53) and 5 (n53) have shown that the sympathetic nervous system acts to days postinfection. Under pentobarbital anesthesia the inhibit detrusor activity presumably through an interseg- animal was perfused transcardially with cold Krebs
solu-mental lumbosacral spinal pathway [7,8]. tion followed by cold 4% paraformaldehyde. The
transec-Therefore, although several lines of anatomical and tions were verified post-mortem as the spinal cord and physiological studies pointed to the existence of the spinal brain were removed. Selected spinal segments were dissec-and the intersegmental lumbosacral spinal reflex pathways, ted and post-fixed for several hours and then placed in no conclusive anatomical evidence had been presented to 25% sucrose–phosphate buffer for cryoprotection. The correlate with the physiological and electrophysiological L6 / S1 and L1 / L2 segments were chosen since they findings. In this report, we extend our previous anatomical represent the origin of the preganglionic fibers in the pelvic findings by reporting that presumed interneurons in the [17] and hypogastric nerves [18], respectively.
ex-amined under epifluorescent illumination. Maps of sections 2.3. Experiment III. Labeling of bladder afferent (Minnesota Datametrics, Shoreview, MN) showed the terminals projecting to presumptive bladder interneurons locations of Pseudorabies virus immunoreactive (PRV-IR), following L6 –S1 ventral root rhizotomy
choline acetyl transferase immunoreactive (ChAT-IR) or
double-labeled neurons. Sprague–Dawley male rats (260–280 g) were
anes-thetized with sodium pentobarbital (40 mg / kg; i.p.) and a dorsal laminectomy exposed the conus medullaris and the 2.2. Experiment 2. Labeling of bladder afferent fibers, L6 and S1 dorsal root ganglia. The dura was opened and lumbosacral parasympathetic neurons and spinal the L6 and S1 ventral roots were identified and cut.
interneurons in intact animals Gelfoam (Upjohn; Kalamazoo, MI) was placed over the
conus medullaris, the muscle incision was sutured using Sprague–Dawley male rats (n54; 280–380 g) were 4-0 silk and the skin incision was closed using wound anesthetized with halothane and a ventral abdominal clips. The animal was then placed on a supine position and incision was made to expose the bladder. A total of 10ml an incision was made to expose the bladder. The bladder of 1% Ctb (List Biological; Campbell, CA) was injected was injected with Ctb and with PRV as already described into the bladder detrusor muscle in several injections to in Section 2.1. The abdominal muscle and skin incisions label the bladder afferent fibers. In addition, the major were closed as described previously. The animals were pelvic ganglion (MPG) was exposed bilaterally using blunt allowed to recover from the anesthesia and their bladders dissection and Fluorogold (1–2 ml; 1% in dH O) was2 were expressed manually twice daily.
injected into each ganglion using a hand-held glass mi- Animals were reanesthetized with sodium pentobarbital cropipette in order to label the parasympathetic neurons (80 mg / kg; i.p.) 96 h after the initial procedure and projecting to the MPG. Finally, pseudorabies virus (Bartha perfused transcardially as previously described. The sur-strain; two injections, 2 ml each) was injected into the vival time of 4 days was based on the results from the bladder muscle in order to label, transneuronally, the spinal L6–S1 ganglionectomy experiment (I above), which did interneurons involved in the micturition pathway. The not reveal any PRV-labeled neurons in the L6–S1 spinal incisions were closed with sutures and clips as already cord segments until 4 days postinfection. The ventral root described and a topical antibiotic ointment (Bacitracin zinc rhizotomies were confirmed post mortem by careful dissec-and Polymyxin B sulfate; Fougera) was applied to the skin tion of the L6–S1 DRG and dorsal and ventral roots. All incision. The animals were allowed to recover from the animals showed bilateral section of the L6 and S1 ventral
halothane anesthesia and returned to their cages. roots. The L1–L2 and L6–S1 spinal segments were
The rats were reanesthetized with sodium pentobarbital identified and removed. Coronal sections (20 mm) of the 72 h after the injections and perfused transcardially as L6–S1 spinal segments were processed for: (1) PRV and described above. The survival time was chosen based on Ctb immunohistochemistry as outlined above; (2) coronal previous experiments [19] that showed the first labeling of sections (20 mm) of the L6–S1 and L1–L2 spinal seg-presumptive interneurons in the IML region at 3 days ments were processed for PRV immunohistochemistry and postinfection with PRV. The spinal cord and brain were ChAT as described in Expt. I.
removed and spinal cord segments L6–S1 were identified and placed in fixative overnight at 48C followed by a 25% sucrose–phosphate buffer solution.
A series (1 / 6 for transverse and 1 / 2 for horizontal) of
3. Results transverse (n53) or horizontal (n51) 20-mm sections of
the L6 and S1 segments were processed for
immuno-3.1. PRV-IR and ChAT-IR neurons in spinal segments histochemistry as follows. The sections were rinsed with
L6 /S1 and L1 /L2 in rats with L6 /S1 ganglionectomies 0.1 M phosphate-buffered saline (PBS) and placed in PBS
containing 3% normal donkey serum. The sections were
then exposed to the following primary antisera: Goat Lumbosacral spinal cord neurons were found that were anti-cholera toxin b (List Biological; Campbell, CA; pseudorabies virus-immunoreactive (PRV-IR), choline 1:2000) and rabbit anti-PRV (generously donated by L. acetyltransferase-immunoreactive (ChAT-IR) or were Enquist; 1:10 000;) in PBS containing 0.3% Triton X-100 doubly labeled. The analysis was restricted to neurons in and 1% normal donkey serum overnight at 48C. After the intermediolateral cell nucleus (IML), the dorsal gray rinsing in PBS, the sections were exposed to the secondary commissure (DGC) and the dorsal horns. Motor neurons in antisera as described in Expt. 1. Sections were mounted on the ventral horn (showing ChAT-IR but not PRV-IR) were gelatinized slides, coverslipped with a fade-retardant noted but excluded from this examination although they medium [23] and examined under epifluorescent illumina- demonstrate restriction of viral infection to autonomic
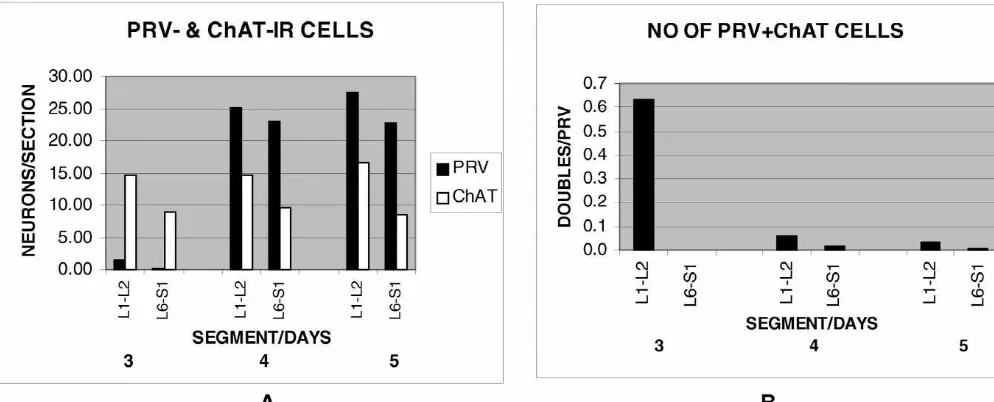
3.1.1. Three days postinfection segments. In addition, a few doubly labeled neurons were At 3 days postinfection there were very few PRV-IR usually found in each tissue section.
neurons in the L6–S1 segments (Fig. 1A) and none of In general, the numbers of PRV-IR neurons increased as these were located in the intermediolateral areas (IML) the postinfection incubation time increased whereas the where preganglionic neurons are found. A representative numbers of ChAT-IR neurons remained constant (Fig. 3A). sample of 43 sections (16% of the total number) contained In particular, at 3 days postinfection, there were very few 13 PRV-labeled neurons all of which were small and none PRV-IR neurons in either the L1–L2 or the L6–S1 were also cholinergic. On the other hand, the IML con- segments. On the other hand, at 4 and 5 days postinfection, tained many ChAT-IR neurons (but not PRV-IR) whose both of these pairs of spinal cord segments contained a size and location identified them as preganglionic cells. comparatively large number of PRV-IR neurons. The Larger ChAT-IR motor neurons were found in the ventral frequency of doubly labeled neurons as a function of time horns and smaller ones in lamina X around the central are illustrated in Fig. 3B. At 3 days postinfection only the canal, however these neurons were not PRV-IR and are not L1–L2 segments contained doubly labeled (SPNs that also
included in this analysis. showed PRV-IR) cells and there were none in the L6–S1
In the L1–L2 cord (Fig. 1B), many medium-size segments. At later times, the ratio of doubly labeled neurons showing both PRV-IR and ChAT-IR were located neurons to PRV-IR only neurons, dropped significantly in in the dorsal gray commissure region (DGC) and in the the L1–L2 segments due to the increased numbers of IML, suggesting that they were sympathetic preganglionic PRV-IR neurons. The numbers of doubly labeled neurons neurons. A representative sample of 48 sections (17% of in the L6–S1 segments increased slightly.
the total number) contained 97 PRV-IR neurons of which
67% were also cholinergic (Fig. 3B). Examination of 3.2. Anatomical evidence for a spinal pathway double-labeled neurons in more rostral segments showed consisting of bladder afferent terminals, interneurons only a few cells in T13 and T12 and none rostral to those and lumbosacral parasympathetic neurons in the intact segments. No double-labeled neurons were observed in L3. rat
By far the largest number of these neurons were located in
L1–L2. The photographs of afferent terminals, PRV-IR neurons
and fluorogold-labeled preganglionic neurons shown in Fig. 4 were all taken from the same section and without
3.1.2. Four and 5 days postinfection repositioning of the slide.
There was a large increase of PRV-IR neurons in the
L6–S1 segments (Fig. 1C) and these were located in the 3.2.1. Location of bladder afferent terminals within the dorsal half of the spinal cord. In particular, a group of lumbosacral cord
these were commonly seen to lie just dorsal to the Bladder afferent terminals labeled with cholera toxin (b preganglionic ChAT-IR neurons in the IML. Additionally, subunit) were located in the superficial layers of the dorsal a few PRV-IR neurons (not ChAT-IR) were always located horn and along the side of the dorsal horn (lateral pathway in the superficial layers of the dorsal horns. A map of a [17]) of the L6 and S1 spinal segments (Fig. 4A). These typical section taken from a five-day animal demonstrating afferent fibers appeared to cluster in the rostrocaudal the relative locations of PRV-IR neurons and ChAT-IR direction at the lateral edge of the dorsal horn.
neurons and photomicrographs of these two groups are
shown in Fig. 2. It can be clearly seen that the group of 3.2.2. Location of parasympathetic preganglionic PRV-IR neurons which are not ChAT-IR (most likely neurons in the lumbosacral cord
interneurons) lies dorsal to the cholinergic preganglionic Neurons retrogradely labeled with FG were located in
neurons of the ChAT-IR group. the intermediolateral nucleus area, in lamina VII at the
In L1–L2 segments (Fig. 1D) there was also a large edge of the gray matter (Fig. 4C). These neurons often increase of PRV-IR neurons and these were also located in were oriented in the mediolateral direction with dendrites the dorsal half of the spinal cord. However the distribution extending medially, as described by others [17]. Some of of these neurons was more spread out than in the L6–S1 these neurons also showed immunoreactivity for PRV. A
Fig. 1. Representative distributions of PRV-IR (green3) and ChAT-IR (red1) neurons in the L6–S1 (A,C) and L1–L2 (B,D) spinal cord segments at 3 days postinfection (A,B) and at 4 days postinfection (C,D). Note the relative scarcity of PRV-IR neurons at 3 days postinfection and the large increase of these neurons 4 days postinfection. Doubly labeled neurons (blue *) are present only in the L1–L2 segments (B,D). ChAT-IR motor neurons can be seen in the ventral horns. Calibration bar: 0.5 mm.
Fig. 3. PRV- and ChAT-IR neurons in the L1–L2 and L6–S1 spinal cord segments as a function of time after L6–S1 bilateral ganglionectomy. (A) Solid bars are PRV-labeled neurons and open bars are ChAT-labeled neurons. Note the absence of significant numbers of PRV-IR neurons in both areas 3 days postinfection and the large increase at 4 and 5 days postinfection. The numbers of ChAT-labeled neurons remains constant throughout the time period since their numbers are not affected by viral infection. (B) Doubly labeled neurons expressed in terms of the numbers of PRV-labeled cells. The number of doubles is high only in L1–L2 at 3 days postinfection because at that time most of the PRV-IR neurons are preganglionic neurons. At later times most of the PRV-IR neurons are interneurons and therefore not cholinergic and the ratio of doubles to PRV-IR neurons falls considerably. In L6–S1 there always were very few labeled preganglionics and there was a large increase in PRV-IR interneurons and so the number of doubles remains very small.
few Ctb-labeled terminal fibers were sometimes observed transported retrogradely from the MPG (Fig. 4C). In in close proximity to PPNs. No PPNs were observed to be addition, other presumed interneurons (PRV-labeled neu-doubly labeled with Ctb and FG, suggesting that the Ctb rons not retrogradely labeled with Fluorogold) were found injections were restricted to the bladder and did not leak dorsal to the central canal in the dorsal gray commissure,
onto the MPGs. as well as interspersed throughout laminae I–VI.
3.2.3. Location of presumptive bladder interneurons in 3.3. Afferent terminals located on presumptive bladder
the lumbosacral cord interneurons in the lumbosacral cord projecting to the
PRV-IR neurons that were not retrogradely labeled with lumbar sympathetic preganglionic neurons Fluorogold were observed interspersed with PPNs in the
IML region, but most were located dorsal to the PPNs, at These results are depicted in Fig. 5. In this figure as in the lateral edge of the gray matter (Fig. 4A). Most of the Fig. 4, the pair of micrographs A–B and the pair C–D dense afferent terminal labeling observed in or dorsal to were taken from the same section without moving the slide the IML nucleus was observed to surround these presump- between photographs. In this last group of experiments tive interneurons (Fig. 4A). These interneurons (presumab- where the L6 and S1 ventral roots had been cut prior to the ly part of the spinal pathway innervating the bladder) were application of PRV to the bladder (preventing PPNs in clearly different from the sacral parasympathetic neurons, L6–S1 from being labeled with virus), afferent terminal which were also labeled with PRV and with Fluorogold labeling was similar to that observed in intact animals.
Fig. 4. Anatomical evidence for a ‘pelvic reflex arc’ in the intact rat. All three micrographs were taken from the same section without moving the slide. Therefore the relative positions of the objects within each of the three photographs can be directly compared. (A) Bladder afferent terminals anterogradely labeled with cholera toxin terminate in the IML region of the L6–S1 spinal cord. (B) Pseudorabies virus-labeled cells in the IML region of the L6–S1 spinal cord. (C) Retrogradely labeled parasympathetic preganglionic neurons (PPNs) in the IML region of the L6–S1 spinal cord. Note the medially oriented dendrites of the PPNs labeled both with Fluorogold (c) and with PRV (b). PRV-labeled cells that do not also show FG labeling appear mainly dorsal to the PPNs. The afferent terminals appear in close apposition to the interneurons in the IML region (A). A drawing of the same section depicted in panels A–C is presented for orientation purposes. The afferent labeling is depicted by the red line in the IML region. PRV-immunoreactive cells are marked with a green3whereas retrogradely labeled (Fluorogold) PPNs are marked with a blue1. Calibration bar: 200mm.
Ctb-labeled fibers were observed in the dorsal horn and IML at L1 / L2 segments in all three experiments, they near the IML area (Fig. 5A–C). PRV neurons were still were only found in the IML at L6 / S1 in the intact rats. observed in the L6 and S1 segments, and especially dorsal Therefore, it is likely that these neurons represent sympa-to the IML region where they came in close apposition sympa-to thetic preganglionic neurons (SPNs; at L1 / L2) and para-the afferent terminal fields (Fig. 5C,D). Double immuno- sympathetic preganglionic neurons (PPNs; at L6 / S1) that staining for PRV and ChAT showed that none of these form part of the sympathetic and parasympathetic innerva-neurons that showed PRV immunoreactivity also showed tion of the bladder. The distribution of SPNs traveling in ChAT immunoreactivity. Therefore, these experiments (as the hypogastric [18] and PPNs projecting in the pelvic [17] in Expt. I) showed two groups of cells in the IML. (1) A nerves have already been described and the present results dorsal group composed of smaller cells that were PRV-IR agree with earlier findings in terms of location, distribution but not ChAT-IR and that received most of the bladder and size of these neurons.
afferent terminals. We presume these neurons to be inter- In addition, a group of PRV-IR but not ChAT-IR neurons. (2) Larger ChAT-IR cells that were seen in the neurons were also observed in the L1 / L2 and L6 / S1 IML area but mostly ventral to the first group and these segments in all three experiments. We propose that these were not PRV-IR. Some bladder afferent terminal fields neurons are interneurons and that they are probably were also seen in this group. We presume these neurons are retrogradely labeled from the labeled SPNs and PPNs sacral parasympathetic preganglionic neurons with axons described earlier. Anatomical evidence for interneurons in in the pelvic nerve, some of which are part of the the L6 / S1 spinal segments and their possible involvement parasympathetic innervation of the bladder (through their in bladder function is available from several earlier studies.
projections to the MPG). Previous PRV injections into the bladder revealed a similar
location of interneurons in the IML [19]. Likewise, wheatgerm agglutinin injected into the bladder trans-synaptically labeled small neurons in the IML and DGC of 4. Discussion
the rat [11]. Also, terminal fields arising from HRP-labeled afferent fibers in the pelvic nerve were shown to terminate Pseudorabies virus is a neurotropic virus that is
trans-in the same areas as described trans-in the present study [15,17]. ported transneuronally in a retrograde direction [5] while
Finally, using the proto-oncogene marker, c-fos, a similar maintaining circuit specificity as shown by studies
examin-group of interneurons in or near the IML region has been ing different organ systems [12,20,24]. An important and
described in the rat and cat after noxious and innocuous crucial feature of the pseudorabies virus compared to other
stimulation of the bladder [2,10,15]. All these studies methods for neuroanatomical tracing such as fluorescent
suggest that these interneurons may function as secondary dyes or horseradish peroxidase, is that it is transmitted
neurons in the bladder afferent pathway of the micturition from an infected neuron to other neurons only via their
reflex. synaptic connections thus defining the specific pathways
We observed the presumptive interneurons at L6 / S1 in involved in neuron to neuron signaling [4]. Previous
intact animals and in animals whose parasympathetic studies have shown that the Bartha strain of PRV (used in
innervation of the bladder had been surgically abolished this study) travels in the anterograde either very poorly or
(L6 / S1 ganglionectomies or ventral root rhizotomies). at a much slower rate than the retrograde direction when
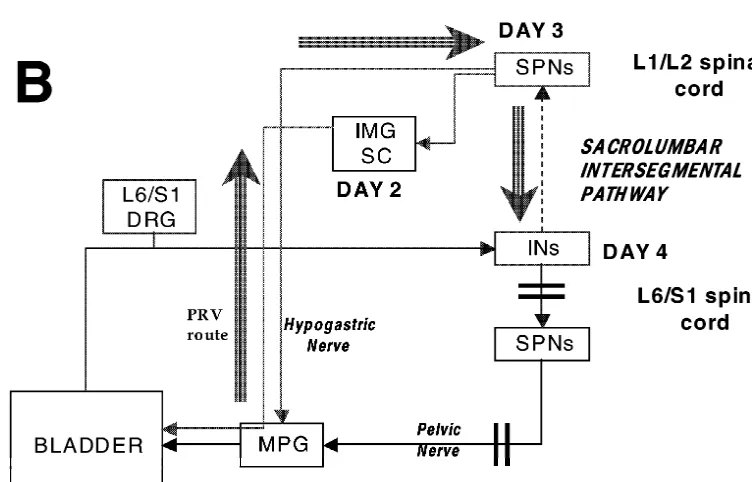
Therefore, we propose two routes for retrograde labeling of compared to the wild strain [25]. Therefore, anterograde
interneurons in the L6 / S1 segments (Fig. 6). In either travel of PRV in the L6 and S1 dorsal roots (or from the
case, these interneurons received bladder afferent terminals L1 and L2 dorsal roots) is an unlikely source of PRV
as seen with the Ctb injections into the bladder. We suggest infection of interneurons in the lumbosacral cord at the
that by combining PRV retrograde labeling of interneurons, survival times employed in the present experiments.
selective surgical procedures to isolate the sympathetic innervation of the bladder, and anterograde labeling of 4.1. Retrograde transneuronal labeling of preganglionic bladder afferent terminals in the L6 / S1 spinal cord, we
neurons and interneurons with PRV present evidence for two spinal reflex pathways involved
4.2. Anatomical evidence for bladder afferent terminals reactivity for the substance P receptor. Therefore, these participating in two possible spinal reflex pathways neurons might receive substance P afferent terminals and
involved in micturition might be involved in bladder nociception. Finally, in
chronic spinal rats (6 weeks post-surgery) transurethral Fig. 6 summarizes the two proposed routes of retrograde catheterization and isovolumetric bladder distension for 2 h labeling of interneurons in the L6–S1 spinal cord from results in an increase in the number of c-fos-positive cells injections of PRV into the bladder, as observed in the in the L6 and S1 cord, predominantly over the lateral present experiments, along with a proposed timeline. dorsal horn and the IML area, when compared to controls. Presumably this is due to activation of second order 4.2.1. Retrograde transport of PRV and anterograde neurons from bladder stimulation, and perhaps represent-transport of Ctb in the pelvic nerves: The ‘pelvic nerve ing spinal re-organization following injury [3]. Therefore,
reflex pathway’ the ‘pelvic nerve pathway’ described in these experiments
In intact animals that received PRV injections into the might be present during normal micturition, but become bladder, FG into the MPG and Ctb into the bladder, at 3 dominant during pathological conditions such as irritation days postinfection there were PRV-IR and FG-labeled or spinal cord injury
neurons in the IML at the L6 / S1 segments (presumably
PPNs traveling in the pelvic nerve). In addition, there were 4.2.2. Sympathetic retrograde transport of PRV and neurons that were PRV-IR but not FG-labeled (presumably anterograde transport of Ctb in the pelvic nerves: the interneurons) in the dorsal half of the spinal cord. Most of ‘sacrolumbar intersegmental reflex pathway’
them were located just dorsal to the PPNs in the IML, but There were no PRV-IR neurons in the L6–S1 spinal some were also seen in the dorsal gray commissure and the segments 3 days postinfection in animals that had under-superficial laminae of the dorsal horns. Dense terminal gone L6–S1 ganglionectomies as was expected since the labeling from afferent fibers was seen around the inter- ganglionectomies effectively interrupted the parasympa-neurons in the IML. Since an earlier study [19] showed thetic innervation of the bladder (via the pelvic nerves). that at 2 days postinfection only PPNs are labeled in The L1–L2 segments, however, displayed the normal L6 / S1 from PRV injections into the bladder and the pattern of PRV-IR neurons and the numbers of PRV-IR / earliest interneuronal labeling is seen at 2.5–3 days ChAT-IR neurons in those segments were comparable to postinfection, we propose that the interneurons observed in those found previously using horseradish peroxidase ap-the present study were probably retrogradely labeled from plied to the hypogastric nerve [18]. Therefore, at 3 days PPNs in the intermediolateral nucleus at L6 / S1. If so, postinfection only SPNs in the L1 / L2 cord that innervate since the bladder afferent terminals travel in the pelvic the bladder via the hypogastric nerve or by connections to nerve and have terminal fields that are in close apposition the lumbar sympathetic chain were double-labeled. to these interneurons (present study) it appears that the At 4 and 5 days postinfection the double-labeled neu-afferent and the efferent arms of this pathway are restricted rons were still detected. However, a large number of to the pelvic nerve. We term this ‘the pelvic nerve reflex PRV-IR neurons, which were not cholinergic, appeared in
arc’ (Fig. 6A). both the L1–L2 and L6–S1 segments. These neurons were
We outline the following timetable for retrograde trans- restricted to the dorsal half of the cord and located in the port of these interneurons with PRV and the anterograde superficial dorsal horn (lamina I), the dorsal gray commis-labeling of bladder afferent terminals in L6 / S1 in the sure, and in the intermediolateral area just dorsal to the intact rat (Fig. 6A). Day1 postinfection: injection into the preganglionic neurons in the sacral parasympathetic nu-bladder labels post-ganglionic neurons in the major pelvic cleus [17]. In addition, in animals with ventral root ganglia (MPG); day2 postinfection: transneuronal labeling rhizotomies that received PRV and Ctb injections into the of parasympathetic preganglionic neurons (PPNs) in the bladder, a similar pattern of interneuronal labeling was L6–S1 spinal cord that project to the MPG via the pelvic observed and these interneurons in the IML region re-nerves; day3 postinfection: transneuronal retrograde label- ceived a dense terminal field innervation from the bladder, ing in interneurons in the L6–S1 cord that project to PPNs. as was observed in the intact animals.
The afferent information from the bladder also travels in The presence of interneurons in the L1–L2 segments the pelvic nerve to terminate at the L6 and S1 segments. was not surprising as this occurred in the normal non-Electrophysiological studies in the rat [16] described ganglionectomized animal as reported earlier [20]. How-both a ‘long’ spinal-supraspinal electrophysiological ever, in the L6–S1 segments of animals with gang-micturition reflex in anesthetized rats, and also a ‘short’ lionectomies or ventral root rhizotomies, these neurons spinal reflex in approximately 15% of the rats but present could not have been labeled retrogradely from neurons in
¨
days postinfection. This finding suggests the existence of cephalon having cutaneous receptive fields and also re-an intraspinal pathway from L6–S1 interneurons to SPNs sponding to visceral stimulation (colon and vaginal disten-(some of which are located in the L1–L2 spinal segment sion). These various studies all point to a group of and project to the bladder via the hypogastric nerves). interneurons similar to those described in the present Interneurons in this pathway also receive bladder afferent study. Although there is no anatomical evidence for this, terminals as described for the ‘pelvic nerve reflex arc’ given that the area in and around IML receives dense
above. bladder afferent termination (this study and Ref. [22]), it is
We outline the following timetable for sympathetic possible that some of these interneurons also receive retrograde transport of these interneurons in L6 / S1 with bladder afferent terminals and project to these areas. While PRV and the anterograde labeling of bladder afferent spinal transections were not conducted in the present series terminals via the pelvic nerves in animals with L6 / S1 of experiments, we consider it unlikely that such inter-ventral root rhizotomies as follows (Fig. 6B). Day 1 neurons were labeled retrogradely with PRV from sup-postinfection: injection in the bladder labels post-gan- raspinal structures since the survival times (3 days in intact glionic sympathetic neurons in the MPG, IMG and the and 4 days in L6 / S1 ganglionectomy experiments) were sympathetic chain; day 3 postinfection: transneuronal chosen to restrict the infection to the spinal cord area or labeling of sympathetic preganglionic neurons that travel the first appearance in the brainstem.
in the hypogastric nerve or in the lumbar sympathetic
chain; day 4 postinfection: transneuronal labeling of 4.3. Concluding remarks interneurons in the L6–S1 and L1–L2 cord that project to
L1–L2 SPNs. The bladder afferent information is also The present findings of interneurons in the L6–S1 IML carried by the pelvic nerve in this pathway, but the area that receive bladder afferents and that either influence interneurons project to the L1 and L2 spinal cord segments the bladder by acting on parasympathetic preganglionic to innervate the sympathetic preganglionic neurons that neurons (‘pelvic nerve pathway’) or relay the bladder
eventually influence bladder function. information to lumbar sympathetic preganglionic neurons
Electrophysiological studies in the cat [7,8] showed that (‘sacrolumbar intersegmental pathway’) conform to previ-excitation of afferent fibers (either from isovolumetric ous anatomical and electrophysiological findings, as re-contractions of the bladder or electrical stimulation of the viewed above. The present study does not resolve the issue pelvic nerves) traveling in the pelvic nerve of the cat result of whether the interneurons are ‘pathway specific’ or in excitation of lumbar SPNs. Therefore, they suggested whether there is just one set of interneurons that serves the presence of a sacrolumbar intersegmental pathway that both functions. This last possibility seems unlikely, since it conveyed bladder afferent information to the lumbar would be presumed that the ‘sacrolumbar intersegmental sympathetic preganglionic nerves. Activation of this sac- pathway’ acts to inhibit bladder function [6] whereas the rolumbar spinal pathway resulted in depression of detrusor ‘pelvic nerve pathway’ may serve to activate the detrusor activity and closing of the internal urethral sphincter. This (equivalent to the ‘short’ spinal pathway of Mallory et al. pathway was observed in cats with intact spinal cords and [17]). In fact, both inhibitory and excitatory interneurons after acute transection of the spinal cord at the T10–T12 have been described in vitro as affecting the activity of level and therefore, appears limited to the sacrolumbar parasympathetic neurons in the L6–S1 cord of the rat [1], spinal cord. This pathway might provide a negative suggesting that perhaps there are two groups of inter-feedback mechanism that may play a part in the mainte- neurons involved in these pathways. Whether these inter-nance of urinary incontinence. The findings of PRV-labeled neurons also relay information supraspinally and are thus interneurons in the L6–S1 spinal cord segments of the rat involved in a spinal–bulbospinal reflex pathway, remains following an L6–S1 ventral root resection and that receive to be determined.
bladder afferent terminals provide direct anatomical sup-port for the sacrolumbar intersegmental pathway.
There is also anatomical evidence for interneurons in the Acknowledgements L6 / S1 spinal segments projecting to supraspinal locations.
Ding et al. [9] showed projections from lumbosacral We thank Gary A. Smith, Jr. for his excellent technical interneurons to the PMC and to the ventrolateral PAG. assistance.
These interneurons are in the same general location as the interneurons described here and also in the terminal fields for bladder afferents. Moreover, Li et al. [14] described
References interneurons in the L6–S1 IML area that project to the
hypothalamus and that also show immunoreactivity for the
[1] I. Araki, W.C. de Groat, Unitary excitatory synaptic currents in
substance P receptor. In addition, Katter et al. [13] preganglionic neurons mediated by two distinct groups of inter-described interneurons in the L6–S2 segments of the rat neurons in neonatal rat sacral parasympathetic nucleus, J.
[2] L. Birder, J. Roppolo, V. Erickson, W.C. de Groat, Increased c-fos [14] J.-L. Li, T. Kaneko, R. Shigemoto, N. Mizuno, Distribution of expression in spinal lumbosacral projection neurons and pregan- trigeminohypothalamic and spinohypothalamic tract neurons dis-glionic neurons after irritation of the lower urinary tract in the rat, playing substance P receptor-like immunoreactivity in the rat, J. Brain Res, 834 (1999) 55–65. Comp. Neurol. 378 (1997) 508–521.
[3] P. Callsen-Cencic, S. Mense, Increased spinal expression of c-Fos [15] Y. Lu, S.-X. Jin, T.-L. Xu, B.-Z. Qin, J.-S. Li, Y.-Q. Ding, R.¨ following stimulation of the lower urinary tract in chronic spinal Shigemoto, N. Mizuno, Expression of c-fos protein in substance P cord-injured rats, Histochem. Cell Biol. 112 (1999) 63–72. receptor-like immunoreactive neurons in response to noxious stimuli [4] J.P. Card, L.W. Enquist, The use of pseudorabies virus for definition on the urinary bladder: an observation in the lumbosacral cord
of synaptically linked population of neurons, in: K.W. Adolph (Ed.), segments of the rat, Neurosci. Lett. 198 (1995) 139–142. Methods in Molecular Genetics, Molecular Virology Techniques, [16] B. Mallory, W. Steers, W.C. de Groat, Electrophysiological study of Part A, Academic Press, San Diego, 1994, pp. 363–382. micturition reflexes in rats, Am. J. Physiol. 257 (1989) R410–R421. [5] J.P. Card, L. Rinaman, J.S. Schwaber, R.R. Miselis, L.W. Enquist, [17] I. Nadelhaft, A.M. Booth, The location and morphology of pre-Neurotropic properties of Pseudorabies virus: uptake and trans- ganglionic neurons and the distribution of visceral afferents from the neuronal passage in the rat central nervous system, J. Neurosci. 10 rat pelvic nerve: A horseradish peroxidase study, J. Comp. Neurol.
(1990) 1974–1994. 226 (1984) 238–245.
[6] W. de Groat, Neuroanatomy and neurophysiology: innervation of the [18] I. Nadelhaft, K.E. McKenna, Sexual dimorphism in sympathetic lower urinary tract, in: S. Raz (Ed.), Female Urology, 2nd Edition, preganglionic neurons of the rat hypogastric nerve, J. Comp. Neurol. Saunders, Philadelphia, 1996, pp. 28–42. 256 (1987) 308–315.
[7] W.C. de Groat, P. Lalley, Reflex firing in the lumbar sympathetic [19] I. Nadelhaft, P.L. Vera, Central nervous system neurons infected by outflow to activation of vesical afferent fibres, J. Physiol. 226 pseudorabies virus injected into the rat urinary bladder following (1972) 289–309. unilateral transection of the pelvic nerve, J. Comp. Neurol. 359 [8] W.C. de Groat, R.J. Theobald, Reflex activation of sympathetic (1995) 443–456.
pathways to vesical smooth muscle and parasympathetic ganglia by [20] I. Nadelhaft, P.L. Vera, J.P. Card, R.R. Miselis, Central nervous electrical stimulation of vesical afferents, J. Physiol. 259 (1976) system neurons labeled following the injection of Pseudorabies virus 223–237. into the rat urinary bladder, Neurosci. Lett. 143 (1992) 271–274. [9] Y.-Q. Ding, H.-X. Zheng, L.-W. Gong, Y. Lu, H. Zhao, B.-Z. Qin, [21] W. Neuhuber, The central projections of visceral primary afferent
Direct projections from the lumbosacral spinal cord to Barrington’s neurons of the inferior mesenteric plexus and hypogastric nerve and nucleus in the rat: a special reference to micturition reflex, J. Comp. the location of the related sensory and preganglionic cell bodies in Neurol. 389 (1997) 149–160. the rat, Anat. Embryol. 164 (1982) 413–425.
[10] W. Grill, B. Wang, S. Hadziefendic, Identification of the spinal [22] J. Pascual, R. Insausti, L.M. Gonzalo, Urinary bladder innervation in neural network involved in coordination of micturition in the male male rat: termination of primary afferents in the spinal cord as cat, Brain Res. 796 (1998) 150–156. determined by transganglionic transport of WGA-HRP, J. Urol. 150 [11] P.J. Janetta, I. Nadelhaft, K.E. McKenna, Urinary bladder afferents (1993) 500–504.
may make direct contacts with lumbosacral intermediolateral neu- [23] J.L. Platt, A.F. Michael, Retardation of fading and enhancement of rons: A wheat germ agglutinin (WGA) immunohistochemical study intensity of immunofluorescence by p-phenylenediamine, J. His-in the rat, Soc. Neurosci. Abstr. 10 (1984) 604. tochem. Cytochem. 31 (1983) 840–842.
[12] L. Jasmin, E. Carstens, A.L. Basbaum, Interneurons presynaptic to [24] A.M. Strack, A.D. Loewy, Pseudorabies virus: a highly specific rat tail-flick motoneurons as mapped by transneuronal transport of transneuronal cell body marker in the sympathetic nervous system, pseudorabies virus: few have long ascending collaterals, Neuro- J. Neurosci. 10 (1990) 2139–2147.
science 76 (1997) 859–876. [25] M. Weiss, S. Chowdhury, The renal afferent pathways in the rat: a [13] J. Katter, R. Dado, E. Kostarczyk, G. Giesler, Spinothalamic and pseudorabies virus study, Brain Res. 812 (1998) 227–241.