The Effect of Inoculum Types On The Yeast Growth Pattern During Tempe Fermentation
Samsul Rizal1), Maria Erna Kustyawati1), Murhadi1), Udin Hasanudin1), and Fatimah2)
1)
Lecturers of Department of Agricultural Product Technology, Faculty of Agriculture, University of Lampung
2)
Graduated of Department of Agricultural Product Technology, Faculty of Agriculture, University of Lampung
Email: marrizal @yahoo.com
ABSTRACT
Tempe is a food made of soybeans fermentation and inoculated with Rhizopus oligosporus in solid fermentation. Besides R. oligosporus, other microorganisms such as bacteria and yeasts were found during fermentation. This study aimed to know the effect of inoculum types on the pattern of yeast growth during tempe fermentation with addition of Saccharomyces cerevisiae. The research was done by Randomized Complete Block Design (RCBD) with two factors and three replications. The first factor was the types of tempe inoculum, consist of 4 levels, i.e. commercial inoculum, Saccharomyces cerevisiae, Rhizopus oligosporus, and mixture of Rhizopus oligosporus and Saccharomyces cerevisiae. The second factor was fermentation time, consist of 6 levels, i.e. 0, 8, 16, 24, 32, and 40 hours. The results showed that yeast grew during the soybean fermentation with commercial tempe inoculum, Saccharomyces cerevisiae, and mixture of Rhizopus oligosporus and Saccharomyces cerevisiae, but they didn’t grow during soybean fermentation with addition of R. oligosporus only. After lag phase until 8 hours of fermentation time, the yeast growth increased until the end of fermentation, although it decreased at 32 hours of fermentation with mixture of Rhizopus oligosporus and Saccharomyces cerevisiae.
Keywords: Tempe, yeast, Saccharomyces cerevisiae. Pattern of Growth
INTRODUCTION
Tempe is a food made by fermenting soybeans inoculated with Rhizopus
oligosporus in solid fermentation. The fermentation of tempe is a two-stage
fermentation, which is fermentation by bacterial activity that takes place during
being inoculated with mold (Kustyawati, 2009). R. oligosporus plays a major role
in making tempe because it can maintain most of the nutrients contained in
soybeans, increase its protein digestibility, and increase levels of several kinds of
vitamin B (Muchtadi, 2010 in Mursyid, 2014). In addition to R. oligosporus,
during tempe fermentation there are also the presence of other microorganisms
such as lactic acid bacteria (LAB) and yeast (Efriwati et al., 2013).
Yeast participates in tempe fermentation (Feng et al., 2007; Kustyawati, 2009).
Kustyawati (2009) states that yeast can grow with indigenous bacteria and R.
oligosporus during tempe fermentation. The presence of yeast has been reported
by Samson et al. (1987) in commercial tempeh in the Netherlands, including
Trichosporon beigelii, Clavispora (Candida) lusitaniae, C. maltosa, C.
intermedia, Yarrowia lipolytica, etc. Among these yeasts, Saccharomyces
cerevisiae is a yeast that have the potential to produce tempeh containing beta
glucans due to its ability to produce beta glucans (Pengkumsri et al., 2017).
Beta-glucan is a compound that has a positive effect on body health, especially its
ability to increase immunity (Hetlan, et al., 2013).
The discovery of yeast in tempe fermentation shows that yeast can grow with R.
oligosporus and bacteria during the fermentation process and is possible that the
yeast has an important role in the fermentation. According to research by Rizal, et
al. (2018), which added S. cerevisiae as an inoculum, showed that the S.
cerevisiae can grow with other microbes and produce tempeh containing
beta-glucans. Therefore, the deliberate addition of S. cerevisiae in tempe fermentation
is expected to produce tempeh containing beta-glucans so that the tempeh
produced will have more value due to the beta-glucan content. How is the growth
pattern of S. cerevisiae during the tempe fermentation process given the addition
of various types of inoculum is one of the problems that need to be studied. In this
study, we will study the effect of various types of tempeh inoculum on yeast
growth patterns during tempe fermentation.
MATERIALS AND METHODS
Materials
The materials used in this research include commercial yeast (Raprima
trademark), pure culture of Rhizopus oligosporus FNCC 6010 and Saccharomyces
cerevisiae FNCC 3012 obtained from the UGM Inter-University Food and
Nutrition Center, imported soybeans with the trademark Soybean USA no. 1
obtained from Mount Sulah in Bandar Lampung, distilled water, peptone water,
physiological salt 0.85%, alcohol 70%, Nutrient Broth (NB), Nutrient Agar (NA),
Malt Extract Agar (MEA), Dextrose Agar (PDA), aluminum foil, and cotton.
Research methods
This study used a Complete Randomized Block Design (RCBD) with two factors
and three replications. The first factor is the type of inoculum tempe, consisting of
4 levels, namely tempe commercial yeast (RAPRIMA trademark), Saccharomyces
cerevisiae, Rhizopus oligosporus, and mixture of Rhizopus oligosporus +
Saccharomyces cerevisiae. The second factor was fermentation time, consisting of
6 levels, namely 0 hours, 8 hours, 16 hours, 24 hours, 32 hours, and 40 hours.
Parameters observed were yeast growth patterns during the fermentation process
0, 8, 16, 24, 32, and 40 hours . The data obtained were analyzed by variance to
determine whether there was influence between treatments. The data was further
tested using Orthogonal Polynomial - Orthogonal Comparison (OP-OC) test at the
level of 5%.
Preparation of Saccharomyces cerevisiae culture
Saccharomyces cerevisiae from sloping agar was cultured into Malt Extract Agar
(MEA) media in a petri dish so that S. cerevisiae was obtained in the form of
colonies in the media. The S. cerevisiae colonies were transferred into a test tube
containing 9 mL of De Man, Rogosa and Sharpe Broth (MRSB) media, then
speed of 3000 rpm for 10 minutes. Centrifugation was carried out twice, using
sterile distilled water in the second centrifugation process. After that, the number
of S. cerevisiae cells was calculated using haemacytometer. The number of S.
cerevisiae was adjusted to 107 cells / gram.
Preparation of Rhizopus oligosporus culture
Rhizopus oligosporus from sloping agar was cultured into Potato Dextrose Agar
(PDA) media in a petri dish, then incubated for 5-7 days at 25oC so that R. oligosporus was in the form of a colony. The R.oligosporus colonies were
harvested using drygalski by adding 5-10 mL of sterile distilled water.
Furthermore, R. oligosporus spores were centrifuged at 3000 rpm for 10 minutes.
After that, a solid R. oligosporus spore was obtained, then diluted in a diluent
solution. The number of R. oligosporus spores in the diluent solution was
calculated using haemacytometer and it was adjuted to 105 cells / gram.
Production of Tempe
The process of making tempe followed the procedure of Kustyawati (2009). A
total of 300 grams of soybeans are soaked in clean water at room temperature for
one night, then the skin is removed. Furthermore, they were boiled use clean
water with a ratio of 1: 3 (soybeans: water) for 30 minutes, drained and dried until
the soybean temperature reaches room temperature. The fermentation stage was
done by mixing every 100 grams of boiled soybean with tempeh inoculum
according to the treatment. After being mixed, the soybean seeds were inserted in
a packing plastic that had been perforated regularly for aeration purposes, then
incubated at 32 ° C for 40 hours, observed every 8 hours during fermentation.
Enumeration of Yeast Amount
Calculation of yeast count was carried out using the Total Plate Count (TPC)
method with Malt Extract Agar (MEA) media following the Lay (1994) method.
The calculation of the number of yeasts was carried out at 0, 8, 16, 24, 32, and 40
hours of fermentation time. Each sample was sampled and a series of dilutions
Kustyawati method (2009). A total of 10 grams of tempe sample were mixed with
90 ml of peptone water, then homogenized. After that, a series of dilutions is
made to a certain concentration. Furthermore, yeast cultivation was done using the
spread plate method on Malt Extract Agar (MEA) media. Incubation was carried
out at 32oC for 24-48 hours.
RESULTS AND DISCUSSION
Growth Pattern of Saccharomyces cerevisiae in Tempe Inoculated with Various Types of Inoculum
The results of the calculation of the number of Saccharomyces cerevisiae cells
during the tempe fermentation process inoculated with various types of inoculums
showed that Saccharomyces cerevisiae was able to grow during tempe
fermentation with the addition of commercial tempe yeast inoculum, inoculum of
Saccharomyces cerevisiae, or inoculum mixture of Rhizopus oligosporus and
Saccharomyces cerevisiae. On the contrary, there was no growth of
Saccharomyces cerevisiae during tempeh fermentation which was inoculated with
Rhizopus oligosporus only. The highest number of yeast cells was found in
soybeans which were inoculated with Saccharomyces cerevisiae after 40 hour
fermentation time, which was 4.82 x 109 CFU/g, while the lowest number of yeast cells was found in tempeh inoculated with Rhizopus oligosporus because there
was no yeast growth. The number of Saccharomyces cerevisiae cells during
soybean fermentation with the addition of tempe yeast, Saccharomyces cerevisiae,
Rhizopus oligosporus, and a mixture of Rhizopus oligosporus and Saccharomyces
Table 1. Number of S. cerevisiae cells during soybean fermentation by inoculum of tempeh yeast, S. cerevisiae, R.oligosporus, and mixture of R. oligosporus and S. cerevisiae.
Types of Inoculum
Number of S.cerevisiae cells (CFU/g) on the each fermentation duration
0 8 16 24 32 40
Tempe yeast 1,05 x 103 1,17 x 103 3,87 x 103 3,93 x 104 3,07 x 105 2,63 x 105
S. cerevisiae 1,00 x 107 1,50 x 107 1,73 x 108 3,33 x 109 2,77 x 109 4,82 x 109
R.oligosporus - - - -
R.oligosporus
+ S. cerevisiae 1,0 x 107 1,43 x 107 1,24 x 108 4,07 x 108 1,31 x 108 3,43 x 108 The results showed that yeast could grow during tempeh fermentation process
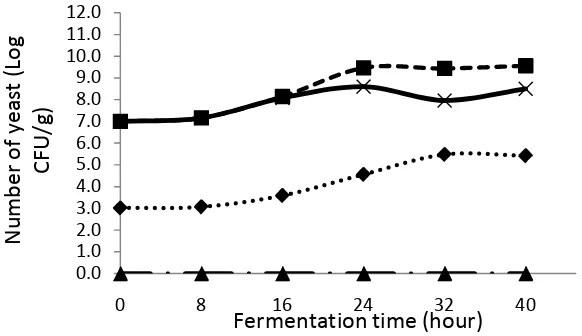
using inoculum of tempe yeast (Figure 1). Based on the picture, the adaptation
phase of yeast growth started from the beginning of fermentation up to 8 hours of
fermentation. Kusmiati et al. (2011) stated that the phase of S. cerevisiae
adaptation in the media using carbon sources of glucose ended in fermentation at
4 o'clock. Meanwhile, S. cerevisiae grown on Yeast Nitrogen Base (YNB) media
containing 30% glucose showed that the adaptation phase was in the first 6 hours
fermentation (Ishmayana et al., 2012). The phase of S. cerevisiae adaptation in
this study was longer than the research conducted by Kusmiati et al. (2011) and
Ishmayana et al. (2012) due to the absence of additional carbon sources which are
the main nutrients needed for the growth of S. cerevisiae. After 8 hours, the
growth of S. cerevisiae enters the exponential phase until the 32nd hour, then
enters the stationary phase until the 40 hours. The death phase of yeast cell was
estimated to occur after fermentation lasting more than 40 hours. The yeast
presence in the soybean treatment which is inoculated with tempeh yeast is
Figure 1. Yeast growth in various types of inoculum during tempe fermentation. ■ Soybean + S. cerevisiae
˟ Soybean + R. oligosporus + S. cerevisiae ▲ Soybean + R. oligosporus
♦ Soybean + tempe yeast (Merk RAPRIMA)
Yeast could grow during the soybean fermentation process which was inoculated
with S. cerevisiae eventhough tempeh was not formed. Khamir grew by utilizing
existing nutrients in soybean substrate. Kustyawati (2010) stated that almost all
foods provide adequate nutrition to support yeast growth. In this research
treatment, yeast growth was in the adaptation phase from 0 to 8 hours of
fermentation time, then increased in the number of yeast cells (logarithmic phase)
until 24 hours of fermentation. After this, yeast enters the stationary phase from
24 hours until 40 hours of fermentation time. The yeast death phase was estimated
to occur after fermentation lasting more than 40 hours.
Soybeans inoculated with R. oligosporus did not show yeast growth until the
fermentation time reach 40 hour. This is because there is no addition of yeast
during inoculation. This research is in line with Kustyawati (2009) which states
that yeast was not found during tempe fermentation using R. oligosporus as
inoculum. Thus, this study revealed that the presence of yeast in tempeh can be
found if fermented soybeans are added with yeast. However, Nisa (2016) stated
that there were the presence of yeast on SDBR tempeh (Sindang Barang) and
WJB (Warung Jambu) during fermentation 24 and 48 hours. Research conducted
by Nisa (2016) did not specifically calculate the number of yeasts during tempe
fermentation. Efriwati et al. (2013) found the presence of yeast from the
beginning of soaking, the end of soaking, the beginning of incubation,
mid-incubation, and the end of incubation on tempeh made by tempe EMP (once
boiling) and EMP tempe (two boiling). The difference between this study and the
research conducted by Efriwati et al. (2013) because this study looked at yeast
growth patterns during soybean fermentation which was inoculated with R.
oligosporus, while research conducted by Efriwati et al. (2013) observed the
presence of yeast in the soybean immersion process and during fermented
soybeans which were inoculated with tempeh yeast. This study showed that
tempeh which was inoculated with R. oligosporus had no yeast growth, while
soybean added with yeast tempeh contained yeast growth. Meanwhile, research
conducted by Efriwati et al. (2013) used tempe yeast as an inoculum in making
tempeh so that yeast was detected. This indicates that the presence of yeast in
soybean added with tempeh yeast comes from the yeast of tempe used or from the
environment during fermentation.
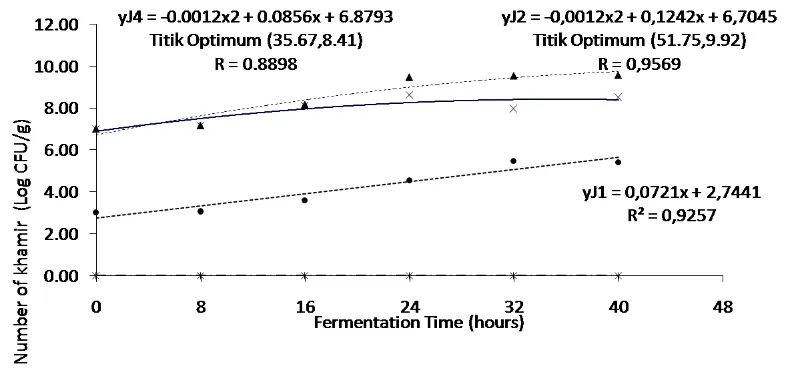
The growth of S. cerevisiae in tempeh which was inoculated with the mixture of
R. oligosporus and S. cerevisiae was in the adaptation phase at fermentation time
0 until 8 hour. After that, S. cerevisiae growth increased until 24 hours of
fermentation (logarithmic phase), then dropped to a population of 8.0 Log CFU/g
at the fermentation time reach 32 hours (Figure 2). Furthermore, yeast growth
increased after fermentation time reach 40 hours. This growth pattern was in line
with the growth pattern of S. boulardi which was inoculated together with R.
oligosporus as tempeh inoculum in research conducted by Kustyawati (2009). The
yeast growth pattern in this treatment was similar to the yeast growth pattern in
soybeans inoculated with S. cerevisiae only. This showed that S. cerevisiae
utilizes nutrients in soybeans for growth and there was a mutually beneficial
symbiosis between R. oligosporus and S. cerevisiae during fermentation.
terms of nutrient availability between R. oligosporus and S. cerevisiae during
tempe fermentation.
Figure 2. Response of yeast growth to the duration of fermentation in each type of inoculum.
Informations: ● soybean + tempe yeast
▲ soybean + S. cerevisiae ӿ soybean + R. oligosporus
x soybean + R. oligosporus + S. cerevisiae
The results of variance analysis showed that the amount of yeast during tempe
fermentation was influenced by the type of inoculum and the time of
fermentation, and there was an interaction between the two. Further test results
showed that the number of yeasts during tempe fermentation with the addition of
inoculums of R. oligosporus and S. cerevisiae was significantly different from the
tempe yeast inoculum, S. cerevisiae inoculum, and R. oligosporus inoculum.
Research conducted by Kustyawati (2009) showed that soybeans for tempe
fermentation added with different yeast inoculums had different yeast growth
patterns.
The results of variance analysis showed that the number of yeasts during tempe
fermentation was influenced by the type of inoculum and fermentation time, and
there was interaction between. The results of further tests showed that yeast
quadratic, but significantly in linear so that linear regression was used to
determine the effect of tempeh yeast inoculum and fermentation time on yeast
growth (Sugiyono, 2007). Linear regression showed that yeast growth in soybean
which was inoculated with tempeh yeast, increased until fermentation took place.
Based on linear regression, the equation y = 0.0721x + 2.7441 was obtained. This
equation means that an increase in fermentation time every 8 hours can increase
the amount of yeast by 7.21%. The results of further tests on the treatment of
addition of R. oligosporus as an inoculum in soybean fermentation were not
significantly different in quadratic or linear terms, meaning that the addition of R.
oligosporus during soybean fermentation did not affect yeast growth. This is due
to the absence of yeast growth in this treatment. Further test results showed that
the number of yeasts during tempe fermentation with the addition of inoculums of
R. oligosporus and S. cerevisiae was significantly different from the tempe yeast
inoculum, S. cerevisiae inoculum, and R. oligosporus inoculum. Research
conducted by Kustyawati (2009) showed that soybean for tempe fermentation
added with different yeast inoculums had different yeast growth pattern.
The treatment of adding S. cerevisiae as an inoculum in tempe making was
significantly different in quadratic terms. This shows that during fermentation,
there is an increase and decrease in the number of yeasts. Based on the equation y
= -0.0012x2 + 0.1242x + 6.7045, the optimum growth point of yeast was obtained
at 51.75 hours with a yeast number of 9.92 CFU / g (Figure 6). Similarly, the
addition of R. oligosporus and S. cerevisiae treatments were significantly different
quadratically. This quadratic difference is because during fermentation, the
number of yeasts has increased, then decreased and experienced an increase again.
Regression equation in this treatment is y = -0.0012x2 + 0.0856x + 6.8793, so that
the optimum point of yeast growth was obtained at 35.67 hours with the number
of yeasts was 8.41 CFU / g (Figure 11). The optimum point of addition of R.
oligosporus and S. cerevisiae as inoculums was faster than treatment with addition
of S. cerevisiae. This is presumably because in the addition of R. oligosporus and
S. cerevisiae as inoculums in soybean fermentation, S. cerevisiae uses the
with addition of S. cerevisiae. This research is in line with Kustyawati (2009)
which states that there may be mutually beneficial symbiosis in terms of nutrient
availability between R. oligosporus and S. cerevisiae during tempe fermentation.
Tempe Appearance Inoculated with Various Types of Inoculum during fermentation.
Miselia produced by R. oligosporus was formed on the surface of soybeans which
were inoculated with a mixture of R. oligosporus and S. cerevisiae with a 24-hour
fermentation period (Figure 3). Start from 24 to 32 hour of fermentation time,
mycelia partially covered the surface of the soybean, while at the fermentation
time reach 40 hours, mycelia covered the entire surface of the soybean. The
appearance of mycelia is similar to mycelia in the treatment of soybeans which are
inoculated using R. oligosporus, which is grayish white and there is a black color.
The black color of this tempe was R. oligosporus spore. Spores grew because of
the oxygen entering through holes in plastic packaging. According to Bintari et al.
(2008), tempe mold is microaerophilic, which requires enough oxygen to grow.
Black color decreases in fermentation at the 40th hour, presumably because at the
40th hour of fermentation the spores have formed mycelia which is increasingly
compacted so that it covers the surface of the soybean and shows a white
appearance.
v
Figure 3. Soybeans inoculated with mixture inoculum of R.oligosporus and S.cerevisae
CONCLUSION
The conclusion of this study is that yeast growth in tempe with the addition of
Saccharomyces cerevisiae has increased until the end of fermentation, although it
had decreased in the 32nd hour of fermentation time. Further research is needed to see the yeast growth in tempeh with the addition of Saccharomyces cerevisiae
which is added with a carbon source (flour or sugar).
BIBLIOGRAPHY
Bintari, S.H., D.P. Anisa, E.J. Veronika, dan C.R. Rivana. 2008. Efek Inokulasi Bakteri Micrococcus luteus Terhadap Pertumbuhan jamur Benang dan Kandungan Isoflavon Pada Proses Pengolahan Tempe. Jurnal Biosantifika 1 : 1-8.
Efriwati, A. Suwanto, G. Rahayu, L. Nuraida. 2013. Populations Dinamic of Yeast and Lactic Acid Bacteria (LAB) during Tempeh Production. Hayati Journal of Biosciences 20 (2) : 57-64.
Feng, X.M., T.O. Larsen, and J Schnürer. 2007. Production of Volatile
Compounds by Rhizopus oligosporus during Soybean and Barley Tempeh Fermentation. Journal of Food Microbiology 113 (2) : 133–141.
Hetland, G., E. Johnson, D.M. Eide, B. Grinde, A.B.C. Samuelsen, dan H.G. Wiker. 2013. Antimicrobial effects of β-glucans and pectin and of the Agricus blazei Based Mushroom Extract, AndoSanTM. Examples of Mouse Models for Pneumococcal, Fecal Bacterial, and Mycobacterial Infections. Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education (A. Mendez-Vilas, Ed.) Formatex pp. 880-898
Ishmayana, S., A.S. Djajasoepana, S.D. Rachman, dan A. Safari. 2012. Kinerja Fermentasi Ragi Saccharomyces cerevisiae Pada Media VHG dengan Variasi Konsentrasi Ekstrak Ragi Sebagai Sumber Nitrogen Untuk Produksi Bioetanol. (Conference Paper). Universitas Padjadjaran. Bandung.
Kusmiati, A. Thontowi, dan S. Nuswantara. 2011. Efek Sumber Karbon Berbeda terhadap Produksi â-Glukan oleh Saccharomyces cerevisiae pada Fermentor Air Lift. Jurnal Natur Indonesia, 13(2) : 138-145.
Kustyawati, M.E. 2009. Kajian Peran Yeast dalam Pembuatan Tempe. Jurnal Agritech 29 (2) : 64-70.
Lay, B. W. 1994. Analisis Mikrobia di Laboratorium. Raja Grafindo Persada. Jakarta.
Mursyid. 2014. Kandungan Zat Gizi dan Nilai Gizi Protein Tepung Tempe Kedelai Lokal dan Impor serta Aktivitas Antioksidannya. (Tesis). Institut Pertanian Bogor. Bogor.
Nisa, A.K. 2016. Isolasi dan Identifikasi Khamir Asal Tempe Serta Uji Aktivitas Enzim β-Glukosidasenya. (Skripsi). Institut Pertanian Bogor. Bogor.
Pengkumsri, N., B.S. Sivamaruthi, S.Sirilun, S. Peerajan, P. Kesika, K. Chaiyasut, C.t Chaiyasut. 2017. Extraction of Β-Glucan From Saccharomyces
cerevisiae : Comparison of Different Extraction Methods and In Vivo Assessment of Immunomodulatory Effect in Mice. Journal of Food Sci. Technol, Campinas, 37 (1) : 124-130. ISSN 0101-2061.
Rizal, S., Kustyawati, M.E., Murhadi, Hasanudin, U., and Marniza. 2018. Pengaruh Konsentrasi Saccharomyces cerevisiae terhadap Kadar Abu, Kadar Protein, Kadar Lemak dan Kandungan Beta-Glukan Tempe
Samson, R.A., V. Kooij, dan E. deBoer. 1987. Microbiological Quality of Commercial Tempeh in The Netherlands. Journal of Food Protection 50 : 92-94.