www.elsevier.com / locate / bres
Research report
Manganese induces neurite outgrowth in PC12 cells via upregulation
of
a
vintegrins
a a a a b ,
*
Pamela Lein , Patrick J. Gallagher , Jeffrey Amodeo , Heather Howie , Jerome A. Roth
a
Department of Biology, Canisius College, Buffalo, NY 14208, USA b
Department of Pharmacology and Toxicology, State University of New York, Buffalo, NY 14214, USA Accepted 12 September 2000
Abstract
Previous studies have demonstrated that the divalent cation manganese (Mn) causes PC12 cells to form neurites in the absence of NGF. Since divalent cations modulate the binding affinity and specificity of integrins, and integrin function affects neurite outgrowth, we tested the hypothesis that Mn induces neurite outgrowth through an integrin-dependent signaling pathway. Our studies support this hypothesis. Function-blocking antisera specific forb1integrins block the neurite-promoting activity of Mn by 90–95%. Bioassays and biochemical studies with antisera specific for theav,a5, ora8integrin subunit suggest that thea bv 1heterodimer is one of the principalb1integrins mediating the response of PC12 cells to Mn. This is corroborated by studies in which Mn failed to induce neurite outgrowth in a clone of PC12 cells that does not express av at levels detectable by immunoprecipitation or immunocytochemistry. SDS–PAGE analysis of biotinylated surface proteins immunoprecipitated from Mn-responsive PC12 cells, as well as confocal laser microscopy of PC12 immunostained for surfaceavindicate that Mn increases the surface expression ofavintegrins. This increase appears to be due in part to synthesis ofavsince specific inhibitors of RNA and protein synthesis block the neurite-promoting activity of Mn. These data indicate that Mn induces neurite outgrowth in PC12 cells by upregulating av integrins, suggesting that Mn potentially represents an additional mechanism for regulating the rate and direction of neurite outgrowth during development and regeneration. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Process outgrowth, growth cones, and sprouting
Keywords: PC12 cell; Manganese; Integrin; Neurite outgrowth; Vitronectin receptor
1. Introduction presence of NGF, PC12 cells undergo mitotic arrest and
differentiate morphologically and biochemically into cells Precise regulation of the rate and direction of neurite that display many properties of differentiated sympathetic outgrowth is essential to the development of functional neurons, including an extensive network of neuronal-like neural circuits both during development and regeneration projections [16]. The mechanisms by which NGF induce [10,11,28]; thus, considerable efforts are being made to neuronal differentiation in these cells involves binding of identify and characterize the mechanisms that control this NGF to trkA receptors [37] which results in sequential process. Rat pheochromocytoma cells (PC12) have served activation of ras [27,53], MEK kinase (raf) [61] and MEK, as a useful model system for this purpose [16]. In the with eventual phosphorylation of the MAP kinases ERK1 and 2 (p44 / 42) [43]. Previous studies in our laboratory [36] have revealed that Mn can similarly induce neurite outgrowth and upregulate the neuronal marker proteins,
*Corresponding author. Department of Pharmacology and Toxicology, peripherin [2] and GAP-43 [46] in PC12 cells grown in the 102 Farber Hall, State University of New York, Buffalo School of
absence of NGF. We have also recently demonstrated that,
Medicine, Buffalo, NY 14214, USA. Tel.:11-716-829-3236; fax: 1
1-as is true for NGF, Mn-induced neurite outgrowth requires
716-829-2801.
E-mail address: [email protected] (J.A. Roth). activation of ERK1 and ERK2 [60]. However, there are
also significant differences in the response patterns of concentrations may represent a mechanism for controlling PC12 cells to Mn and NGF. Mn elicits a much more rapid the rate and direction of neurite outgrowth.
outgrowth of neurites than NGF (hours versus days, respectively) and, unlike NGF, Mn does not support
neuronal survival [36]. Such observations suggest that 2. Materials and methods there are likely to be differences between the Mn and NGF
signaling pathways. At least one point of divergence is at 2.1. Materials the level of initiation of the signaling pathway since there
is no evidence to suggest that Mn triggers signaling All tissue culture reagents were obtained from
Gibco-through a trk-dependent mechanism. BRL (Grand Island, NY) with the exception of b-NGF,
Integrins represent one class of transmembrane mole- which was purchased from Harlan Bioproducts (Madison, cules that could function to transduce the Mn signal in WI). Poly-D-lysine, cycloheximide and actinomycin D
PC12 cells. Integrins comprise a family of heterodimeric were obtained from Sigma (St. Louis, MO), and MnCl2
ab receptors, which mediate cellular interactions with was purchased from J.T. Baker (Phillipsburg, NJ). Goat extracellular matrix (ECM) and cell-surface ligands antiserum anti-gp140 known as anti-ECMR was raised [21,25]. Binding of ligand to integrin receptors activates against purified adhesion-related, 140-kDa, integral mem-intracellular signaling cascades that may alter gene tran- brane glycoproteins from BHK cells [30] and was the scription [21,22] and thus ECM–integrin interactions generous gift of Dr. Karen Knudsen (Lankenau Medical influence numerous aspects of neural development and Research Center, Philadelphia, PA). Polyclonal antisera differentiation [35,45,58]. One such aspect is neurite raised against the human fibronectin receptor,a b5 1, or the outgrowth, and changes in cellular adhesion to the ECM human vitronectin receptors, a bv 3 and a bv 5, were ob-are thought to play an important role in differentiation and tained from Gibco-BRL. Monoclonal antibody (mAb) to guidance of axons [31,35,45,58]. Neuronal interactions the vitronectin receptor (clone Vr147) was purchased from with the ECM can be altered by changing integrin–ligand Chemicon (Temecula, CA). mAb specific for the a8 affinity [21,29] or by regulating either the amount or integrin subunit was generously donated by Dr. Lynn subcellular distribution of integrins [7,8,13,19,39]. One Schnapp (The Mount Sinai School of Medicine, New factor that has been shown to modulate both the affinity York, NY). The polyclonal goat anti-bovine GFAP was and specificity of integrins for ECM ligands is Mn. obtained from Axell (Westbury, NY); the rhodamine-Moreover, there is evidence to implicate integrins in Mn- conjugated goat anti-rabbit IgG secondary antibody was induced neurite outgrowth in PC12 cells. Studies per- from Boehringer-Mannheim (Indianapolis, IN).
formed in serum-free media indicate that serum or purified
vitronectin or fibronectin are required to support Mn- 2.2. Maintenance and treatment of PC12 cell cultures induced neurite outgrowth [36]. The pentapeptide GRGDS,
which blocks RGD-mediated interactions of integrins with PC12 cells that respond to Mn with increased neurite vitronectin or fibronectin [9,65], inhibits not only Mn- outgrowth were originally obtained from Dr. John Wagner induced neurite outgrowth but also activation of ERK1 and (Harvard University School of Medicine), and a variant ERK2 [36,60]. These observations further suggest that the clone of PC12 cells that does not respond to Mn (nrPC12) effects of Mn are mediated by av integrins since this was obtained from Dr. John Aletta (SUNY at Buffalo family of integrins binds to both vitronectin and fibronectin School of Medicine, Buffalo, NY). The responsive clone in an RGD-dependent manner [6], and their function has of PC12 cells was maintained on tissue culture plastic in been shown to be upregulated by Mn in nonneuronal cells DMEM supplemented with 10% fetal calf serum (FCS),
[9,14,17,18,50,51,68]. 5% heat-inactivated horse serum (HS), penicillin (100
Although av integrins have been reported to play an units / ml), and streptomycin (100 units / ml). The nrPC12 integral role in regulating the differentiation and migration cells were maintained in RPMI supplemented with 10% of neuronal and glial cells [3,26,36–38], their expression HS, 5% FCS, penicillin (100 units / ml), and streptomycin in PC12 cells has yet to be demonstrated. Therefore, the (100 units / ml) on substrates precoated with rat tail col-validity of these interpretations depends upon demon- lagen as described in Ref. [15]. For both clones, culture strating that PC12 cells express av integrins and that media was changed 3 times per week, and cultures were blocking av integrin function is sufficient to inhibit the passaged at 80–90% confluence. All experiments were neurite-promoting activity of Mn. The data reported herein performed on cells between passage numbers 3–20. supports the hypothesis that Mn-induced neurite outgrowth For neurite-outgrowth studies, cells of either clone were is mediated byavintegrins and further suggests a model in subcultured onto 18-mm glass coverslips precoated with
5
which activation ofavintegrins by Mn results in increased poly-D-lysine (100mg / ml) at a density of 2310 cells per
serum-free medium and replacing the culture medium with these data, films were scanned and band density deter-DMEM (or RPMI for nrPC12 cells) containing 1% FCS mined (as absorption units) using the MacBas software and the experimental agent(s). Experiments were termi- program (version 2.31, Fuji Film).
nated at various time points by fixing cultures in 4%
paraformaldehyde in 0.1 M phosphate buffer for 10 min, 2.4. Immunocytochemistry then mounting cultures in Elvanol (DuPont, Wilmington,
DE) after rinsing with phosphate-buffered saline (PBS, 5 Cells were subcultured onto poly-D-lysine-coated
cover-mM phosphate, 150 cover-mM NaCl, pH 7.4). Neurite outgrowth slips as described above, and incubated for 48 h in the was analyzed by phase contrast microscopy (3200). presence or absence of 0.1 mM MnCl . Subsequently,2
Processes were scored as neurites if they exhibited a cultures were rinsed twice in PBS and fixed in 4% growth cone, and their length was at least equal to the paraformaldehyde in 0.1 mM phosphate buffer. The av diameter of the cell body. Data are presented as the integrin subunit was localized to the surface of non-mean6S.E.M. and were evaluated by ANOVA followed by permeabilized cells by indirect immuno fluorescence using
Fisher’s LSD test. previously described procedures [33]. Polyclonal rabbit
antiserum that specifically cross-reacts with theavsubunit
2.3. Immunoprecipitation (1:100) was used as the primary antibody;
rhodamine-conjugated goat anti-rabbit IgG (1:450), as the secondary PC12 cells cultured in 100-mm dishes in the presence or antibody. Immunostained cultures were mounted in Slow-absence of MnCl2 were removed from the substrate by fade (Molecular Probes, Eugene, OR) as per the manufac-gentle trituration and rinsed sequentially with ice-cold PBS turer’s instructions and analyzed by confocal laser micro-and labeling buffer (5 mM NaPO , 150 mM NaCl, 1 mM4 scopy (BioRad 1024 confocal with a krypton–argon laser CaCl , and 1 mM MgCl ). Pelleted cells were resuspended2 2 linked to a Nikon Optiphot microscope). Images were and incubated in labeling buffer supplemented with 10 obtained using a 340 lens with a numerical aperture of
mg / ml biotin–X-NHS (Calbiochem, Cambridge, MA) for 1.3.
1 h at 48C. Biotinylated cells were extracted for 1 h at 48C in extraction buffer (1% Triton X-100, 5 mM NaPO , 1504
mM NaCl, 1 mM CaCl , 1 mM MgCl , and 1 mM PMSF)2 2 3. Results then incubated with Protein-A complexed to sepharose
beads (Sigma, St. Louis, MO) to reduce background. 3.1. Mn-induced neurite outgrowth is mediated byb1 Pre-cleared extracts were incubated overnight at 48C with integrins
100 ml Protein-A beads plus relevant antisera (1–10 mg /
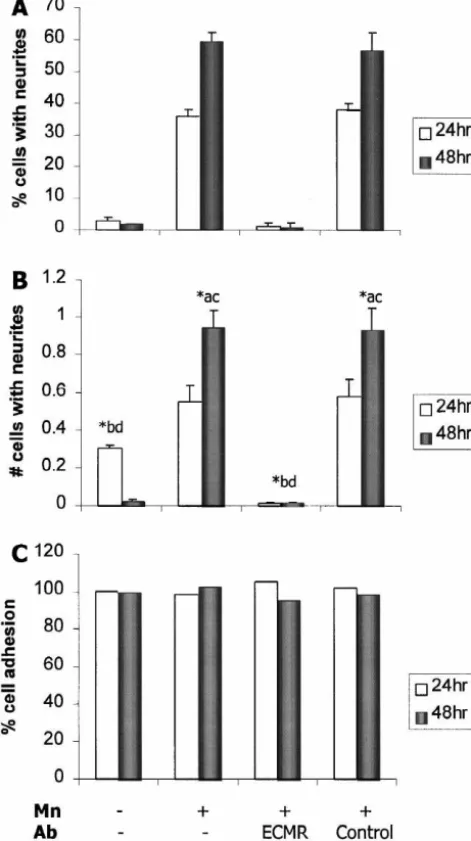
dent mechanism. First, PC12 cells do not extend neurites in response to Mn unless grown on substrates treated with vitronectin, fibronectin, or culture medium containing serum. Second, the morphological effects of Mn are blocked by the pentapeptide GRGDS, an analog of the RGD binding sequence recognized by various integrins [9,65], but not by GRGES, the biologically inactive sequence. To further examine the role of integrins in Mn-induced morphological differentiation, we tested the ability of function-blocking antibodies specific for integ-rins to inhibit the neurite-promoting effects of Mn. Anti-ECMR serum has been shown to selectively interfere with the function of b1 integrins in both nonneuronal and neuronal tissues [1,34,38,52], and in PC12 cells, this antiserum immunoprecipitates b1 integrins [55,56]. As indicated in Figs. 1 and 2, addition of anti-ECMR (0.2%) almost completely blocks neurite outgrowth in response to Mn at both 24 and 48 h as measured by the percentage of cells with neurites (Fig. 2A) and the number of neurites per cell (Fig. 2B). Isotype-matched control serum added to the culture medium at the same concentration has no effect on process growth. The attachment of PC12 cells to coverslips precoated with poly-D-lysine is not affected by anti-ECMR
(Fig. 2C), and the effects of anti-ECMR on Mn-induced neurite outgrowth are reversible after removal of the antibody (not shown).
3.2. av Integrins play a significant role in Mn-induced neurite outgrowth
We next wanted to determine the identity of the a integrin subunit(s) mediating the Mn response. The av represented a likely candidate since previous studies of PC12 cells suggested that Mn-induced neurite outgrowth is mediated by b1 integrins that recognize the RGD ligand motif [36]. In addition, anti-ECMR was shown to immuno-precipitate the av integrin subunit from oligodendrocyte precursor cells [38]. To determine ifa bv 1 is expressed by PC12 cells, and if it is recognized by anti-ECMR, PC12 cell extracts were immunoprecipitated with anti-ECMR or with antibodies specific for the av integrin subunit. As indicated in Fig. 3, anti-ECMR immunoprecipitates two
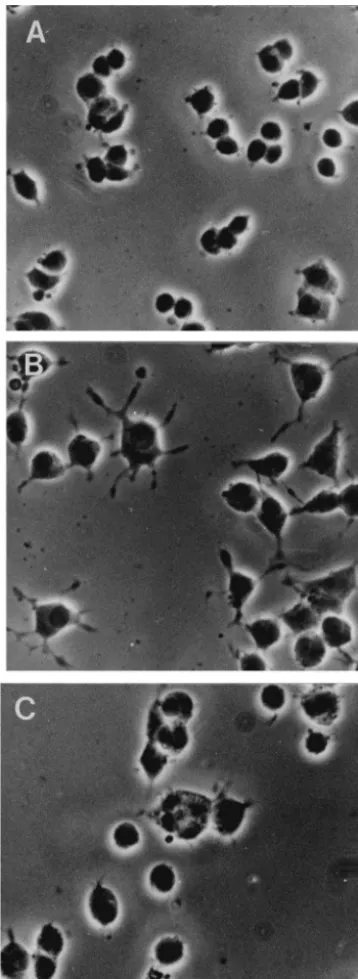
Fig. 1. Mn induces neurite outgrowth in PC12 cells grown in the absence strong bands with molecular weights of approximately 150
of NGF and this activity is significantly inhibited by function-blocking and 125 kDa (lane 1). Polyclonal antibody to the a v antisera specific forb1 integrins. (A) Phase contrast photomicrographs of
integrin subunit immunoprecipitates two bands; one strong
PC12 cells grown in the absence of Mn exhibit a rounded morphology
band at approximately 150 000 and a weaker band at
with very few neuritic extensions. (B) PC12 cells exposed to Mn (0.1
115 000 Da (Fig. 3, lane 2). As illustrated in lane 3, the
mM) for 48 h typically appear flattened with multiple neurites exhibiting
growth cones. (C) The addition of antisera specific for b1 integrins higher molecular weight protein also appears to be
precipi-(anti-ECMR, 0.2%) significantly reduces Mn-induced neurite outgrowth tated by the monoclonal antibody (mAb) specific for a . v but does not interfere with cell spreading. Bar, 50mm.
Fig. 3. Immunoprecipitation of integrin subunits from surface biotinylated PC12 cells. Anti-ECMR serum (lane 1) immunoprecipitates integrin subunits whose electrophoretic properties are similar to those immunoprecipitated by polyclonal av integrin antiserum (lane 2) and anti-av mAb (lane 3).
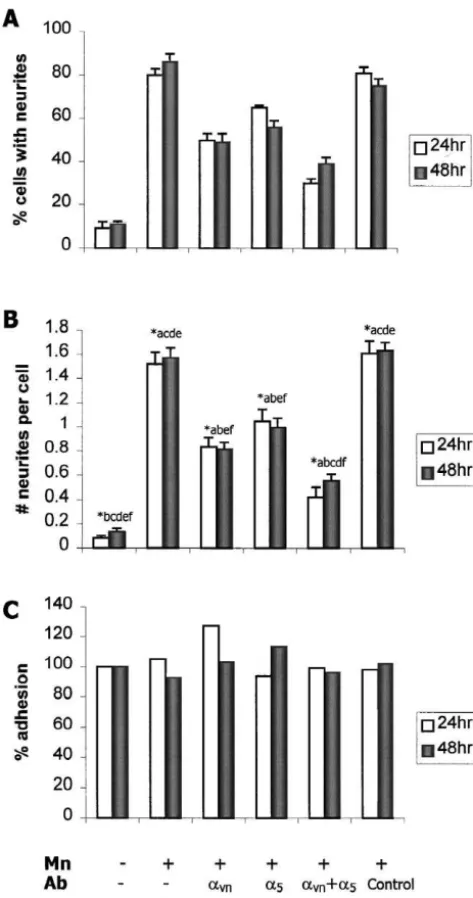
the presence of av antibodies did not interfere with cell adhesion (Fig. 4C). However, the extent of inhibition exerted by av-specific antibodies is not as robust as that observed in the presence of anti-ECMR (90–95%). There are at least two possible interpretations. First, there may be significant differences in the function-blocking capabilities of the anti-ECMR andavantibodies. Second, it is possible that multiple b1 integrins mediate the morphological response to Mn. In addition to theav integrins, plausible candidates include the fibronectin receptor, a b5 1, which also binds the RGD sequence, and a b8 1 which has been shown to function as a receptor for vitronectin and fibronectin [40,47]. Immunoblot analyses of PC12 cell extracts suggest that these cells expressa b , but nota b
Fig. 2. Mn-induced neurite outgrowth is inhibited by an antiserum to the 5 1 8 1
(data not shown), and antibody blocking experiments
b1 integrin subunit (anti-ECMR). Addition of anti-ECMR serum (0.2%,
column c) to PC12 cell cultures exposed to Mn (100mM) significantly indicate that a b functions in Mn-induced neurite out-5 1
inhibited the percentage of cells with neurites (A) and the number of growth (Fig. 4A,B). Simultaneous exposure to botha and v neurites per cell (B). Exposure to similar concentrations of an antiserum
a5 antibodies causes a significantly greater inhibition of
to an intracellular antigen (GFAP, column d) had no effect on
Mn-neurite outgrowth than either antibody alone, and these
induced neurite outgrowth. Data are expressed as mean6S.E.M. of three
effects are independent of changes in cell adhesion.
trials (n$50 per experimental condition per trial). *Indicates a significant
difference at P,0.01 (ANOVA); the lower case letters over the bars Although these data suggest that each of these integrins is indicate which data sets are significantly different from the set repre- important in the response of PC12 cells to Mn, the sented by the bars. (C) Anti-ECMR serum does not alter adhesion of
remainder of our study focuses on the av integrins since
PC12 cells to the culture substratum. Cell adhesion was evaluated by
these appear to play a more significant role in axonal
counting the number of cells per culture following fixation with 4%
outgrowth and synaptogenesis than a integrins in many
paraformaldehyde. Data are expressed as the percentage of adherent cells 5 relative to control cultures (n53 coverslips per experimental condition). neuronal cell types [35,41,42,45,58].
Fig. 5. Characterization of a stable clone of PC12 cells (nrPC12) that does not respond to the neurite-promoting effects of Mn. Exposure of nrPC12 cells to varying concentrations of Mn (10mM to 1 mM) fails to elicit neurite outgrowth as measured by the percentage of cells with neurites (A) and the number of neurites per cell (B). However, nrPC12 cells did respond to NGF (100 ng / ml) with significant neuritic outgrowth. Data are expressed as the mean6S.E.M. of three separate trials (n$50 per experimental condition per trial).
in response to Mn not because they are deficient in the cytoskeletal and membrane elements necessary to sustain neurite outgrowth but rather because they lack the factor(s) necessary to trigger neurite outgrowth in response to Mn. Immunoprecipitation studies indicate that nrPC12 cells express negligible levels of theavintegrin subunit on their surface (Fig. 6A) and immunocytochemical analyses fail to
Fig. 4. Anti-avanda5integrin sera inhibit neurite outgrowth in response detect av (data not shown). These data support the to Mn. The addition of polyclonal antibodies toav and / ora5 integrin hypothesis that a integrins are necessary to trigger the
v subunits (1:100, columns c, d, e) to PC12 cell cultures exposed to Mn
morphological response of PC12 cells to Mn.
(100mM) significantly reduces the percentage of cells with neurites (A) and the number of neurites per cell (B). Addition of isotype-matched
3.3. Mn increases surface expression ofa integrins
antisera specific for GFAP (column f) at a similar concentration had no v effect on neurite outgrowth. Data are expressed as the mean6S.E.M. of
three trials (n$50 per condition per trial). *Indicates a significant One mechanism by which Mn could modulate the difference at P,0.01 (ANOVA, Fisher’s LSD test); the lower case letters
function of av integrins is by altering their level of
over the bars indicate which data sets are significantly different from the
expression in the plasma membrane. To determine if Mn
set represented by the bars. (C) The addition ofavanda5antibodies did
upregulates surface expression ofa integrins, PC12 cells
not alter the adhesion of PC12 cells to the culture substratum as v
determined by counting the number of cells per culture. Data are grown in the absence or presence of Mn were immuno-expressed as a percentage of adherent cells relative to control cultures precipitated witha antibodies subsequent to biotinylation
v (n53 coverslips per condition).
of surface proteins. SDS–PAGE analysis of these immuno-precipitates indicates that Mn causes an|100-fold increase
Fig. 6. SDS–PAGE of proteins immunoprecipitated from surface biotinylated PC12 cells with polyclonalav antibodies. (A)av Integrin expression by the nrPC12 cell subclone (lane 1) is significantly less than in Mn-responsive PC12 cells (lane 2). (B) Comparison of the amount of avimmunoprecipitated in responding PC12 cells not exposed to Mn (lane 1) versus comparable cultures exposed to Mn (100mM) for 24 h (lane 2) suggests that Mn upregulatesavexpression.
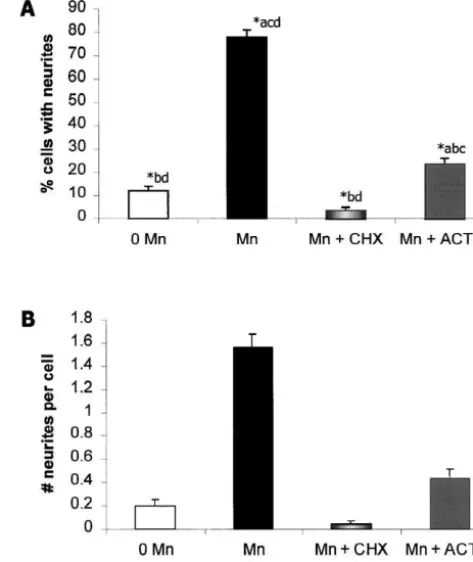
Fig. 8. Mn-induced neurite outgrowth requires de novo mRNA and protein synthesis. Addition of actinomycin D (ACT-D, 0.1mg / ml) or cycloheximide (CHX, 1.0mg / ml) to cultures exposed to Mn (0.1 mM) significantly inhibits the percentage of cells with neurites (A), and the number of neurites per cell (B). Data are expressed as the mean6S.E.M. (n$100 per experimental condition). *Indicates a significant difference at
P,0.01 (ANOVA, Fisher’s LSD test); the lower case letters over the bars indicate which data sets are significantly different from the set repre-sented by the bars.
confocal images of surface immunoreactivity for av in non-permeabilized PC12 cells support the hypothesis that Mn specifically increases surface levels of av in PC12 cells (Fig. 7). This upregulated surface expression may result from de novo protein synthesis of av integrin subunits since addition to the culture medium of the RNA synthesis inhibitor, actinomycin D, or the protein synthesis inhibitor, cycloheximide, markedly reduces neurite out-growth in the presence of Mn (Fig. 8).
Fig. 7. Mn alters the amount and distribution ofavimmunoreactivity on
the surface of PC12 cells. PC12 cells grown for 48 h in the absence (A) or 4. Discussion presence of Mn at 100 mM (B) were immunostained with polyclonal
anti-av serum (1:100) and analyzed using confocal microscopy. (A)
Previous studies have demonstrated that Mn promotes
Confocal image of a clump of five cells grown in the absence of Mn
neuronal differentiation in PC12 cells grown in the absence
reveal diffuse low levels ofav immunoreactivity with scattered
aggre-gates of intense staining localized primarily to points of cell–cell and of NGF as evidenced by upregulation of peripherin and
cell–substrate contact. The nucleus is evident as an area clear of any GAP-43 and the extension of neurites [36]. The purpose of immunoreactivity, and as is typical of PC12 cells grown under these this study was to determine the role of integrins in this conditions, these cells have no neurites. (B) Confocal image of a single
phenomenon. The most direct evidence to support the
PC12 cell exposed to Mn indicates a dramatic increase in fluorescence
hypothesis that Mn induces neurite outgrowth in PC12
intensity forav along the surface of the cell body as well as bright
observa-tions that anti-ECMR antibodies, which block the function neuronal cells indicate that integrin function can be of b1 integrins [1,34,38,52], effectively abolish neurite modulated by changes in the number of receptors ex-outgrowth in response to Mn. That this is not due to pressed on the cell surface or in the subcellular distribution deleterious effects on cell viability is suggested by ob- of integrins as well as by alterations in receptor affinity servations that anti-ECMR does not alter cell number in [23,25,29,39,48]. Our studies of biotinylated surface pro-exposed cultures relative to cultures grown in the absence teins immunoprecipitated with antibodies to av clearly of these antibodies, and that the inhibition of neurite indicate that Mn increases surface expression ofav integ-outgrowth is reversible upon removal of anti-ECMR. The rins in PC12 cells. These data are corroborated by confocal effects of anti-ECMR are specific in that similar con- laser microscopy of non-permeabilized PC12 cells im-centrations of isotype-matched antisera raised against munostained forav. Preliminary observations suggest that intracellular antigens did not alter the neurite-promoting this effect is restricted to a subset of integrin subunits in
activity of Mn. that Mn similarly increases surface expression ofa b5 1, but
Previous studies of PC12 cells grown on collagen not a b1 1 in PC12 cells (unpublished results). Since substrates demonstrated that anti-ECMR immuno- increased expression of integrin at the cell surface is precipitates a b1 1 and a b3 1 integrins, which function associated with increased neurite outgrowth [7,8], this primarily as receptors for laminin and collagen IV through suggests a plausible mechanism by which Mn effects on RGD-independent mechanisms [57]. However, such find- integrin function lead to neurite outgrowth. In the absence ings are inconsistent with observations that vitronectin and of published data indicating similar effects of Mn or other fibronectin supports NGF- and Mn-induced neurite out- cations on integrin expression in neuronal or nonneuronal growth in PC12 cells grown on plastic [12,36], and that the systems, these data also suggest a novel mechanism by neurite-promoting activity of Mn is dependent on cell– which Mn modulates integrin function.
matrix interactions mediated by the RGD ligand binding Observations that specific inhibitors of transcription and sequence [36]. Sinceav integrins have been demonstrated translation significantly inhibit Mn-induced neurite out-to bind RGD amino acid sequences in both vitronectin and growth suggest that the increased surface levels of av fibronectin [6,49,59,64,67], these data strongly suggest that integrin are due in part to de novo synthesis of the av anti-ECMR may also block the function of av integrins. subunits. Alternatively, actinomycin D and cycloheximide Consistent with this observation, we found that anti-ECMR may block the neurite promoting activity of Mn by immunoprecipitates a protein from PC12 cells whose inhibiting the synthesis of non-integrin molecules that are molecular weight is comparable to that of proteins im- necessary to support neurite outgrowth, such as proteins of munoprecipitated from PC12 cells by av antibodies and the cytoskeleton or plasma membrane. However, prelimin-that is recognized by these sameav antibodies in Western ary data from Northern blot analyses confirm that Mn blots. Anti-ECMR has also been shown to immuno- upregulates av mRNA (unpublished results). Similarly, precipitate av integrins in oligodendrocyte precursor cells NGF has been shown to transcriptionally regulate the [38]. Further evidence that the effects of anti-ECMR result expression of integrins in PC12 cells and this effect is from inhibition of a bv 1 integrins is the suppression of required for NGF-induced neurite outgrowth in these cells Mn-induced neurite outgrowth by polyclonal antibodies to [66]. Our studies do not exclude the possibility that Mn av. However, our data clearly show that a bv 1 is not the may also recruit integrins sequestered in the cytoplasm to only b1 integrin responsible for Mn-induced neurite out- the surface of PC12 cells [29]. Indeed, alterations in ECM growth since complete or near complete inhibition of ligand concentration regulate the amount of integrin ex-neurite outgrowth is not achieved with this antibody. pressed on the surface of sensory neurons through post-Antibodies to a b5 1 also inhibit neurite outgrowth and translational mechanisms [7], and Mn upregulates the these effects are additive with the effects of a bv 1 anti- surface expression of the peripheral-type benzodiazepine bodies. The importance of the a bv 1 integrin in initiating receptor (PTBR) on astrocytes through recruitment of Mn-induced neurite outgrowth is further suggested by receptors from intracellular stores [20].
experiments utilizing a non-responsive PC12 cell clone. Immunocytochemical analysis of av expression reveals These cells do not express detectable levels ofav integrin that Mn changes not only the number ofav integrins but and they fail to produce neurites when challenged with also the subcellular distribution of these receptors. In cells Mn. Since these cells do respond to NGF with robust exposed to Mn, a bv 1 appears as aggregates distributed neurite outgrowth, their inability to respond to Mn reflects around the periphery of the cell body and along the length the importance of theavintegrins in mediating the neurite- of the neurite. This localization pattern is consistent with a promoting activity of Mn and not a general deficiency in prominent role for integrins in neurite outgrowth. It is not the cytoskeletal elements or signal transduction elements possible to determine from these studies whether Mn
necessary for process outgrowth. directly causes aggregation and redistribution of these
observed in sympathetic neurons in response to NGF [13], These data suggest that differences in the extracellular the latter interpretation seems more likely. concentrations of Mn could influence neurite outgrowth via Another mechanism by which Mn could alter integrin effects on integrins. Whether such effects are manifest only function is via effects on receptor affinity. It has been when the cell body is exposed to varying concentrations of extensively documented in a variety of model systems that Mn, as in our culture paradigm, or if variations in Mn binding of Mn to the extracellular domain of theaintegrin concentrations at the level of the growth cone are sufficient subunit significantly increases the affinity of a bv 1 integ- to alter the rate or direction of neurite outgrowth is not rins for ligand [4,6,9,14,17,18,23,44,50,63]. Moreover, known. The relevance of the latter is suggested by reports activation of integrins by Mn is sufficient to trigger that changes in the distribution and functional status of signaling pathways that control cell function and structure integrins in the growth cone are associated with changes in [29,51]. Although we did not experimentally address the directed neurite outgrowth [13,62]. Another consideration effects of Mn on the binding affinity of av integrins in relevant to determining the physiological relevance of these studies, recently published work from this laboratory these studies concerns the levels of Mn found in situ under [60] strongly suggests that Mn directly activates a bv 1 both normal and pathological states. The total body burden integrins in PC12 cells. RGD-dependent Mn induction of of Mn in the standard 70-kg man is estimated to be ERK1 and 2 phosphorylation occurs within 2 h of Mn approximately 10–20 mg and Mn concentrations in most exposure and maximal phosphorylation is observed at 12 adult tissues range from 3 to 20 mM [24]. We observed h. These changes precede neurite outgrowth in Mn-respon- effects on neurite outgrowth after 72 h of exposure to sive PC12 cells, and Mn-induced increases in phos- concentrations of total Mn as low as 1.0 mM, with phorylated ERK1 and 2 are not observed in the nrPC12 maximal effects observed at total Mn concentrations of clone shown here to be unresponsive to the neurite-pro- 10–20 mM. Since serum (1%) is present in our culture moting effects of Mn and to not express a bv 1 integrins system the concentrations of free Mn are not known, but [60]. If, as in NGF signaling, integrins were involved in the levels of total Mn are certainly within the range the Mn signaling pathway downstream but not upstream of observed in most adult tissues. Brain tissue concentrations ERK1 and 2, then exposing cells to the GRGDS penta- of Mn are considerably higher in the early stages of peptide would not be expected to block Mn-induced development than in the adult organism, and can reach phosphorylation of ERK1 and 2. Moreover, levels of concentrations of over 200mM in specific areas of the rat phosphorylated ERK1 and 2 would not be expected to brain such as the striatum [5]. These data, in conjunction differ between the Mn-responsive and non-responsive with our observations that Mn concentrations of 100mM PC12 cell clones upon exposure to Mn. Data to the elicited significant neurite outgrowth in non-NGF primed contrary [60] argues for a role of integrins upstream of PC12 cells within 24 h, suggest the potential for Mn to
ERK1 and 2 in Mn signaling. regulate neurite outgrowth in the developing organism.
Based on these data, we propose the following model to Levels of Mn are also increased in healing skin wounds explain how Mn induces neurite outgrowth in PC12 cells. [32], which may function to promote reinnervation of Mn binds to the extracellular domain of the av integrin injured skin.
accumulation of b integrin at the tips of filopodia in the growth
this activity. These data suggest that Mn induces neurite 1
cones of sympathetic neurons, J. Neurosci. 17 (1997) 5455–5465.
outgrowth in PC12 cells by upregulating surface
expres-[14] M. Grazia, M.G. Lampugnani, S. Bernasconi, P. Neri, L. Lozzi, I.
sion ofav integrins, in part through de novo synthesis of Gavazzi, P.C. Marchisio, E. Dejana, Role of manganese in MG-63 integrin subunits. In light of this, we propose that Mn may osteosarcoma cell attachment to fibrinogen and von Willebrand
represent a mechanism for regulating the rate and / or factor, Lab Invest. 65 (1991) 96–103.
[15] L.A. Greene, M.M. Sobeih, K.K. Teng, Methodologies for the
direction of neurite outgrowth through effects on integrin
culture and experimental use of the PC12 rat pheochromocytoma
function.
cell line, in: G. Banker, K. Goslin (Eds.), Culturing Nerve Cells, MIT Press, Cambridge, MA, 1991, pp. 207–226.
[16] L.A. Greene, A.S. Tischler, PC12 pheochromocytoma cultures in neurobiological research, Adv. Cell Neurobiol. 3 (1982) 373–414.
Acknowledgements
[17] F. Grinnell, Manganese-dependent cell-substratum adhesion, J. Cell Sci. 65 (1984) 61–72.
This work was supported by the National Institutes of [18] F. Grinnell, R. Backman, Role of integrin receptors in manganese-Health (AREA grant [1 R15 NS / OD36401-01 to PL), a dependent BHK cell spreading on albumin-coated substrata, Exp.
Cell Res. 195 (1991) 218–223.
Research Fellowship to Patrick Gallagher from the Howard
[19] T. Hato, N. Pampori, S.J. Shattil, Complementary roles for receptor
Hughes Medical Institution Undergraduate Biological
Sci-clustering and conformational changes in the adhesive and signaling
ences Education Grant (to Canisius College), and the
functions of integrinaIIbb3, J. Cell Biol. 141 (1998) 1685–1695.
Environmental Protection Agency (grant [ R826248 to
[20] A.S. Hazell, P. Desjardins, R.F. Butterworth, Chronic exposure of
JAR). rat primary astrocyte cultures to manganese results in increased
binding sites for the ‘peripheral-type’ benzodiazepine receptor 3
ligand H-PK 11195, Neurosci. Lett. 271 (1999) 5–8.
[21] M.E. Hemler, VLA proteins in the integrin family: structure,
References functions, and their role on leukocytes, Annu. Rev. Immunol. 8
(1990) 365–400.
[22] M.J. Humphries, Integrin activation: the link between ligand binding [1] S.M. Albelda, M. Daise, E.M. Levine, C.A. Buck, Identification and
and signal transduction, Curr. Opin. Cell Biol. 8 (1996) 632–640. characterization of cell-substratum adhesion receptors on cultured
[23] M.J. Humphries, A.P. Mould, D.S. Tuckwell, Dynamic aspects of human endothelial cells, J. Clin. Invest. 83 (1989) 1992–2002.
adhesion receptor function — integrins both twist and shout, [2] J.M. Aletta, M.L. Shelanski, L.A. Greene, Phosphorylation of the
BioEssays 15 (1993) 391–397. peripherin 58-kDa neuronal intermediate filament protein: regulation
[24] L. Hurley, Teratogenic aspects of manganese, zinc and copper by nerve growth factor and other agents, J. Biol. Chem. 264 (1989)
nutrition, Physiol. Rev. 61 (1981) 249–295. 4619–4627.
[25] R.O. Hynes, Integrins: versatility, modulation, and signaling in cell [3] E.S. Anton, J.A. Kreidberg, P. Rakic, Distinct functions of alpha(3)
adhesion, Cell 69 (1992) 11–25. and alpha(v) integrin receptors in neuronal migration and laminar
[26] T.S. Jacques, J.B. Relvas, S. Nishimura, R. Pytela, G.M. Edwards, organization of the cerebral cortex, Neuron 22 (1999) 277–289.
C.H. Streuli, C. Ffrench-Constant, Neural precursor cell chain [4] G. Bazzoni, D.-T. Shih, C.A. Buck, M.E. Hemler, Monoclonal
migration and division are regulated through differentb integrins,
antibody 9EG7 defines a novel b1 integrin epitope induced by 1
Development 125 (1998) 3167–3177. soluble ligand and manganese, but inhibited by calcium, J. Biol.
[27] H. Kamata, C. Tanaka, H. Yagisawa, S. Matsuda, Y. Gotoh, E. Chem. 270 (1995) 25570–25577.
Nishida, H. Hirata, Suppression of nerve growth factor-induced [5] A.W.K. Chan, M.J. Minski, L. Lim, J.C.K. Lai, Changes in brain
neuronal differentiation of PC12 cells, J. Biol. Chem. 271 (1996) regional manganese and magnesium levels during postnatal
develop-33018–33025. ment: modulation by chronic manganese administration, Metab.
[28] R. Keynes, G.M.W. Cook, Axon guidance molecules, Cell 83 (1995) Brain Dis. 7 (1992) 21–33.
161–169. [6] I.F. Charo, L. Nannizzi, J.W. Smith, D.A. Cheresh, The vitronectin
receptor avb3 binds fibronectin and acts in concert witha b5 1 in [29] L.T. Kim, K.M. Yamada, The regulation of expression of integrin promoting cellular attachment and spreading on fibronectin, J. Cell receptors, Proc. Soc. Exp. Biol. Med. 214 (1997) 123–131. Biol. 111 (1990) 2795–2800. [30] K.A. Knudsen, P.E. Rao, C.H. Damsky, C.A. Buck, Membrane [7] M.L. Condic, P.C. Letourneau, Ligand-induced changes in integrin glycoproteins involved in cell-substratum adhesion, Proc. Natl.
expression regulate neuronal adhesion and neurite outgrowth, Nature Acad. Sci. USA 78 (1981) 6071–6075.
389 (1997) 852–856. [31] A.D. Lander, Molecules that make axons grow, Mol. Neurobiol. 1 [8] M.L. Condic, D.M. Snow, P.C. Letourneau, Embryonic neurons (1987) 213–245.
adapt to the inhibitory proteoglycan aggrecan by increasing integrin [32] A.B.G. Landsdown, B. Sampson, A. Rowe, Sequential changes in expression, J. Neurosci. 19 (1999) 10036–10043. trace metal, metallothionein and calmodulin concentrations in [9] M.J. Elices, L.A. Urry, M.E. Hemler, Receptor functions for the healing skin wounds, J. Anat. 195 (1999) 375–386.
integrin VLA-3: fibronectin, collagen, and laminin binding are [33] P.J. Lein, D. Higgins, Laminin and a basement membrane extract differentially influenced by ARG-GLY-ASP peptide and by divalent have different effects on axonal and dendritic outgrowth from cations, J. Cell Biol. 112 (1991) 169–181. embryonic rat sympathetic neurons in vitro, Dev. Biol. 136 (1989) [10] A. Faissner, Glial derived extracellular matrix components: im- 330–345.
portant roles in axon growth and guidance, Neuroscientist 3 (1997) [34] P.J. Lein, D. Higgins, D.C. Turner, L.A. Flier, V.P. Terranova, The
371–380. NC1 domain of type IV collagen promotes axonal growth in
[11] C.S. Goodman, C.J. Shatz, Developmental mechanisms that generate sympathetic neurons through interaction with the a b1 1integrin, J. precise patterns of neuronal connectivity, Cell 10 (1993) 77–98. Cell Biol. 113 (1991) 417–428.
[12] P.W. Grabham, P.H. Gallimore, R.J.A. Grand, Vitronectin is the [35] P.C. Letourneau, M.L. Condic, D.M. Snow, Interactions of develop-major serum protein essential for NGF-mediated neurite outgrowth ing neurons with the extracellular matrix, J. Neurosci. 14 (1994) from PC12 cells, Exp. Cell Res. 202 (1992) 337–344. 915–928.
Marcucci, J.A. Roth, Manganese induces spreading and process Brugge, Ras is essential for nerve growth factor- and phorbol outgrowth in rat pheochromocytoma (PC12) cells, J. Neurosci. Res. ester-induced tyrosine phosphorylation of MAP kinases, Cell 68
34 (1993) 546–561. (1992) 1031–1040.
[37] D.M. Loeb, J. Maragos, D. Martin-Zanca, M.V. Chao, L.F. Parada, [54] J. Thongphasuk, L.W. Oberley, T.D. Oberley, Induction of superox-L.A. Greene, The trk proto-oncogene rescues NGF responsiveness in ide dismutase and cytotoxicity by manganese in human breast mutant NGF-nonresponsive PC12 cell lines, Cell 66 (1991) 961– cancer cells, Arch. Biochem. Biophys. 365 (1999) 317–327.
966. [55] K.J. Tomaselli, C.H. Damsky, L.F. Reichardt, Interactions of a
[38] R. Milner, G. Edwards, C. Streuli, C. Ffrench-Constant, A role in neuronal cell line (PC12) with laminin, collagen IV, and fibronectin: migration for the a bv 1 integrin expressed on oligodendrocyte identification of integrin-related glycoproteins involved in attach-precursors, J. Neurosci. 16 (1996) 7240–7252. ment and process outgrowth, J. Cell Biol. 105 (1987) 2347–2358. [39] S. Miyamoto, S.K. Akiyama, K.M. Yamada, Synergistic roles for [56] K.J. Tomaselli, C.H. Damsky, L.F. Reichardt, Purification and
receptor occupancy and aggregation in integrin transmembrane characterization of mammalian integrins expressed by a rat neuronal function, Science 267 (1995) 883–885. cell line (PC12): evidence that they function asa/bheterodimeric [40] U. Muller, B. Bossy, K. Venstrom, L.F. Reichardt, Integrina b8 1 receptors for laminin and type IV collagen, J. Cell Biol. 107 (1988)
promotes attachment, cell spreading, and neurite outgrowth on 1241–1252.
fibronectin, Mol. Biol. Cell. 6 (1995) 433–448. [57] K.J. Tomaselli, D.E. Hall, L.A. Flier, K.R. Gehlsen, D.C. Turner, S. [41] K.M. Neugebauer, C.J. Emmett, K.A. Venstrom, L.F. Reichardt, Carbonetto, L.F. Reichardt, A neuronal cell line (PC12) expresses Vitronectin and thrombospondin promote retinal neurite outgrowth: twob1-class integrins —a b1 1anda b3 1— that recognize different developmental regulation and role of integrins, Neuron 6 (1991) neurite outgrowth-promoting domains in laminin, Neuron 5 (1990)
345–358. 651–662.
[42] S.L. Nishimura, K.P. Boylen, S. Einheber, T.A. Milner, D.M. [58] K.A. Venstrom, L.F. Reichardt, Extracellular matrix 2: role of Ramos, R. Pytela, Synaptic and glial localization of the integrin extracellular matrix molecules and their receptors in the nervous a bv 8in mouse and rat brain, Brain Res. 791 (1998) 271–282. system, FASEB J. 7 (1993) 996–1003.
[43] L. Pang, T. Sawada, S.J. Decker, A.R. Saltiel, Inhibition of MAP [59] B.E. Vogel, G. Tarone, F.G. Giancotti, J. Gailit, E. Ruoslahti, A kinase kinase blocks the differentiation of PC12 cells induced by novel fibronectin receptor with an unexpected subunit composition nerve growth factor, J. Biol. Chem. 270 (1995) 13585–13588. (a bv 1), J. Biol. Chem. 265 (1990) 5934–5937.
[44] A.J. Pelletier, T. Kunicki, V. Quaranta, Activation of the integrin [60] J. Walowitz, J.A. Roth, Activation of ERK1 and ERK2 is required a bv 3involves a discrete cation-binding site that regulates conforma- for manganese-induced neurite outgrowth in rat pheochromocytoma tion, J. Biol. Chem. 271 (1996) 1364–1370. (PC12) cells, J. Neurosci. Res. 57 (1999) 847–854.
[45] L.F. Reichardt, K.J. Tomaselli, Extracellular matrix molecules and [61] K.W. Wood, H. Qi, G. D’Arcangelo, R.C. Armstrong, T.M. Roberts, their receptors: Functions in neural development, Annu. Rev. S. Halegoua, The cytoplasmic raf oncogene induces a neuronal Neurosci. 14 (1991) 531–570. phenotype in PC12 cells: a potential role for cellular raf kinases in [46] F.R.A. San, M.H. Michels, B.M. Spruijt, A.B. Ostreicher, P. neuronal growth factor signal transduction, Proc. Natl. Acad. Sci.
Schotman, W.H. Gispen, Quantitation of the growth-associated USA 90 (1993) 5016–5020.
protein B-50 / GAP-43 and neurite outgrowth in PC12 cells, J. [62] D.-Y. Wu, L.-C. Wang, C.A. Mason, D.J. Goldberg, Association of Neurosci. Res. 29 (1991) 149–154. b1 integrin with phosphotyrosine in growth cone filopodia, J. [47] L.M. Schnapp, N. Hatch, D.M. Ramos, I.V. Klimanskaya, D. Neurosci. 16 (1996) 1470–1478.
Sheppard, R. Pytela, The human integrin a b8 1 functions as a [63] T. Yanai, T. Shimo-Oka, I. Ii, Manganese ion elicits a binding receptor for tenascin, fibronectin, and vitronectin, J. Biol. Chem. 270 activity of placenta vitronectin receptors to fibronectin cell-binding (1995) 23196–23202. domain, Cell Struct. Funct. 16 (1991) 49–156.
[48] M.A. Schwartz, C. Lechene, D.E. Ingber, Insoluble fibronectin [64] J.T. Yang, R.O. Hynes, Fibronectin receptor functions in embryonic activates the Na / H antiporter by clustering and immobilizing cell deficient ina5b1 integrin can be replaced byavintegrins, Mol. integrina b5 1, independent of cell shape, Proc. Natl. Acad. Sci. USA Biol. Cell. 7 (1996) 1737–1748.
88 (1991) 7849–7853. [65] P.M. Yip, X. Zhao, M.P. Montgomery, C.-H. Siu, The Arg-Gly-Asp [49] J.W. Smith, D.A. Cheresh, Integrin (a bv 3)-ligand interaction, identi- motif in the cell adhesion molecule L1 promotes neurite outgrowth fication of a heterodimeric RGD binding site on the vitronectin via interaction with the a bv 3 integrin, Mol. Biol. Cell 9 (1998) receptor, J. Biol. Chem. 265 (1990) 2168–2172. 277–289.
58
[50] J.W. Smith, D.A. Cheresh, Labeling of Integrina bv 3with Co(III). [66] Z. Zhang, G. Tarone, D.C. Turner, Expression of integrin alpha 1 Evidence of metal ion coordination sphere involvement in ligand beta 1 is regulated by nerve growth factor and dexamethasone in binding, J. Biol. Chem. 266 (1991) 11429–11432. PC12 cells. Functional consequences for adhesion and neurite [51] J.W. Smith, R.S. Piotrowicz, D. Mathis, A mechanism for divalent outgrowth, J. Biol. Chem. 268 (1993) 5557–5565.
cation regulation ofb3 integrins, J. Biol. Chem. 269 (1994) 960– [67] Z. Zhang, A.O. Morla, K. Vuori, J.S. Bauer, R.L. Juliano, E. 967. Ruoslahti, Thea bv 1integrin functions as a fibronectin receptor but [52] A.E. Sutherland, P.G. Carlarco, C.H. Damsky, Expression and does not support fibronectin matrix assembly and cell migration on
function of cell surface extracellular matrix receptors in mouse fibronectin, J. Cell Biol. 122 (1993) 235–242.