www.elsevier.com / locate / bres
Research report
Medial prefrontal transection enhances social interaction
I: Behavioral studies
*
Luis E. Gonzalez , Maria Rujano, Sonia Tucci, Daniel Paredes, Elizabeth Silva,
German Alba, Luis Hernandez
Laboratory of Behavioral Physiology, Department of Physiology, School of Medicine, Los Andes University, Merida, Venezuela Accepted 29 August 2000
Abstract
Behavioral effects of a medial prefrontal cortex (MPFC) transection were assessed in animal tests of anxiety. Social investigation and plus-maze open arm exploration increased in MPFC damaged animals relative to sham ones. MPFC lesions preventedD-amphetamine (2
mg / kg, i.p.) induced social investigation decrease and exaggerated general locomotion increase. Diazepam (1 mg / kg, i.p.) and MPFC synergistically increased open arm exploration on a second (repeated) plus-maze trial. These results suggest that the MPFC would be implicated in a generalized mechanism of warning enabling emission of appropriate responses to anxiogenic stimuli. Although, this lesion did not modify motor activity itself, the pattern of the motor activation induced by amphetamine was altered. The role of the MPFC areas in the behavioral response associated with fear is discussed. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behaviour
Topic: Motivation and emotion
Keywords: Anxiety; Prefrontal cortex; Amphetamines; Benzodiazepines; Animal model
1. Introduction action test. A single plus-maze trial (Experiment 3) allowed a comparative study, in which an independent It has been proposed that reciprocal neural circuits group of animals are confronted with a novel situation. linking the MPFC, the extended amygdala and the hypo- Because it has been previously found that systemic thalamus are directly involved in fear processing and the administration of amphetamine decreases social interaction behavioral response to stress in rats [16,20,30]. In support [40] we assessed whether or not MPFC damage suppresses of this hypothesis, Fos protein has been selectively induced amphetamine effect. To compare the behavioral changes in the MPFC, amygdala, lateral septum and hypothalamus induced by amphetamines [25,40] additional behavioral in anxiety related situations but not in many other types of parameters (rearing and self-grooming) were scored in behavioral activation [8,43]. Moreover, positron emission control and lesioned animals (Experiment 2).
tomography studies have shown that the MPFC is con- Finally, systemic administration of benzodiazepines sistently activated during anxiety [18,19,36,52]. does not evoke anxiolytic effect in the plus-maze in mice Although marked effects on social behavior have been or rats with a 5 min previous experience in this test noted [6,45], to the best of our knowledge the social [10,31,37]. However, lesions of subcortical structures interaction test of anxiety has not been used to discriminate interconnected to the MPFC such as the amygdala [12] and effects of MPFC lesions on anxiety. This study evaluates hypothalamus [13] restate sensitivity to the anxiolytic the effect of a surgical MPFC lesion in the social inter- effect of benzodiazepines in this test. On the basis of these observations it was tested whether or not MPFC transec-tion might abolish the typical loss of benzodiazepine effect
*Corresponding author. Tel.:158-74-403-110; fax:158-74-638-304.
E-mail address: [email protected] (L.E. Gonzalez). on the plus-maze second trial. Thereby, MPFC damaged
8 L.E. Gonzalez et al. / Brain Research 887 (2000) 7 –15
rats were treated with benzodiazepines or vehicle and 1900 h. Animals were anesthetized by co-administration of tested on a repeated trial of the elevated plus-maze ketamine and penthothal (110 and 10 mg / k i.p., respec-(Experiment 4). tively) and positioned in a stereotaxic frame (David-Kopf Instruments). The skull was exposed and the incisor bar adjusted such that bregma and lambda were at the same
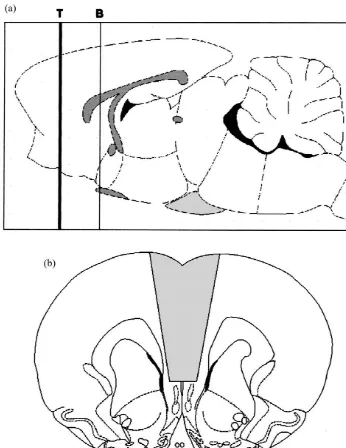
2. Materials and methods height. To lesion the MPFC a knife blade was positioned at 2.5 mm anterior to bregma. The sharp pointed tip of a 2.1. Animals and surgery 3-mm wide scalp blade was lowered 7.0 mm below dura such that each side of the blade extended to a maximum of Male Wistar rats weighing 250–300 g were single 1.5 mm lateral to the midsagital suture (see Fig. 1). housed with food and water ad libitum, and the room Sham-operated had a craniotomy and the longitudinal sinus temperature was kept at 228C. Lights were on from 0700 to was cut. In both type of surgical procedure, bleeding from
Fig. 1. (continued )
sinus was stopped by pressing over the area for about 1 line crossing and rears using a computational timer / coun-min. Animals were allowed to recover for 10 days prior to ter apparatus.
behavioral testing.
2.3.2. The plus-maze
The plus-maze was made of wood and consisted of two 2.2. Drugs
opposite open arms 50310 cm, and two opposite arms enclosed by 40 cm high walls. The arms were connected Diazepam and D-amphetamine sulphate (Sigma, St.
by a 10310 cm central square, and thus the maze formed a Louis, MO, USA) were prepared in 0.9% sodium chloride
‘plus’ shape. The maze was elevated 50 cm above the solution. A drop of Tween 80 was added to disolve
floor. A closed circuit TV camera was vertically mounted diazepam.
over the maze and the behavior was scored from a monitor in an adjacent room. All scores were directly entered into 2.3. Apparatus an IBM computer. Changes in the % of time spent on the
open arms indicate changes in anxiety [34] whereas the 2.3.1. The social interaction test arena number of closed arm entries is the best measure of The social interaction test arena was a wooden box general activity in the maze [4,11]. Testing took place in a 60360 cm, with 35 cm high walls and was lit by low light room lit by dim light (,50 lux) from a light source (50 lux). A camera was vertically mounted above the arena directly above the plus-maze.
and the rats were observed on a monitor in an adjacent
room. Test sessions were also video recorded for sub- 2.4. Behavioral testing sequent analysis. The arena was divided into nine squares
2
10 L.E. Gonzalez et al. / Brain Research 887 (2000) 7 –15
2.4.1. The social interaction test 2.6. Histology Rats were tested under low light, unfamiliar test
con-ditions for anxiogenic or anxiolytic effects [9]. A day prior At the end of the behavioural testing all animals were to testing, operated animals were weighed and allocated to overdosed with chloroform and the brains perfused in-pairs; the partners were non-operated, untreated rats of tracardially with 0.9% saline followed by 4% formalde-similar weight, which had been singly housed for the same hyde solution. Brains were removed from the skull and length of time as the operated animals. Both animals of a fixed with paraffin. Coronal 25 mm microtome sections, pair were placed in low light (50 lux) in the social stained by hematoxylin-eosin, were performed to evaluate interaction arena for a 5-min period. An observer blind to the position and extensions of the lesion [33]. To further rat condition scored the time spent in social interaction. visualize the extension of the mechanical damage and Behaviors were only scored when initiated by the operated gliosis, preliminary studies included horizontal brain sec-animal and consisted of sniffing, following, allogrooming, tions (Fig. 1C).
wrestling, biting and kicking. A surgical scar on the head allowed the scorer to distinguish the operated animals from
2.7. Statistics their partner.
Behavioral scores from experiments 1 and 3 were 2.4.2. Elevated plus-maze
analyzed by Student t-tests. Data from experiments 2 and 4 Each rat was placed in the central square of the
plus-were subjected to analyses of variance with drug and maze facing a closed arm and its behavior observed for 5
lesion as independent factors (Two-way ANOVA). The min by an observer blind to the treatment. The number of
significance obtained by Newman-Keuls’ post-hoc test is entries onto open and closed arms and the times spent in
shown in the figures. open and closed arms and in the central square were
scored. The arena was thoroughly wiped with damp tissue after each trial.
3. Results
2.5. Allocation to experimental groups
3.1. Histology Except for experiments 3 and 4, different sets of rats
were allocated to each experiment. Numbers in brackets The stereotaxic knife cut produced lesions of uniform correspond to animals with correct lesion location. size and comparable volumes. Data from animals with predominant damage on the left (n54: two from experi-2.5.1. Experiment 1 ment 1 and two from experiment 2) and right (n52, from
Effect of the MPFC lesion on the behavior in the social experiments 3 and 4) sides were excluded from the study.
interaction test. Rats were allocated to sham (n59) and The corpus callosum and nucleus accumbens were spared MPFC lesion (n59) groups. in all the animals included in the study. Fig. 1A shows a
diagram of the lesion target area. 2.5.2. Experiment 2
Effect of amphetamine(2 mg /kg) on the behavior in the
3.2. Experiment 1
social interaction test. Rats were allocated to the following groups: sham1vehicle (n58); sham1amphetamine (n58);
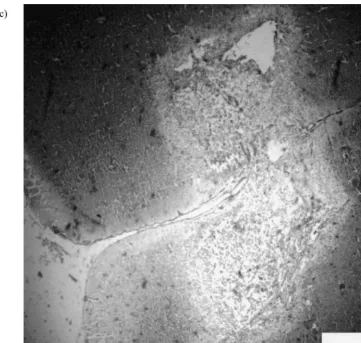
Rats with MPFC lesions significantly spent more time in MPFC lesion1vehicle (n57); MPFC lesion1amphetamine
social interaction than controls (P,0.002, Fig. 2). No (n57).
significant difference in locomotor activity was observed, see Fig. 2. MPFC damaged rats increased social interaction 2.5.3. Experiment 3
by displaying extensive social investigation (sniffing,
Effect of the MPFC lesion on the behavior in the allogrooming, and following). Aggressive behavioral fea-plus-maze. Rats were allocated to sham (n510) and MPFC tures (wrestling, biting and kicking) were not observed. lesion (n510) groups.
2.5.4. Experiment 4 3.3. Experiment 2
Effect of diazepam (1 mg /kg) on the behavior in a
plus-maze second trial. Rats tested on experiment 3 were The effect of amphetamine on time spent in social retested in the plus-maze apparatus for 5 min. Animals interaction varied depending on whether the animal brain were allocated to the following groups: sham1vehicle was intact or transected [drug3lesion, F(1,26)54.85, P,
(n55); sham1diazepam 1 mg / kg (n55); MPFC lesion1 0.01; lesion, F(1,26)516.67, P,0.0001; drug, F(1,26)5
amphet-Fig. 2. Mean (6S.E.M.) time (s) spent in social interaction by rats with medial prefrontal cortex lesions and sham in low light unfamiliar test condition. Unpaired t-test: T(16)53.7, **P,0.002. There was no differ-ence in locomotor activity [T(16)50.3, P50.1].
amine decreased the time spent in social interaction in sham but not in MPFC damaged (Fig. 3A). As in experi-ment 1, MPFC damaged rats increased social interaction exclusively at the expense of social investigation.
Amphetamine increased locomotor activity [F(1,26)5
16.3, P,0.0001] see Fig. 3B. However, this effect was greater in lesioned than in sham animals and there was a significant drug3lesion interaction [F(1,26)53.34, P,
0.05]. However, the lesion itself had no effect on locomotor activity [F(1,26)51.6, NS].
There were effects of the lesions [F(1,26)513.59, P,
0.001], and drug treatment [F(1,26)55.81, P,0.02] on number of rearing and there was significant drug3lesion
Fig. 3. Behavioral measures in the social interaction test by MPFC and
interaction [F(1,26)54.7, P,0.05]. However, post-hoc sham rats after IP injections of saline or d-amphetamine (2 mg / kg) in low analysis showed that rearing only increased in sham but light, unfamiliar test conditions. (A) Mean (6S.E.M.) time (s) spent in
not in lesioned rats following amphetamine administration. social interaction. Sham-amphetamine and lesion-vehicle groups differ significantly from sham-vehicle (*P,0.05 and 0.01, respectively). (B)
In contrast, only the drug factor was significant for time
Mean (6S.E.M.) number of line crossing. *P,0.05 sham-amphetamine
spent in self-grooming [F(1,26)516.97, P,0.0001],
vs. sham-vehicle. **P,0.001 lesioned-amphetamine vs. sham-vehicle
whereas the lesion and drug3lesion factors were not and lesioned-vehicle. (C) Mean (6S.E.M.) number of rearing. *P,0.01 significant [F(1,26)50.01, NS and F(1,26)50.1, NS, sham-amphetamine vs. sham vehicle. (D) Mean (6S.E.M.) time spent
respectively]. Post-hoc analysis showed that amphetamine grooming. *P,0.05 both groups (sham and lesioned) amphetamines groups vs. their controls.
abolished self-grooming in both lesioned and sham animals to the same extent.
It is worth noting that number of rears and time spent in
self-grooming from lesioned and sham animals were 3.5. Experiment 4 similar (Figs. 3C and D).
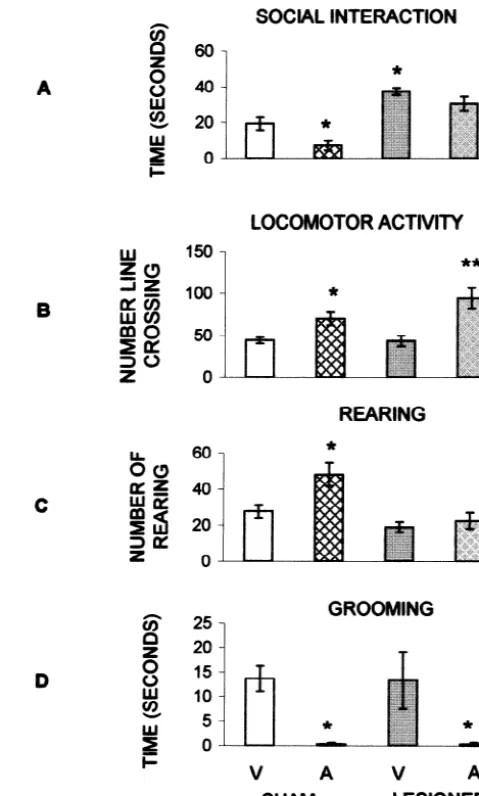
The effect of diazepam on percentage of time spent on the open arms varied depending on whether the animal 3.4. Experiment 3 brain was intact or transected [drug3lesion, F(1,16)53.39,
P,0.04; lesion, F(1,16)56.23, P,0.02; drug, F(1,16)5
12 L.E. Gonzalez et al. / Brain Research 887 (2000) 7 –15
was confirmed that the lesion produced a primary change in anxiety measures, which does not depend on a particular motor activity pattern. Lesioned animals reduced open arms exploration as did sham ones in the second trial of the elevated plus-maze. This implies that MPFC transected rats conserved their ability for spatial discrimination and for changing exploratory strategy.
Increased social behavior in rats following MPFC lesions have been reported elsewhere [6,23]. However, a behavioral analysis on anxiety related measures in the social interaction test was lacking. The increase in social interaction reported here was at the expense of social investigation and not related to augmented aggression.
The results in the plus-maze trial 1 confirm the be-havioral pattern observed in the social interaction test. MPFC transected animals explore the open arms more than controls without altering the entries to closed arms, which is considered the best measure of locomotion on this model [4,11]. During a plus-maze trial rodents progressively reduce open arm exploration due to their natural aversion to open spaces [12,13,17,38,46]. Therefore, the animal identifies and avoids an anxiogenic area. In contrast, social interaction increases as a function of familiarity with the
Fig. 4. Mean (6S.E.M.) time (s) spent on, and number of entries onto,
arena that becomes less aversive over the time [9,35].
open arms of the elevated plus-maze by MPFC and sham rats. (A) TRIAL
1, there was a significant difference between groups [% time open: T(18) The fact that MPFC transections selectively decrease 3.2, **P,0.005; % number open: T(18)52.7, *P,0.01]; (B) TRIAL 2, anxiety as measured by tests whose situational arrange-after IP injections of saline or diazepam (1 mg / kg). *P,0.01
lesioned-ments serve distinct cognitive processes suggests that the
diazepam vs. sham-vehicle group.
MPFC transections primarily altered the general emotional response. Further support for this view comes from the No effects were observed in other plus-maze measures (see
plus-maze second trial experiment. In this test situation Fig. 4B and Table 1).
both sham and MPFC transected animals learned to avoid the open arms. However, MPFC transected animals sig-nificantly differed from sham-vehicle group when treated
4. Discussion
with diazepam. This indicates that MPFC lesions facilitate the anxiolytic effects of benzodiazepines.
MPFC transection reduced anxiety as measured by the
Neuroanatomical studies have shown reciprocal con-social interaction and elevated plus-maze tests. In both
nections between the amygdala, hypothalamus and MPFC models, the lesion did not modify motor activity measures.
[1,21,28,41,42,49]. Lesions in the basolateral amygdala Following systemic administration of amphetamine, rats
and dorsomedial hypothalamus increased sensitivity to with MPFC lesions increased locomotor activity but their
anxiolytic effects of chlordiazepoxide on the second trial in levels of social interaction did not decrease. Therefore, it
the plus-maze [12,13]. It is believe that both of these structures are part of a larger neural circuitry that includes
Table 1 other forebrain (cingulate cortex, MPFC and septum) and
Mean (6S.E.M.) number of entries onto closed arms of the plus-maze by
midbrain (periaqueductal gray, VTA, locus coeruleus,
rats with medial prefrontal cortex lesions (L) and sham (S). On trial 2
raphe nuclei) structures [3,15,20,21,30]. If the anxiety
animals were IP injected with vehicle (V) or diazepam 1 mg / kg (DZP).
There was not significant difference between groups [Trial 1: T(18)51.6, depends on neuronal circuits interconnecting these struc-P50.1; Trial 2: F(3,16)51.1, P50.4] tures, damage of any component of this circuitry would
impair processing and reduce expression of anxiety or
Trial Condition No. of closed arm entries
would facilitate inhibition of this neuronal system by
Mean SDM
diazepam and other CNS depressants.
1 S 9.4 60.7
The finding that lesion did not alter locomotor measures
L 7.7 60.9
is also consistent with previous studies [24,26]. Animals with large MPFC lesions not only were similar to controls
2 S1V 10.4 61.4
S1DZP 10 61.1 in their swimming performance and escape latency during L1V 13 62.0 the spatial training phase but learned to locate the hidden
L1DZP 8.8 61.9
Our results show that D-amphetamine enhanced century. These patients did not have motor alterations and
locomotor activity in lesioned rats but did not change the intellectual capability as measured by conventional test social investigation time. Neonatal excitotoxic lesions of of intelligence was slightly affected. In contrast, their the MPFC enhance sensitivity to the amphetamine-induced emotional life was profoundly touched showing lack of locomotion [14]. Additional destruction of the nucleus drives or lack of inhibitions [27,48]. Similarly, patients accumbens blocked amphetamine-induced locomotion in with traumatic injuries involving prefrontal structures MPFC damaged rats, suggesting that this action depended scored well on perception, language, memory and in-upon the integrity of the nucleus acumbens [51]. In the telligence. However, they had difficulty reassuming a task accompanying paper we reported that MPFC enhanced after a delay, exhibited lack of control and their social amphetamine-induced dopamine release in the nucleus behavior was inappropriate. Detailed analysis on special accumbens, which may underlay amphetamine-induced tasks also revealed that these patients were resistant to locomotion [47]. modify established behaviors when facing changes of The level of vertical activity measured as number of contingencies [39,41,42]. Recently, Tucker et al. [48] have rearing was not different between sham and lesioned suggested that MPFC dorsomedial and ventral lesions animals. However, D-amphetamine increased rearing in produce apathy and disinhibition, respectively.
sham but not in MPFC lesioned animals. Thus, lesioned Cutting across MPFC should interrupt monoaminergic animals showed an altered motor response pattern to projections impairing generalized mechanisms of warning amphetamines. [44,45,53]. As a result the animal exhibits behavioral It has been reported that dorsal MPFC lesions enhance disinhibition in tasks where warning and caution should be timidity and conditioned fear in the rat [22,23,32]. Also, in play. Studies in monkeys and humans support this view Jinks and McGregor [26] suggested that lesions in pre- [39,48,50]. In contrast, lesion in dorsal or other discreet limbic and infralimbic cortex enhanced anxiety as mea- areas within the MPFC would not interrupt most fibers sured by the elevated plus-maze and a modified open field. passing through, thus, preserving functional input to It is possible that restricted lesions of these areas within anterior prefrontal areas.
the MPFC could lead to anxiogenic instead of anxiolytic In summary, MPFC transections have reliable effects on effects. However there are many controversial data that anxiety measures, which do not depend on general motor preclude this conclusion. For instance, dorsal MPFC lesion activity changes or alterations in particular cognitive tasks, had no effect in the elevated plus-maze, prepulse inhibition suggesting a crucial role of the MPFC in the emotional or two-way active avoidance [29]. Furthermore, this lesion response to threatening stimuli.
did not alter the incidence of freezing in a box associated with shock, increased activity in a novel open field,
increased time ‘in contact’ with other rats and did not Acknowledgements induce neo-phobia [22,23,29]. Rats with ventral prefrontal
damage showed significantly step-down shorter latencies in CONICIT G-97000820 and CDCHT-ULA M-653-9903-a pM-653-9903-assive M-653-9903-avoidM-653-9903-ance test, exhibited M-653-9903-a significM-653-9903-antly lower A grants support this research. The authors are grateful for shock-induced respiratory quotient relative to controls [26] the expert assistance of Dr. Carlos E. Mendoza, De-and reduced several other autonomic responses to an- partamento de Histologıa, Universidad de los Andes,´ xiogenic stimuli [15] similar to humans with injures in this Merida, Venezuela.
region [2,5]. Morgan and LeDoux [30] have suggested a functional dissociation between dorsal and ventral aspects of the MPFC on the basis that dorsal but not ventral
References
lesions of the MPFC increased fear reactivity during acquisition of conditioned fear. Collectively, these studies
[1] D.G. Amaral, J.L. Price, Amygdalo-cortical projections in the
suggest, in fact, that ventral but not dorsal damage of the monkey (Macaca fascicularis), J. Comp. Neurol. 230 (1984) 465– MPFC may reduce some types of anxiety. In this respect, 496.
lesions of the ventral MPFC probably produces more [2] A. Bechara, D. Tranel, H. Damasio, A.R. Damasio, Failure to respond autonomically to anticipated future outcomes following
damage along the dorsoventral axis of the MPFC and,
damage to prefrontal cortex, Cereb. Cortex 6 (1996) 215–225.
therefore, behavioral effect of ventral MPFC lesion may
[3] M.J. Christie, L.B. James, P.M. Beart, An excitatory amino acid
resemble those of the large lesion seen in the present study. projection from rat prefrontal cortex to periaqueductal gray, Brain Monkeys with anterior cingulate cortex lesions loss their Res. Bull. 16 (1986) 127–129.
fear and timidity toward humans or co-specifics increasing [4] A.P. Cruz, F. Frei, F.G. Graeff, Ethopharmacological analysis of rat behavior on the elevated plus-maze, Pharmacol. Biochem. Behav. 49
interactive and exploratory behavior [50]. To reduce
(1994) 171–176.
emotional response in patients with obsessive compulsive
[5] A.R. Damasio, D. Tranel, H. Damasio, Individuals with sociopathic
disorder, generalized anxiety or pain, prefrontal psycho- behavior caused by frontal damage fail to respond autonomically to surgery (lobotomy, leukotomy, tractotomy, cingulotomy, social stimuli, Behav. Brain Res. 41 (1990) 81–94.
14 L.E. Gonzalez et al. / Brain Research 887 (2000) 7 –15
following lesions of the orbital prefrontal cortex in male rats, Behav. dopamine system: behavioral and neurochemical evidence, Psycho-Brain Res. 10 (1983) 209–232. pharmacology (Berl.) 138 (1998) 89–95.
[7] J.P. de Bruin, F. Sanchez-Santed, R.P. Heinsbroek, A. Donker, P. [26] A.L. Jinks, I.S. McGregor, Modulation of anxiety-related behaviours Postmes, A behavioural analysis of rats with damage to the medial following lesions of the prelimbic or infralimbic cortex in the rat, prefrontal cortex using the Morris water maze: evidence for be- Brain Res. 772 (1997) 181–190.
havioural flexibility, but not for impaired spatial navigation, Brain [27] E. Kandel, J. Schwartz, T. Jessel (Eds.), The Principles of Neural Res. 652 (1994) 323–333. Science, 3rd Edition, Elsevier, Amsterdam, 1991.
[8] G.E. Duncan, D.J. Knapp, G.R. Breese, Neuroanatomical characteri- [28] J.E. Krettek, J.L. Price, Projections from the amygdaloid complex to zation of Fos induction in rat behavioral models of anxiety, Brain the cerebral cortex and thalamus in the rat and cat, J. Comp. Neurol.
Res. 713 (1996) 79–91. 172 (1977) 687–722.
[9] S.E. File, The use of social interaction as a method for detecting [29] L. Lacroix, L.M. Broersen, I. Weiner, J. Feldon, The effects of anxiolytic activity of chlordiazepoxide-like drugs, J. Neurosci. excitotoxic lesion of the medial prefrontal cortex on latent inhibi-Methods 2 (1980) 219–238. tion, prepulse inhibition, food hoarding, elevated plus maze, active [10] S.E. File, One-trial tolerance to the anxiolytic effects of chlor- avoidance and locomotor activity in the rat, Neuroscience 84 (1998)
diazepoxide in the plus-maze, Psychopharmacology (Berl.) 100 431–442.
(1990) 281–282. [30] J.E. LeDoux, Brain mechanisms of emotion and emotional learning, [11] S.E. File, Behavioural detection of anxiolytic action, in: J.M. Elliot, Curr. Opin. Neurobiol. 2 (1992) 191–197.
D.J. Heal, C.A. Marsden (Eds.), Experimental Approaches to [31] R.G. Lister, The use of a plus-maze to measure anxiety in the Anxiety and Depression, John Wiley, Chichester, UK, 1992, pp. mouse, Psychopharmacology (Berl.) 92 (1987) 180–185. 25–44. [32] M.A. Morgan, J.E. LeDoux, Differential contribution of dorsal and [12] S.E. File, L.E. Gonzalez, R. Gallant, Role of the basolateral nucleus ventral medial prefrontal cortex to the acquisition and extinction of
of the amygdala in the formation of a phobia, Neuropsychophar- conditioned fear in rats, Behav. Neurosci. 109 (1995) 681–688. macology 19 (1998) 397–405. [33] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, [13] S.E. File, L.E. Gonzalez, R. Gallant, Role of the dorsomedial Academic Press, Sydney, 1982.
hypothalamus in mediating the response to benzodiazepines on trial [34] S. Pellow, P.H. Chopin, S.E. File, M. Briley, Validation of open: 2 in the elevated plus-maze test of anxiety, Neuropsychopharmacol- closed arm entries in an elevated plus-maze as a measure of anxiety ogy 21 (1999) 312–320. in the rat, J. Neurosci. Methods 14 (1985) 149–167.
[14] G. Flores, G.K. Wood, J.J. Liang, R. Quirion, L.K. Srivastava, [35] R.J. Primus, C.K. Kellogg, Pubertal-related changes influence the Enhanced amphetamine sensitivity and increased expression of development of environment-related social interaction in the male dopamine D2 receptors in postpubertal rats after neonatal excitotox- rat, Dev. Psychobiol. 22 (1989) 633–643.
ic lesions of the medial prefrontal cortex, J. Neurosci. 16 (1996) [36] M. Reivich, R. Gur, A. Alavi, Positron emission tomographic 7366–7375. studies of sensory stimuli, cognitive processes and anxiety, Hum. [15] R.J. Frysztak, E.J. Neafsey, The effect of medial frontal cortex Neurobiol. 2 (1983) 25–33.
lesions on cardiovascular conditioned emotional responses in the rat, [37] R.J. Rodgers, J.K. Shepherd, Influence of prior maze experience on Brain Res. 643 (1994) 181–193. behaviour and response to diazepam in the elevated plus-maze and [16] L.E. Goldstein, A.M. Rasmusson, B.S. Bunney, R.H. Roth, Role of light / dark tests of anxiety in mice, Psychopharmacology (Berl.) 113
the amygdala in the coordination of behavioral, neuroendocrine, and (1993) 237–242.
prefrontal cortical monoamine responses to psychological stress in [38] R.J. Rodgers, N.J. Johnson, J.C. Cole, C.V. Dewar, G.R. Kidd, P.H. the rat, J. Neurosci. 16 (1996) 4787–4798. Kimpson, Plus-maze retest profile in mice: importance of initial [17] L.E. Gonzalez, S.E. File, A 5-min experience in the elevated stages of trail 1 and response to post-trail cholinergic receptor
plus-maze alters the state of the benzodiazepine receptor in the blockade, Pharmacol. Biochem. Behav. 54 (1996) 41–50. dorsal raphe nucleus, J. Neurosci. 17 (1997) 1505–1511. [39] E.T. Rolls, J. Hornak, D. Wade, J. McGrath, Emotion-related [18] L.A. Gottschalk, M.S. Buchsbaum, J.C. Gillin, J. Wu, C.A. learning in patients with social and emotional changes associated Reynolds, D.B. Herrera, Positron-emission tomographic studies of with frontal lobe damage, J. Neurol. Neurosurg. Psychiatry 57 the relationship of cerebral glucose metabolism and the magnitude (1994) 1518–1524.
of anxiety and hostility experienced during dreaming and waking, J. [40] F. Sams-Dodd, Effect of continuous D-amphetamine and Neuropsychiatry Clin. Neurosci. 3 (1991) 131–142. phencyclidine administration on social behavior, stereotyped be-[19] L.A. Gottschalk, M.S. Buchsbaum, J.C. Gillin, J. Wu, C.A. havior and locomotor activity in rats, Neuropsychopharmacology 19
Reynolds, D.B. Herrera, The effect of anxiety and hostility in silent (1998) 18–25.
mentation on localized cerebral glucose metabolism, Compr. Psychi- [41] S.R. Sesack, V.M. Pickel, Prefrontal cortical efferents in the rat atry 33 (1992) 52–59. synapse on unlabeled neuronal targets of catecholamine terminals in [20] F.G. Graeff, Minor tranquilizers and brain defense systems, Braz. J. the nucleus accumbens septi and on dopamine neurons in the ventral
Med. Biol. Res. 14 (1981) 239–265. tegmental area, J. Comp. Neurol. 320 (1992) 145–16041. [21] H.J. Groenewegen, Organization of the afferent connections of the [42] T. Shallice, P.W. Burgess, Deficits in strategy application following
mediodorsal thalamic nucleus in the rat, related to the mediodorsal- frontal lobe damage in man, Brain 114 (Pt. 2) (1991) 727–741. prefrontal topography, Neuroscience 24 (1988) 379–431. [43] M.C. Silveira, G. Sandner, F.G. Graeff, Induction of Fos immuno-[22] R.R. Holson, Mesial prefrontal cortical lesions and timidity in rats. I. reactivity in the brain by exposure to the elevated plus-maze, Behav.
Reactivity to aversive stimuli, Physiol. Behav. 37 (1986) 221–230. Brain Res. 56 (1993) 115–118.
[23] R.R. Holson, C. Walker, Mesial prefrontal cortical lesions and [44] C.J. Stam, J.P. de Bruin, A.M. van Haelst, J. van der Gugten, A. timidity in rats. II. Reactivity to novel stimuli, Physiol. Behav. 37 Kalsbeek, Influence of the mesocortical dopaminergic system on (1986) 231–238. activity, food hoarding, social-agonistic behavior, and spatial de-[24] G.E. Jaskiw, D.R. Weinberger, Ibotenic acid lesions of the medial layed alternation in male rats, Behav. Neurosci. 103 (1989) 24–35. prefrontal cortex potentiate FG-7142-induced attenuation of ex- [45] J.W. Tidey, K.A. Miczek, Social defeat stress selectively alters ploratory activity in the rat, Pharmacol. Biochem. Behav. 36 (1990) mesocorticolimbic dopamine release: an in vivo microdialysis study,
695–697. Brain Res. 721 (1996) 140–149.
[47] S. Tucci, Q. Contreras, X. Paez, L. Gonzalez, P. Rada, L. Her- structures compete for behavioral expression? Evidence from am-nandez, Medial prefrontal transection enhances social interaction: II. phetamine-induced behavior, microdialysis, and caudate-accumbens Neurochemical studies. Submitted to Brain Research, 2000. lesions in medial frontal cortex damaged rats, Brain Res. 576 (1992) [48] D.M. Tucker, P. Luu, K.H. Pribram, Social and emotional self- 1–11.
regulation, Ann. NY Acad. Sci. 769 (1995) 213–239. [52] J.C. Wu, M.S. Buchsbaum, T.G. Hershey, E. Hazlett, N. Sicotte, J.C. [49] S.R. Vincent, T. Hokfelt, L.R. Skirboll, J.Y. Wu, Hypothalamic Johnson, PET in generalized anxiety disorder, Biol. Psychiatry 29
gamma-aminobutyric acid neurons project to the neocortex, Science (1991) 1181–1199.
220 (1983) 1309–1311. [53] M. Yoshioka, M. Matsumoto, H. Togashi, H. Saito, Effects of [50] A.A. Ward, The cingular gyrus: Area 24, J. Neurophysiology 11 conditioned fear stress on 5-HT release in the rat prefrontal cortex,
(1948) 13–23. Pharmacol. Biochem. Behav. 51 (1995) 515–519.