Summary Multiple shoots were obtained from nodal seg-ments of mature trees of Elaeagnus angustifolia L. cultured on MS medium (Murashige and Skoog 1962) supplemented with 0, 0.88 or 2.22 µM N6-benzyladenine. When nodal segments taken from the in vitro proliferated shoots were cultured under the same conditions, additional multiple shoots were obtained. Rooting of the in vitro propagated shoots was achieved on full strength MS medium or on MS supplemented with 2.46 µM indole-3-butyric acid. Regenerated plantlets were acclimatized and successfully transplanted to soil.
Keywords: Elaeagnaceae, in vitro culture, mature explants, nodal explants, oleaster, rooting.
Introduction
Elaeagnus angustifolia L. (Elaeagnaceae), commonly named oleaster, has been cultivated for centuries in the desert and subdesert regions of Asia; however, no commercial cultivars are available. It is valued as an ornamental plant in the Occi-dent because of its silvery leaves and fragrant flowers. Its fruits have been used as a source of food and medicine. Elaeagnus angustifolia grows to a height of 10 m, is tolerant to low-tem-perature, drought and pollution, and requires little care. The species is not susceptible to any of the major plant diseases of the Mediterranean region. The species is fertile from the age of 4--6 years and lives for 60 to 80 years. It can be propagated from seeds in the fall and from soft-wood cuttings obtained in summer (Vetvicka and Matousova 1991). However, the seeds are difficult to germinate and germination percentages vary between 30 and 60% even after stratification, and propagation by cuttings is not commercially viable.
Micropropagation of tree species offers a rapid means of producing clonal planting stock, woody biomass production, and conservation of elite and rare germplasm (Yeoman 1986, Bonga 1987). It is also a useful tool for the selection and development of new cultivars. We have developed a practical method for the micropropagation of E. angustifolia by induc-tion of shoot proliferainduc-tion in axillary buds of mature trees.
Materials and methods
During the growing season, young shoots were collected from five, field-grown, adult (about 15 years old) E. angustifolia trees. The shoots were defoliated and cut into pieces (1--1.5
cm) each containing a single node. The nodal segments were surface disinfected with 1.6% (v/v) NaOCl for 30 min, rinsed three times in sterile distilled water and cultured in 25 × 150 mm culture tubes each containing 15 ml of MS medium (Mu-rashige and Skoog 1962) plus 3% sucrose and 0.7% agar. The pH was adjusted to 5.8 before autoclaving. The culture me-dium was supplemented with 0, 0.88 or 2.22 µM N6 -benzy-ladenine (BA) for shoot induction. One explant was placed in each culture tube. The cultures were grown at 25 ± 1 °C in a 16-h photoperiod under an irradiance of 60 µmol m−2 s−1 provided by cool-white fluorescent tubes.
Every 30 days, explants (nodal segments from neoformed shoots) were transferred to fresh culture medium. At the third subculture, some of the shoots (approximately 20 mm long) obtained in MS + 0.88 µM BA medium were transferred to full or half strength MS medium without growth regulators or to MS medium supplemented with 0.49, 2.46 or 4.90 µM indole-3-butyric acid (IBA) to induce rooting.
Plantlets obtained from the in vitro rooting phase were washed in water to remove agar and the roots were sprayed with a 0.6 g l−1 Bavistin fungicide solution (0.3 g l−1 carben-dazim, BASF). The plantlets were then transferred to pots containing a 5/2 mixture (v/v) of sterilized peat moss and vermiculite. Plantlets were covered with inverted jars for 6 weeks to minimize water loss. The plants were watered every 2 days.
Thirty replicates (one explant per culture tube) were used per treatment. Analysis of variance (ANOVA) was performed on the collected data.
Results and discussion
During the initial 30-day culture period, we observed minor swelling of buds from nodal segments and the development of a few leaves in explants on all three culture media tested.
After the first transfer to fresh medium, the presence of one or several shoots, of axillary origin, per explant was detected. These neoformed shoots were cut into segments, each contain-ing a node, and transferred to fresh medium. This procedure was repeated at each subculture. No significant differences among the five generations of subcultures were detected at the 5% level. The highest mean number of shoots per explant was obtained on MS + 0.88 µM BA, whereas the highest mean shoot length was found on MS without growth regulators (Table 1). Nevertheless, the differences among the three media
Micropropagation of Elaeagnus angustifolia from mature trees
J. M. IRIONDO, M. DE LA IGLESIA and C. PÉREZ
Departamento Biología Vegetal, E.T.S. Ingenieros Agrónomos, Universidad Politécnica de Madrid, E-28040 Madrid, Spain
Received November 16, 1994 Tree Physiology 15, 691--693
were not significant at the 5% level for either variable. Accord-ing to Economou and Spanoudakis (1988), the addition of 0.5 µM BA to modified woody plant medium sustained the growth of shoot tip explants from a single 35-year-old E. angustifolia tree and induced shoot formation from existent axillary buds, whereas higher BA concentrations (5 and 50 µM) were inef-fective. They found that the number of new shoots produced increased after the first and second subcultures.
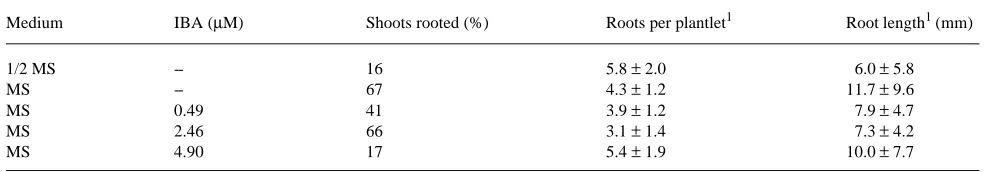
Because shoots obtained on MS + 0.88 µM BA medium were more vigorous and less often etiolated or necrosed than shoots obtained on the other two media, shoots cultured on the MS + 0.88 µM BA medium were chosen for the root induction treatments. The percentage of rooted shoots after 30 days in culture is shown in Table 2. Maximum values were obtained in the basal medium and in MS + 2.46 µM IBA. The reduction of MS salt concentration to 1/2 and the use of MS + 4.90 µM IBA medium had a negative influence on root induction (Table 2). No significant differences in mean number of roots and the mean root length were detected among the rooting media.
Economou and Spanoudaki (1988) reported that micro-shoots harvested from in vitro cultures of E. angustifolia also rooted readily in an auxin-free medium. According to these authors, rooting percentage was reduced by the addition of 0.5 µM IBA to the medium and completely inhibited by high IBA concentrations (5 and 50 µM).
A more diluted salt concentration is commonly used for root production than for shoot proliferation. A reduction in salt has provided satisfactory results in the rooting of different orna-mental, fruit and forest tree species (Moncousin 1991). The positive effect on rooting of a decrease in the mineral concen-tration of the culture medium can be explained by the reduc-tion in nitrogen concentrareduc-tion (Driver and Suttle 1987).
However, an excessive reduction in nitrate (Depommier 1981) and other mineral elements (Nemeth 1986, Gaspar and Cou-mans 1987) can inhibit rooting of shoots in some species. Supra-optimal concentrations of auxins can also inhibit the rooting process (Nemeth 1986, Gaspar and Coumans 1987).
Nodal explants from shoots of all five of the adult trees studied produced healthy plantlets suitable for transplanting to the field when subjected to a protocol of shoot proliferation in MS + 0.88 µM BA and rooting in MS without growth regula-tors. A 70--80% survival rate was achieved during the acclima-tization process. We estimate that, in one year, around 3,000,000 acclimatized plantlets can be produced from a sin-gle explant. Thus, we conclude that E. angustifolia can be effectively micropropagated from mature trees.
References
Bonga, J.M. 1987. Clonal propagation of mature trees: problems and possible solutions. In Cell and Tissue Culture in Forestry. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff, Dordrecht, The Nether-lands, pp 249--271.
Depommier, D. 1981. Micropropagation d’Eucalyptus résistant au froid: influence de quelques facteurs sur l’allongement et l’enrac-inement des plantules. Colloque International sur la Culture In Vitro des Essences Forestières. IUFRO, Fontainebleau, France, pp 127--132.
Driver, J.A. and G.R.L. Suttle. 1987. Nursery handling of propagules.
In Cell and Tissue Culture in Forestry. Eds. J.M. Bonga and D.J.
Durzan. Martinus Nijhoff, Dordrecht, The Netherlands, pp 320--335.
Economou, A.S. and M.J. Spanoudaki. 1988. Regeneration in vitro of oleaster (Elaeagnus angustifolia L.) from shoot tips of mature trees. Acta Hortic. 227:363--368.
Gaspar, T. and M. Coumans. 1987. Root formation. In Cell and Tissue Culture in Forestry. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff, Dordrecht, The Netherlands, pp 202--217.
Moncousin, Ch. 1991. Rooting of in vitro cuttings. In Biotechnology in Agriculture and Forestry 17: High-Tech and Micropropagation I. Ed. Y.P.S. Bajaj. Springer-Verlag, Berlin, Germany, pp 231--261. Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth
and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473--497.
Nemeth, G. 1986. Induction of rooting. In Biotechnology in Agricul-ture and Forestry 1: Trees I. Ed. Y.P.S. Bajaj. Springer-Verlag, Berlin, Germany, pp 49--64.
Vetvicka, V. and V. Matousova. 1991. Arboles y arbustos. Susaeta, Madrid, Spain, 311 p.
Table 1. Effects of BA on shoot production and shoot length of
E. angustifolia nodal segments after 30 days on MS medium at the
third subculture.
BA (µM) Shoots per explant1 Shoot length (mm)1
0 3.00 ± 0.47 21.6 ± 3.8
0.88 3.54 ± 0.65 16.7 ± 3.0
2.22 2.64 ± 1.04 12.9 ± 4.2
1 Mean ± standard error. Thirty replicates were used for each
treat-ment.
Table 2. Responses of in vitro proliferated shoots of E. angustifolia to root induction treatments after 30 days in culture.
Medium IBA (µM) Shoots rooted (%) Roots per plantlet1 Root length1 (mm)
1/2 MS -- 16 5.8 ± 2.0 6.0 ± 5.8
MS -- 67 4.3 ± 1.2 11.7 ± 9.6
MS 0.49 41 3.9 ± 1.2 7.9 ± 4.7
MS 2.46 66 3.1 ± 1.4 7.3 ± 4.2
MS 4.90 17 5.4 ± 1.9 10.0 ± 7.7
1 Mean ± standard error. Thirty shoots were used for each treatment.
Yeoman, M.N. 1986. The present development and future of plant cell and tissue culture in agriculture, forestry and horticulture. In Plant Tissue Culture and Its Agricultural Applications. Eds. L.A. Withers and P.G. Alderson. Butterworths, London, U.K., pp 489--500.