www.elsevier.com / locate / bres
Research report
Ovarian steroids influence the activity of neuroendocrine dopaminergic
neurons
*
Jamie E. DeMaria, John D. Livingstone, Marc E. Freeman
Program in Neuroscience, Department of Biological Science, Florida State University, Tallahassee, FL 32306-4340, USA Accepted 25 July 2000
Abstract
The secretion of prolactin (PRL) from the anterior lobe (AL) of the pituitary gland is tonically inhibited by dopamine (DA) of hypothalamic origin. While ovarian steroids play a role in the regulation of the secretion of PRL, their effect on all three populations of hypothalamic neuroendocrine dopaminergic neurons is not fully understood. In this study we describe the effects of ovarian steroids on regulation of the release of DA from tuberoinfundibular dopaminergic (TIDA), tuberohypophyseal dopaminergic (THDA) and periventricular-hypophyseal dopaminergic (PHDA) neurons. Adult female rats were bilaterally ovariectomized (OVX) and, 10 days following ovariectomy (day 0), injected with corn oil (vehicle), estrogen, or estrogen plus progesterone (day 1). Animals were sacrificed every 2 h from 09.00 to 21.00 h by rapid decapitation. Trunk blood was collected and the concentration of PRL in serum was determined by radioimmunoassay. The median eminence (ME) and the AL, intermediate (IL) and neural (NL) lobes of the pituitary gland were dissected and the concentration of DA and DOPAC in each was measured by HPLC-EC. OVX rats presented small but significant increases in the secretion of PRL at 15.00 and 17.00 h. Replacement of estrogen or estrogen plus progesterone increased the basal concentration of PRL. Moreover, injection of estrogen only, or estrogen plus progesterone increased the concentration of PRL in serum at 15.00 h through 19.00 h, respectively, followed by a decrease to baseline thereafter. The turnover of DA in the ME and NL of OVX rats increased at 13.00 and returned to low levels. Turnover of DA in the IL of OVX rats increased in the morning by 11.00 h and remained elevated before decreasing by 17.00 h. The turnover of DA in the ME, IL and NL of OVX rats increased by 19.00 h. Injection of estrogen advanced the increase of TIDA activity by 2 h in the ME compared to OVX rats. Moreover, administration of estrogen suppressed the activity of THDA and PHDA neurons in the afternoon compared to OVX rats. In estrogen plus progesterone-treated rats, the activity of hypothalamic neuroendocrine dopaminergic neurons terminating in the ME, IL, and NL was inhibited prior to the increase in the secretion of PRL. The concentration of DA in the AL diminished prior to the estrogen-induced increase of PRL. Administration of progesterone, in concert with estrogen, delayed the increase of PRL in serum and the decrease of DA in the AL, compared to estrogen-treated rats, by 4 h. These data suggest a major role for ovarian steroids in controlling increases in the secretion of PRL by not only stimulating PRL release from lactotrophs, but also by inhibiting the activity of all three populations of hypothalamic neuroendocrine DAergic neurons. 2000 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Neuroendocrine: other
Keywords: Estrogen; Prolactin; Pituitary gland; Median eminence; Arcuate nucleus; Periventricular nucleus
1. Introduction gland is stimulated by hormone-specific releasing factors
originating within the hypothalamus [25,46–48]. While a With the exception of prolactin (PRL), the secretion of single PRL releasing factor, analogous to those found for hormones from the anterior lobe (AL) of the pituitary other pituitary hormones, has yet to be identified, several candidates, including ovarian steroids, have been consid-ered [41].
*Corresponding author. Tel.: 11-850-644-3896; fax: 1
1-850-644-The secretion of PRL is tonically inhibited by dopamine
4583.
E-mail address: [email protected] (M.E. Freeman). (DA) [4]. DA arrives at the AL through three distinct
dopaminergic (DAergic) pathways: (1) Tuberoinfundibular libitum. Animal procedures were approved by the Florida DAergic (TIDA) neurons arise from throughout the arcuate State University Animal Care and Use Committee. Rats nucleus and terminate on the primary capillary loops of the were bilaterally ovariectomized (OVX) under Halothane long portal vessels within the external zone of the median anesthesia and subsequently divided into three groups. Ten eminence (ME) [22]. Long portal vessels transport DA days following OVX, rats in group one (estrogen-treated) released from TIDA neurons to the AL. (2) received an injection of estrogen (20 mg, i.p., Sigma, St Tuberohypophyseal DAergic (THDA) neuron cell bodies Louis, MO) at 10.00 h on day zero and corn oil at 12.50 h are located in the rostral portion of the arcuate nucleus and on day one. Rats in group two received an injection of terminate in both the intermediate (IL) and neural (NL) estrogen (20mg, i.p., Sigma) at 10.00 h on day zero and an lobes of the pituitary gland [6]. (III) Periventricular-hypo- injection of progesterone (5 mg, i.p., Sigma) at 12.50 h on physeal DAergic (PHDA) neurons originate in the periven- day one. Rats in group three received an injection of corn tricular nucleus of the hypothalamus and terminate exclu- oil (200ml, i.p., vehicle) at 10.00 h on day zero and 12.50 sively in the IL [23,24]. DA released from THDA and h on day one. Five rats from each treatment group were PHDA neurons is transported to the AL through short sacrificed by rapid decapitation every 2 h from 09.00 to portal vessels. 21.00 h on day one. Rats decapitated at 13.00 h on day 1 The increasing concentration of estradiol in serum on had received corn oil or progesterone 10 min before the afternoon of diestrus-2 of the estrous cycle of the rat decapitation. The ME, AL, IL, and NL from each animal stimulates the proestrous increase of PRL in serum [40]. were dissected and individually stored in cryogenic vials Immunoneutralization of endogenous estrogen during the with 250 ml of homogenization buffer [0.2 N perchloric afternoon of diestrus-2 prevents the increase of PRL on the acid, 26 mM EGTA, 700 pM dihydroxybenzylamine afternoon of proestrus [20,40]. In addition to stimulating (DHBA, internal standard)] at 2408C until day of assay. the secretion of PRL [41], estrogen diminishes and / or Trunk blood was collected, allowed to clot, centrifuged, reverses the inhibitory response of lactotrophs to DA and serum was removed and stored at 2408C until the [8,12,19,34], recruits cells within the pituitary gland to concentration of PRL was measured by RIA.
produce [31] and secrete [7,18,26] PRL and increases transcription of the PRL gene [30–32,36,38,50–53].
Un-like estrogen, the role of progesterone in the regulation of 2.2. HPLC-EC the secretion of PRL is not as thoroughly defined.
How-ever, when administered in concert with estrogen, proges- ME, AL, IL, and NL samples were thawed, homogen-terone is a potent stimulator of the secretion of PRL [10]. ized, and sonicated. Twenty microliters of homogenate It has previously been shown that the activity of were removed for protein assay. The remaining sample dopaminergic neurons terminating in the ME [42] and the was centrifuged (10 min @ 10003g). The supernatant was concentration of DA in portal vessels is decreased [5] prior filtered through a 0.2 mm nylon microfiltration unit (Os-to the peak of PRL on the afternoon of proestrus. In monics, Livermore, CA), and then placed into autosampler addition, we previously reported that the concentration of vials.
DA and DOPAC decreases in the AL and IL, but not the The concentration of DA and DOPAC in each sample NL prior to increased secretion of PRL on the afternoon of was measured using high performance liquid chromatog-proestrus [16]. Moreover, it has been shown that ovarian raphy coupled to electrochemical detection (HPLC-EC), as steroids regulate biosynthesis [2,3,28,49,55] and release previously described [15–17,39]. Twenty microliters of [13,14] of DA from TIDA neurons. each sample was injected by an autosampler (WISP 710 In this study we have described the effects of ovarian Autosampler, Waters Corp., Milford, MA). Mobile phase steroids on the activity of all three populations of hypo- consisting of 75 mM sodium dihydrogen phosphate mono-thalamic neuroendocrine dopaminergic neurons. Changes hydrate (EM Science, Gibbstown, NJ), 1.7 mM 1-octane in the concentrations of DA and DOPAC in the ME, AL, sulfonic acid (Fisher Scientific), 100 ml / l triethylamine IL, and NL and the concentration of PRL in serum were (Aldrich, Milwaukee, WI), 25 mM EDTA (Fisher Sci-monitored following administration of estrogen and es- entific), 6% acetonitrile (EMScience), titrated to pH 3.0 trogen plus progesterone in ovariectomized rats. with phosphoric acid (Fisher Scientific), was delivered by a dual piston pump (Kratos Analytical Instruments, Ram-sey, NJ) at 600 ml / min. Water was purified on a Milli-Q
2. Materials and methods system (Millipore, Bedford, MA) to 18 MVand polished with a C18 Sep-Pak (Millipore).
cell (E :1 255 mV, E :2 2185 mV, ESA 5011 High 3. Results
Sensitivity Analytical Cell, ESA inc.). The change in
current on the second analytical electrode was measured by 3.1. Concentration of PRL in serum a coulometric detector (ESA Coulochem II, ESA Inc.) and
recorded using Baseline 810 software (Waters Corp.). DA The concentration of PRL in serum is shown in Fig. 1. and DOPAC were identified on the basis of their peak
retention times (RT511.0 and 6.5 min, respectively). The amount of catecholamine in each sample was estimated by comparison to the area under each peak generated by known amounts of each catecholamine. The amount of DHBA (RT57.5 min) recovered was compared to the amount of DHBA added as internal standard and corrected for any loss of sample (usually ,5%). The sensitivity of the assay was 30 pg of DA and 33 pg of DOPAC.
2.3. Protein assay
The amount of protein in each sample was measured using a modified form of the Pierce BCA Protein Assay Kit (Pierce, Rockford, IL). Homogenate (10 ml) from sonicated tissue samples was aliquoted into 96-well plates (Corning, Corning, NY) in duplicate and 200 ml of BCA solution was added to each well. The plate was incubated for 30 min at 608C, and the absorbance of each well was measured at 562 nm by a plate spectrophotometer (Molec-ular Devices, Palo Alto, CA). Unknowns were compared against standards of bovine serum albumin.
2.4. Radioimmunoassay (RIA)
The concentration of PRL in serum was determined by RIA as previously described [21] with materials supplied by Dr Albert F. Parlow and the National Hormone and Pituitary Program. Serum concentrations of PRL were expressed as ng / ml in terms of the rat PRL RP-3 standard in assays whose sensitivity averaged 1 ng / ml. The inter-assay coefficient of variation was 10% and the intra-inter-assay coefficient of variation was 5% for both assays.
2.5. Data analysis
Differences in the DOPAC / DA ratio, the concentration of DA in the AL, and the concentration of PRL in serum within individual treatment groups were compared statisti-cally using a one-way ANOVA. Tukey’s honestly signifi-cant difference test was used as a post-hoc test for all data sets. Values are considered significant at P,0.05.
Differences in the DOPAC / DA ratio, the concentration of DA in the AL, and the concentration of PRL in serum between treatment groups were compared statistically
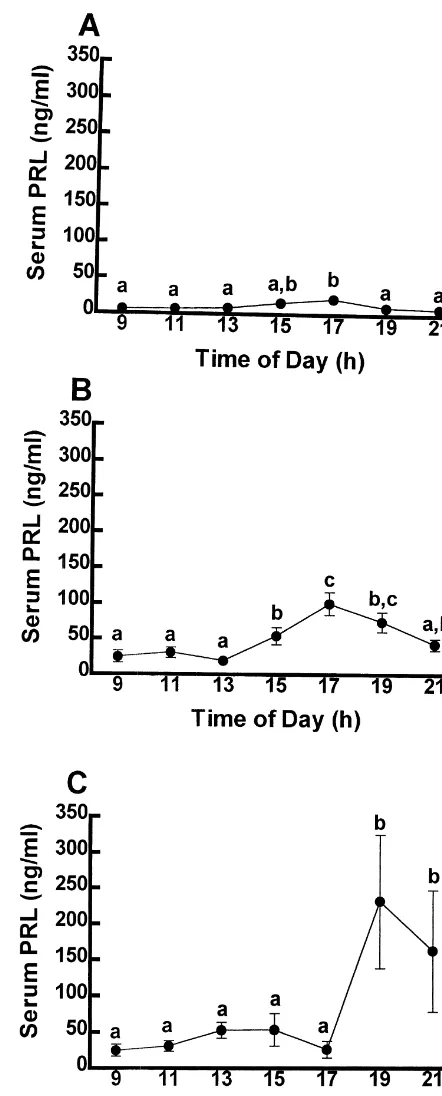
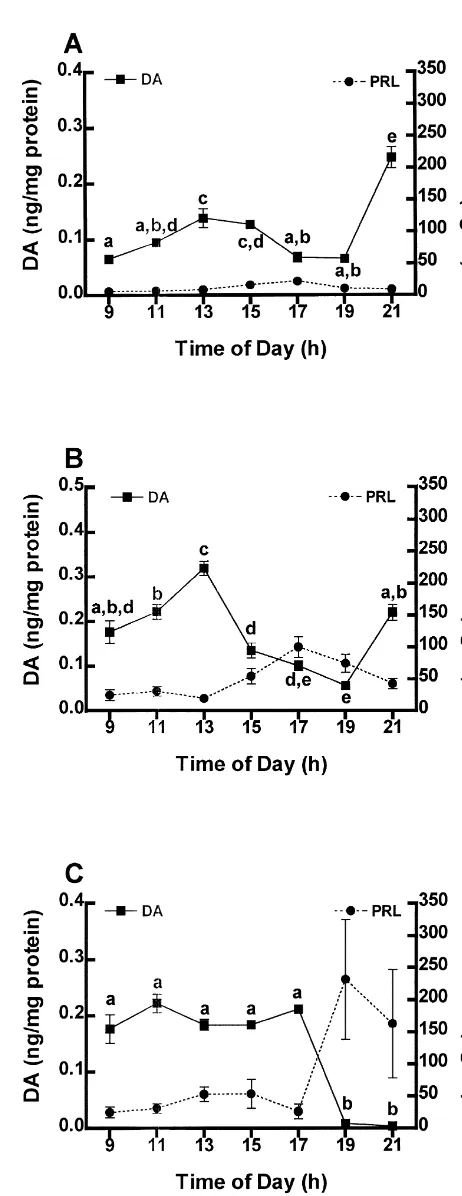
Fig. 1. The concentration of PRL in the serum of OVX (A),
estrogen-using a two-way ANOVA. Tukey’s honestly significant (B), and estrogen plus progesterone- (C) treated rats. Each point difference test was used as a post-hoc test for all data sets. represents the mean6S.E.M. (n55). Points with dissimilar letters are
The concentration of PRL in serum of OVX rats is low and unchanging for most of the day with the exception of a 25% increase from baseline (P,0.05) occurring between 15.00 and 17.00 h (Fig. 1A). Unlike the steady and relatively unchanging levels of PRL in OVX rats, replace-ment of ovarian steroids significantly elevates baseline secretion of PRL. The concentration of PRL in serum of estrogen-treated rats is significantly increased (P,0.05) by 15.00 h, peaks by 17.00 h at a concentration three-fold greater than baseline, and returns to low levels by 21.00 h (Fig. 1B). The concentration of PRL in serum of estrogen plus progesterone-treated rats significantly increases by 19.00 h at a concentration five-fold greater than baseline, and diminishes to low levels thereafter (Figs. 1C).
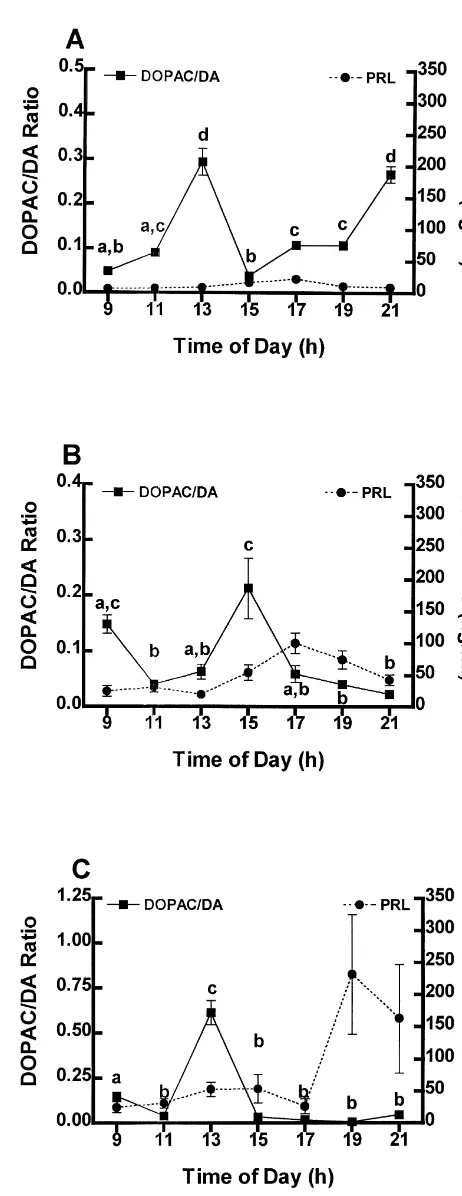
3.2. TIDA neuronal activity: DOPAC /DA turnover in the ME
The turnover of DA in the ME of OVX (A), estrogen-(B) and estrogen plus progesterone- (C) treated rats is shown in Fig. 2 as the mean DOPAC / DA ratio6S.E.M. In this and subsequent figures the serum PRL concentrations shown in Fig. 1 are duplicated for comparison. DA turnover is significantly increased (P,0.05) by 13.00 h in OVX rats (Fig. 2A), diminishes to low levels by 15.00 h, returns to baseline by 17.00 h and subsequently increases by 21.00 h (Fig. 2A). Injection of estrogen to OVX rats decreases the turnover of DA in the ME between 09.00 and 11.00 h (P,0.05), delays the increase in DA turnover seen in OVX rats until 15.00 h concomitant with the initial increase in the secretion of PRL, and prevents the late evening increase in turnover of DA observed in OVX rats by 21.00 h (Fig. 2B). The DOPAC / DA ratio in estrogen plus progesterone-treated rats increases significantly (P,
0.05) by 13.00 h similar to OVX rats, but unlike OVX rats is depressed through the late afternoon coincident with an increase in serum PRL (Fig. 2C).
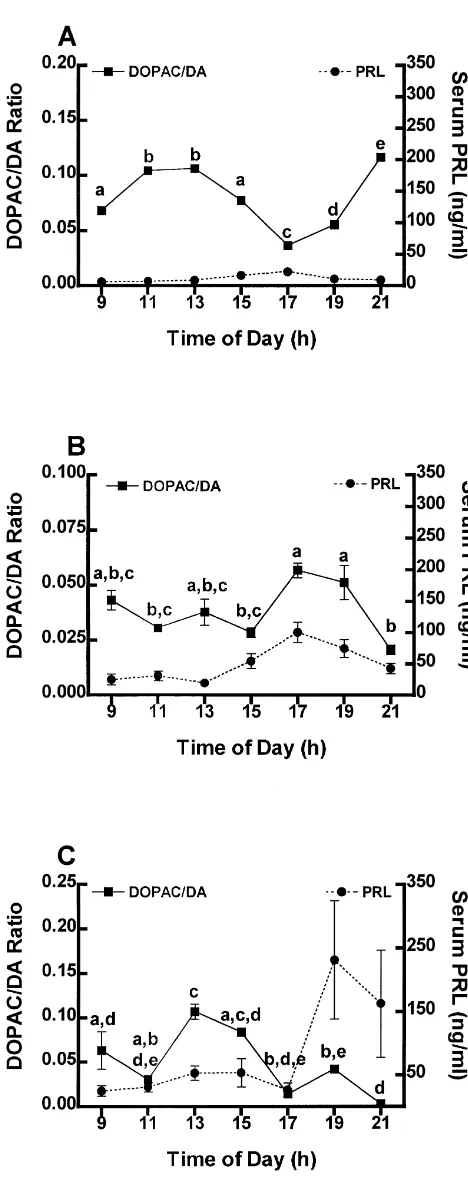
3.3. PHDA neuronal activity: DOPAC /DA turnover in the IL
The turnover of DA in the IL of OVX rats increases by 11.00 h (P,0.05), returns to low levels by 17.00 h, coincident with the increase of serum PRL, and again increases (P,0.05) through 21.00 h (Fig. 3A). In contrast to the turnover of DA in the IL of OVX rats, the turnover of DA in the IL of estrogen-treated rats is unchanged between 09.00 and 15.00 h (Fig. 3B). The turnover of DA in the IL of estrogen-treated rats subsequently increases (P,0.05) by 17.00 h, coincident with the increase of
serum PRL, and returns to low levels concomitant with the Fig. 2. The concentration of PRL in serum and the DOPAC / DA ratio in the median eminence of OVX (A), estrogen- (B), and estrogen plus
levels of PRL in serum (Fig. 3B). This is nearly a
mirror-progesterone- (C) treated rats. Circles indicate mean concentration of
image of the pattern of PRL secretion in OVX rats.
PRL in serum6S.E.M. Significant differences in the concentration of
Estrogen plus progesterone treatment significantly in- PRL are as indicated in Fig. 1. Squares are mean DOPAC / DA creases (P,0.05) DA turnover by 13.00 h, which then ratio6S.E.M. Points with different letters are significantly different (P,
initiation of the increase of PRL in serum (Fig. 3C). This pattern is similar in timing, but differs in magnitude from that of OVX rats. However, unlike OVX rats, the turnover of DA in estrogen plus progesterone-treated rats remains low and unchanging in the IL thereafter (Fig. 3C).
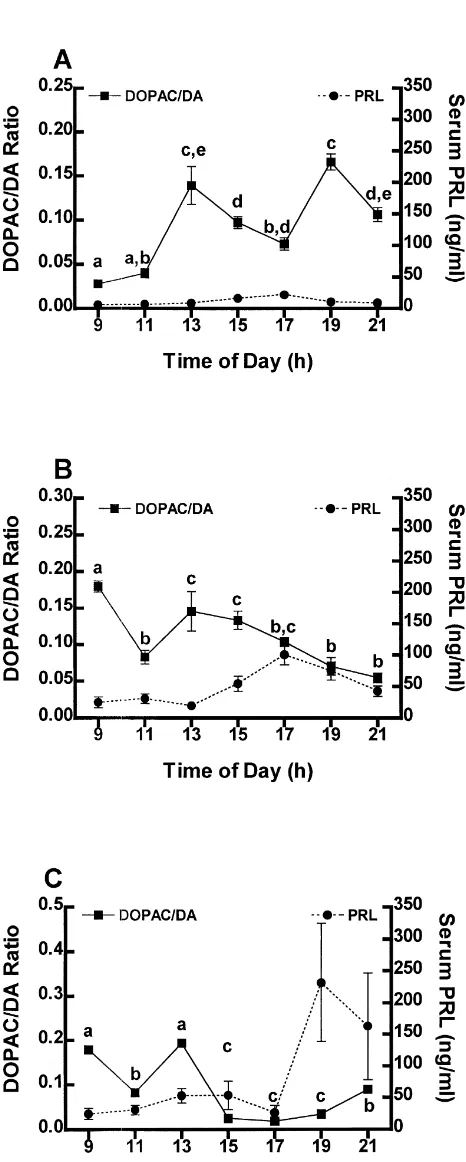
3.4. THDA neuronal activity: DOPAC /DA turnover in the NL
The turnover of DA in the NL of OVX rats increases (P,0.05) twice; first by 13.00 h, prior to the increase of PRL in serum, and the second by 19.00 h, during the waning phase of the peak of serum PRL (Fig. 4A). Unlike OVX rats, estrogen treatment causes a decrease (P,0.05) in the activity of THDA neurons by 11.00 h. However, similar to changes in OVX rats, the turnover of DA in the NL of estrogen plus progesterone-treated rats returns to high levels by 13.00 h (Fig. 4B). The turnover of DA in the NL of estrogen-treated rats decreases (P,0.05) steadi-ly thereafter to low levels at 21.00 h coincident with the increase of PRL in serum (Fig. 4B). During the morning, estrogen plus progesterone-treated rats present a DA turnover profile similar to that of the estrogen-treated rats (Figs. 4B, C). However, following injection of progester-one at 13.00 h, DA turnover is significantly (P,0.05) depressed and remains low through 21.00 h (Fig. 4C), prior to and coincident with the increase of PRL in serum.
3.5. Concentration of DA in the anterior lobe
The concentration of DA in the AL of OVX rats increases slightly from 09.00 to 15.00 h (Fig. 5A). Coincident with the slight increase of PRL in serum at 17.00 h, the concentration of DA in the AL decreases to morning levels (P,0.05) and remains low until it increases significantly (P,0.05) again at 21.00 h (Fig. 5A). Al-though of greater magnitude in estrogen-treated rats, the patterns of DA in the AL are similar between 09.00 h and 13.00 h in the OVX and estrogen-treated groups (Fig. 5A, B). In both groups, the concentration of DA in the AL significantly decreases (P,0.05) coincident with the in-crease in the concentration of PRL in serum at 15.00 h and remains low through 19.00 h (Fig. 5B). Concomitant with the return of PRL to baseline levels, the concentration of DA in the AL increases to levels similar to those of the morning (Fig. 5B). Similar to the concentration of DA in the AL of OVX rats, the concentration of DA in the AL of estrogen plus progesterone-treated rats is unchanged through 17.00 h and then subsequently decreases through 21.00 h (P,0.05). However, in estrogen plus
progester-Fig. 3. The concentration of PRL in serum and the DOPAC / DA ratio in one-treated rats, the rate of decrease is greater than that
the intermediate lobe of OVX (A), estrogen- (B), and estrogen plus seen in the AL of OVX rats. The concentration of DA in progesterone- (C) treated rats. Circles indicate mean concentration of the AL of estrogen and progesterone-treated rats remains PRL in serum6S.E.M. Significant differences in the concentration of
low through 21.00 h while the secretion of PRL increases
PRL are as indicated in Fig. 1. Squares are mean DOPAC / DA
(Fig. 5C; P,0.05), unlike the concentration of DA in the
ratio6S.E.M. Points with different letters are significantly different (P,
4. Discussion
While ovarian steroids play a critical role in the direct regulation of PRL secretion [41], they are not the only
´
participants in what is rapidly becoming a melange of regulatory factors. DA is the physiological inhibitor of the secretion of PRL [4]. However, the interplay between ovarian steroids and DA in regulating PRL secretion has yet to be elucidated. In these experiments we report the effects ovarian steroids have on the activity of three populations of hypothalamic neuroendocrine DAergic neu-rons involved in the control of PRL secretion.
In the absence of ovarian steroids the secretion of PRL is low and relatively stable throughout the day [9,10,27]. Administration of exogenous estrogen stimulates the secre-tion of PRL in OVX rats [9,10]. Immunoneutralizasecre-tion of endogenous estrogen prevents spontaneous secretion of PRL on proestrus [40]. Moreover, administration of pro-gesterone to estrogen primed rats, advances the timing [56] and enhances the secretion of PRL [9,10,27].
The DOPAC / DA ratio in the ME is an effective tool for monitoring the activity of TIDA neurons [35], whereas the DOPAC / DA ratio in the posterior pituitary is an effective tool for measuring the change in activity of THDA and PHDA neurons terminating in the IL and NL [33]. Using DOPAC / DA ratios, we have measured the activity of neuroendocrine dopaminergic neurons terminating in the ME, IL and NL in response to estrogen and estrogen plus progesterone. Additionally, we have measured the con-centration of DA in the AL as a measure of DA arriving at the target tissue.
It has previously been reported that estrogen inhibits the expression of tyrosine hydroxylase in the arcuate nucleus [2,28] and the release of DA into long portal vessels [13]. The data presented here reflect estrogen inhibition of neuroendocrine dopaminergic neurons. TIDA and THDA neuronal activity in estrogen-treated rats is inhibited in the morning between 09.00 and 11.00 h (Fig. 2B and 4B). Subsequently, the turnover of DA in TIDA neurons in the ME of estrogen-treated rats increases coincident with the initiation of the increase of PRL and returns to low levels at the peak concentrations of PRL (Fig. 2B). The turnover of DA in THDA neurons in the NL of estrogen-treated rats decreases steadily through the initiation, peak, and termi-nation of the increased secretion of PRL (Fig. 4B). The turnover of DA in PHDA neurons of the IL of estrogen-treated rats is relatively constant throughout the morning (09.00–15.00 h), increases coincident with the peak of PRL and returns to baseline thereafter (Fig. 3B). These data indicate that estrogen plays a role in the regulation of neuroendocrine dopaminergic neurons by inhibiting the
Fig. 4. The concentration of PRL in serum and the DOPAC / DA ratio in activity of these neurons prior to the initiation of the the neural lobe of OVX (A), estrogen- (B), and estrogen plus progester- increase of PRL. Indeed it has been hypothesized that one- (C) treated rats. Circles indicate mean concentration of PRL in
estrogen down regulates the activity of TIDA neurons by
serum6S.E.M. Significant differences in the concentration of PRL are as
diminishing the activity of tyrosine hydroxylase [28]. In
indicated in Fig. 1. Squares are mean DOPAC / DA ratio6S.E.M. Points
dopa-minergic neuronal activity, estrogen serves as a major stimulator of lactotroph recruitment [26,31], PRL synthesis [30–32,37,50,51,53,54] and PRL secretion [9,10].
Progesterone alone does not have a substantial effect on the secretion of PRL [10,45]. However, when administered in concert with estrogen, progesterone is a potent stimulator of the secretion of PRL [10] and has been shown to reverse the estradiol-induced inhibition of tyrosine hydroxylase mRNA in the hypothalamus [2,3]. Administration of progesterone to estrogen-treated animals caused an increase in the activity of all three populations of neuroendocrine dopaminergic neurons at 13.00 h (Figs 2–4C) compared to estrogen-treated rats. The activity of TIDA and THDA neurons returns to low levels immedi-ately following the increase of their activity at 13.00 h, whereas the activity of PHDA neurons decreases over a four hour period. The activity of all three populations of neuroendocrine dopaminergic neurons remains low throughout the initiation of the increase in PRL secretion (Figs. 2–4C). It has been shown that long-term progester-one treatment will stimulate the release of DA into portal vessels [14]. Following progesterone induced increases in the activity of these neurons, the activity of all three populations of neuroendocrine dopaminergic neurons de-clines rapidly and remains low through the increase of PRL secretion. Progesterone may be acting to deplete neurons of DA so that, concomitant with the increase of PRL secretion, the activity of neuroendocrine dopa-minergic neurons is low and unchanging.
The sum of the activity of these three populations of neurons is reflected by the concentration of DA in the AL of the pituitary gland (Fig. 5A–C). While the concentration of DA in the AL is not an accurate indicator of hypo-thalamic neuroendocrine dopaminergic activity [33,35], it reveals the amount of DA arriving at the target cells from all three populations. The relative contribution of DA from each neuron population to the AL cannot be ascertained from these data. However, based on our previous work indicating that all three populations of neuroendocrine dopaminergic neurons contribute DA to the AL [17], it is likely that each population contributes DA to the regulation of the secretion of PRL. In each treatment group the concentration of DA in the AL decreases prior to the increase in the secretion of PRL (Fig. 5). Moreover, the degree and timing of the decline appears to be mediated by the presence of ovarian steroids. The abundance of DA in the AL of OVX rats is low throughout most of the morning. The concentration of DA in the AL of OVX rats increases slightly (P,0.05) by 13.00 h, then decreases by 17.00 h, coincident with the slight increase in the con-centration of PRL in serum (Fig. 5A). Estrogen treatment
Fig. 5. The concentration of PRL in serum and the concentration of DA
of OVX rats doubles the concentration of DA in the AL
in the AL of OVX (A), estrogen- (B), and estrogen plus progesterone- (C)
compared to oil-treated OVX rats. The concentration of
treated rats. Circles indicate mean concentration of PRL in
DA in the AL of estrogen-treated rats increases by 13.00 h serum6S.E.M. Significant differences in the concentration of PRL are as and subsequently declines concomitant with the initiation indicated in Fig. 1. Squares are mean DA concentration6S.E.M. Points
of estrogen-treated rats remains low through 19.00 h, then Hormone Pituitary Program are thanked for the PRL RIA increases back to values similar to those at 09.00 h (Fig. reagents. This work was supported by NIH DK 43,200 to 5B). The decrease of DA in the AL at 15.00 h in estrogen- MEF.
treated rats is two-fold) greater than that of OVX rats. The apparent decrease of DAergic tone on the lactotroph, in concert with the lactotroph-priming action of estrogen
References
[7,8,12,18,19,26,30–32,34,36,38,41,50–53], initiates an in-crease in the secretion of PRL.
[1] S. F Ali, E.J. Peck, Modulation of anterior pituitary dopamine
Administration of progesterone to estrogen-treated rats
receptors by estradiol 17-beta: dose–response relationship, J.
Neuro-magnifies the inhibitory effects of estrogen only on the
sci. Res. 13 (1985) 497–507.
concentration of DA in the AL. No decrease in the
[2] L.A. Arbogast, J.L. Voogt, Progesterone reverses the
estradiol-concentration of DA in the AL occurs during the morning. induced decrease in tyrosine hydroxylase mRNA levels in the However, the decrease of DA in the AL of estrogen plus arcuate nucleus, Neuroendocrinology 58 (1993) 501–510.
progesterone-treated rats during the afternoon which is [3] L.A. Arbogast, J.L. Voogt, Progesterone suppresses tyrosine hy-droxylase messenger ribonucleic acid levels in the arcuate nucleus
coincident with the increase of PRL secretion, is almost
on proestrus, Endocrinology 135 (1994) 343–350.
three fold greater and more abrupt than that of
estrogen-[4] N. Ben-Jonathan, Dopamine: a prolactin-inhibiting hormone,
Endo-treated animals (Fig. 5C). The delay in the decrease of the cr. Rev. 6 (1985) 564–589.
concentration of DA in the AL of estrogen and progester- [5] N. Ben-Jonathan, C. Oliver, H.J. Winer, R.S. Mical, J.C. Porter, one-treated rats is expected given the delaying effects Dopamine in hypophyseal portal plasma of the rat during the estrous cycle and throughout pregnancy, Endocrinology 100 (1977) 452–
progesterone has on the activity of neuroendocrine
dopa-480.
minergic neurons [56]. These changes initiate an increase
[6] A. Bjorklund, R.Y. Moore, A. Nobin, U. Stenevi, The organization
in the secretion of PRL that is two-fold greater and occurs of tubero-hypophyseal and reticulo-infundibular catecholamine neu-several hours later than that seen in the estrogen-treated ron systems in the rat brain, Brain Res. 51 (1973) 171–191. rat. [7] F.R. Boockfor, J. P Hoeffler, L.S. Frawley, Estradiol induces a shift
in cultured cells that release prolactin or growth hormone, Am. J.
We have demonstrated that PRL plays a role in
activat-Physiol. 250 (1986) E100–E103.
ing all three populations of hypothalamic neuroendocrine
[8] A.M. Brandi, S. Joannidis, F. Peillon, D. Joubert, Changes of
DAergic neurons [15]. It has been shown that the activity
prolactin response to dopamine during the rat estrous cycle,
Neuro-of TIDA, THDA, and PHDA neurons decreases prior to an endocrinology 51 (1990) 449–454.
increase in endogenous PRL [15]. However, the mecha- [9] L. Caligaris, J.J. Astrada, S. Taleisnik, Oestrogen and progesterone influence on the release of prolactin in ovariectomized rats, J.
nism for inactivation of these neurons is still unclear.
Endocrinol. 60 (1974) 205–215.
These data support the notion that ovarian steroids,
espe-[10] C.L. Chen, J. Meites, Effects of estrogen and progesterone on serum
cially estrogen, are responsible for relieving the
dopa-and pituitary prolactin levels in ovariectomized rats, Endocrinology
minergic inhibition of lactotrophs. Indeed, estrogen de- 86 (1970) 503–505.
creases the number of dopamine receptors in the AL [1,43] [11] F.T. Close, M.E. Freeman, Dopamine-induced intracellular calcium
and decreases tyrosine hydroxylase production [2] and responses in single identified rat lactotrophs, Soc. Neurosci. Abstr. 23 (1997) 1249.
activity [28] in the arcuate nucleus. In addition to directly
[12] F.T. Close, M.E. Freeman, Effects of ovarian steroid hormones on
inhibiting the activity of TIDA, THDA, and PHDA
neu-dopamine-controlled prolactin secretory responses in vitro,
Neuroen-rons, estrogen uncouples inhibitory subunits of G-proteins docrinology 65 (1997) 430–435.
coupled to inhibitory DA D receptors on lactotrophs [34],2 [13] O.M. Cramer, R. Parker, J.C. Porter, Estrogen inhibition of
dopa-increases calcium influx into lactotrophs [11,44], and mine release into hypophyseal portal blood, Endocrinology 104 (1979) 419–422.
upregulates PRL-R number on neuroendocrine DAergic
[14] O.M. Cramer, R. Parker, J.C. Porter, Stimulation of dopamine
neurons [29].
release into hypophyseal portal blood by administration of
proges-These data, taken together with other reports, suggest terone, Endocrinology 105 (1979) 929–933.
that while PRL is responsible for activation of all three [15] J.E. DeMaria, A. Lerant, M.E. Freeman, Prolactin activates all three populations of hypothalamic neuroendocrine DAergic neu- populations of hypothalamic neuroendocrine dopaminergic neurons
in ovariectomized rats, Brain Res. 837 (1999) 236–241.
rons, estrogen is responsible for inhibiting their activity
[16] J.E. DeMaria, J.D. Livingstone, M.E. Freeman, Characterization of
prior to an endogenous increase in PRL secretion.
More-the dopaminergic input to More-the pituitary gland throughout More-the estrous
over, administration of progesterone to estrogen-treated
cycle of the rat, Neuroendocrinology 67 (1998) 377–383.
animals intensifies and delays the inhibitory effects of [17] J.E. DeMaria, D. Zelena, M. Vecsernyes, G.M. Nagy, M.E. Freeman,´ estrogen to produce a later and greater secretion of PRL. The effect of neurointermediate lobe denervation on hypothalamic neuroendocrine dopaminergic neurons, Brain Res. 806 (1998) 89– 94.
[18] E. Ellerkmann, G.M. Nagy, L.S. Frawley, Rapid augmentation of Acknowledgements
prolactin cell number and secretory capacity by an estrogen-induced factor released from the neurointermediate lobe, Endocrinology 129
The authors wish to thank DeAnn Scarborough for her (1991) 838–842.
prolactin secretory response to dopamine in vitro, Endocrine 4 prolactin gene expression by estradiol, Prog. Clin. Biol. Res. 322
(1996) 59–63. (1990) 159–169.
[20] M.E. Freeman, L.E. Reichert, J.D. Neill, Regulation of the proestrus [39] G.M. Nagy, J.E. DeMaria, M.E. Freeman, Changes in the local surge of prolactin secretion by gonadotropin and estrogens in the rat, metabolism of dopamine in the anterior and neural lobes but not in Endocrinology 90 (1972) 232–238. the intermediate lobe of the pituitary gland during nursing, Brain [21] M.E. Freeman, J.R. Sterman, Ovarian steroid modulation of prolac- Res. 790 (1998) 315–317.
tin surges in cervically stimulated ovariectomized rats, Endocrinolo- [40] J.D. Neill, M.E. Freeman, S.A. Tillson, Control of the proestrus gy 102 (1978) 1915–1920. surge of prolactin and luteinizing hormone secretion by estrogens in [22] K. Fuxe, Cellular localization of monoamines in the median the rat, Endocrinology 89 (1971) 1148–1453.
eminence and in the infundibular stem of some mammals, Acta [41] J.D. Neill, G.M. Nagy, Prolactin secretion and its control, in: E. Physiol. Scand. 58 (1964) 383–384. Knobil, J.D. Neill (Eds.), The Physiology of Reproduction, Raven [23] J.L. Goudreau, W.M. Falls, K.J. Lookingland, K.E. Moore, Periven- Press Ltd, New York, 1994, pp. 1833–1860.
tricular-S.E.M. dopaminergic neurons innervate the intermediate but [42] C. Pasqualini, F. Bojda, F. Gaudoux, B. Guibert, B. Leviel, E. not the neural lobe of the rat pituitary gland, Neuroendocrinology 62 Teissier, R. Rips, B. Kerdelhue, Changes in tuberoinfundibular (1995) 147–154. dopaminergic neuron activity during the rat estrous cycle in relation [24] J.L. Goudreau, S.E. Lindley, K.J. Lookingland, K.E. Moore, Evi- to the prolactin surge: alteration by a mammary carcinogen,
dence that hypothalamic periventricular dopamine neurons innervate Neuroendocrinology 48 (1988) 320.
the intermediate lobe of the rat pituitary, Neuroendocrinology 56 [43] C. Pasqualini, V. Lenoir, A.E. Abed, B. Kerdelhue, Anterior pituitary (1992) 100–105. dopamine receptors during the rat estrous cycle, Neuroendocrinolo-[25] R. Guillemin, F. Zeytin, N. Ling, P. Bohlen, F. Esch, P. Brazeau, P. gy 38 (1984) 39–44.
Block, W.B. Wehrenberg, Growth-hormone releasing factor: chemis- [44] A.K. Ritchie, Estrogen increases low voltage-activated calcium try and physiology, Proc. Soc. Exp. Biol. Med. 175 (1984) 401– current density in GH anterior pituitary cells, Endocrinology 1323
413. (1993) 1621–1629.
[26] A. Hashi, S. Mazawa, S.-Y. Chen, K. Yamakawa, J. Kato, J. Arita, [45] M. Sar, J. Meites, Effects of progesterone, testosterone cortisol on Estradiol-induced diurnal changes in lactotroph proliferation and hypothalamic prolactin-inhibiting factor and pituitary prolactin their hypothalamic regulation in ovariectomized rats, Endocrinology content, Proc. Soc. Exp. Biol. Med. 127 (1968) 426–429. 137 (1996) 3246–3252. [46] A.V. Schally, A. Arimura, Y. Baba, R.M.G. Nair, H. Matsuo, T.W. [27] A.E. Jimenez, J.L. Voogt, L.A. Carr, Plasma luteinizing hormone and Redding, L. Debeljuk, W.F. White, Isolation and properties of the prolactin levels and hypothalamic catecholamine synthesis in ster- FSH and LH-releasing hormone, Biochem. Biophys. Res. Commun. oid-treated ovariectomized rats, Neuroendocrinology 23 (1977) 43 (1971) 393–399.
341–351. [47] A.V. Schally, T.W. Redding, C.Y. Bowers, J.F. Barrett, Isolation and [28] E.E. Jones, F. Naftolin, Estrogen effects on the tuberoinfundibular properties of porcine thyrotropin-releasing hormone, J. Biochem.
dopaminergic system in the female rat brain, Brain Res. 510 (1990) 244 (1969) 4077–4088.
84–91. [48] A.V. Schally, S. Sawano, A. Arimura, J.F. Barrett, I. Wakabayashi, [29] A. Lerant, M.E. Freeman, Ovarian steroids differentially regulate the C.Y. Bowers, Isolation of growth hormone-releasing hormone(GRH) expression of PRL-R in neuroendocrine dopaminergic neuron from porcine hypothalami, Endocrinology 84 (1969) 1493–1506. populations: a double label confocal microscopic study, Brain Res. [49] C.M. Shaw-Bruha, H.K. Happe, L.C. Murrin, J.F. Rodriguez-Sierra, 802 (1998) 141–154. J.D. Shull, 17b-Estradiol inhibits the production of dopamine by the [30] M.E. Lieberman, R.A. Maurer, P. Claude, J. Gorski, Prolactin tuberoinfundibular dopaminergic neurons of the male rat, Brain Res.
synthesis in primary cultures of pituitary cells: regulation by Bull. 40 (1996) 33–36.
estradiol, Mol. Cell. Endocrinol. 25 (1982) 277–294. [50] J.D. Shull, J. Gorski, Estrogen regulates the transcription of the rat [31] M.E. Lieberman, R.A. Maurer, P. Claude, J. Wiklund, N. Wertz, J. prolactin gene in vivo through at least two independent mechanisms,
Gorski, Regulation of pituitary growth and prolactin gene expression Endocrinology 116 (1985) 2456–2462.
by estrogen, Adv. Exp. Med. Biol. 138 (1981) 151–163. [51] J.D. Shull, J. Gorski, Estrogen regulation of prolactin gene transcrip-[32] M.E. Lieberman, R.A. Maurer, J. Gorski, Estrogen control of tion in vivo: paradoxical effects of 17beta-estradiol dose,
Endo-prolactin synthesis in vitro, Proc. Natl. Acad. Sci. USA 75 (1978) crinology 124 (1989) 279–285.
5946–5949. [52] J.D. Shull, J. Gorski, Regulation of prolactin gene transcription in [33] S.E. Lindley, J.W. Gunnet, K.J. Lookingland, K.E. Moore, 3,4- vivo: interactions between estrogen, pimozide, and a-ergocryptine,
Dihydroxyphenylacetic acid concentrations in the intermediate lobe Mol. Pharm. 37 (1990) 215–221.
and neural lobe of the posterior pituitary gland as an index of [53] J.D. Shull, J.H. Walent, J. Gorski, Estradiol stimulates prolactin gene tuberohypophyseal. dopaminergic neuronal activity, Brain Res. 506 transcription in primary cultures of rat anterior pituitary cells, J.
(1990) 133–138. Steroid Biochem. 26 (1987) 451–456.
[34] J.D. Livingstone, A. Lerant, M.E. Freeman, Ovarian steroids modu- [54] R.T. Stone, R.A. Maurer, J. Gorski, Effect of estradiol-17 beta on late responsiveness to dopamine and expression of G-proteins in preprolactin messenger ribonucleic acid activity in the rat pituitary lactotropes, Neuroendocrinology 68 (1998) 172–179. gland, Biochemistry 16 (1977) 4915–4921.
[35] K. Lookingland, H.D. Jarry, K.E. Moore, The metabolism of [55] T.W. Toney, D.E. Pawsat, A.E. Fleckenstein, K. Lookingland, K.E. dopamine in the median eminence reflects the activity of tuberoin- Moore, Evidence that prolactin mediates the stimulatory effects of fundibular neurons, Brain Res. 419 (1987) 303–310. estrogen on tuberoinfundibular dopamine neurons in female rats, [36] R.A. Maurer, Estradiol regulates the transcription of the prolactin Neuroendocrinology 55 (1992) 282–289.
gene, J. Biol Chem 257 (1982) 2133–2136. [56] S.H. Yen, J.T. Pan, Progesterone advances the diurnal rhythm of [37] R.A. Maurer, J. Gorski, Effects of estradiol-17beta and pimozide on tuberoinfundibular dopaminergic neuronal activity and the prolactin prolactin synthesis in male and female rats, Endocrinology 101 surge in ovariectomized, estrogen-primed rats and in intact
proestr-(1977) 76–84. ous rats, Endocrinology 139 (1998) 1602–1609.