Electrically Mediated Hydrocarbon Conversion Processes
Guido P. Pez 3/6/16
Theme
: Conversion of saturated C
1,C
2&C
3HC’s to chemicals by
any
means
that involves electricity.
1. Limiting economics.
2. Direct hydrocarbon fuel cells (DHCFC’s): Chemicals from FC’s?
3. Electrochemically promoted catalysis (EPC): Methane to syn-gas (CO/H
2) via
(non-Faradaic) “Electroreforming”.
1. Limiting Economics
Natural gas (NG) @ $2.67 / MMBtu (2015 ave. price)
Ideally
Electricity @ 0.9 cents/KWh
Electricity cost: 8-12 cents/KWh (Pa 2015)
Currently NG ,lowest cost fuel for electric power generation.
But electricity is a relatively costly
reagent
if it’s required for endothermic
hydrocarbon (HC) conversion processes (eg CH
4to C
2HC’s or syn gas via plasma).
Consider first an (exothermic) selective
anodic
oxidation of HC’s that provides
both
electricity and chemicals- in a ‘tailored’ direct hydrocarbon fuel cell (DHCFC)*
Fuel Cell Fundamentals: The H
2
-O
2
FC
H2 + ½ O2 = H2O(g) ERev = 1.17 V at 80C
Rev. Efficiency (ηr ) = (ΔH - TΔ�)/ΔH
Polarization Curve ( E Cell vs Current) Losses from: mass transp. res.( ηtx), Ohmic.res. ,but mainly slow O2 redn. kinetics(ηORR ).
Gasteiger, Wagner et al, Appl. Cat. B 56 (2005), 9 Xianguo Li “Principles of FC’s” Taylor &Francis
Publ. 1962
Figure from p 62 of Li’s book
Thermodynamically feasible FC’s for chemicals and energy cogeneration
1. Ethane to ethylene.
C
2H
6+ 1/2O
2= C
2H
4+ H
2O(g) E = 0.693 V at ( 80 °C ); 0.855 V (500 °C)
2. Methane coupling to ethane.
2CH
4+ O
2= C
2H
6+ H
2O(g) E = 0.815 V at 80 °C; 0.695 V (500 °C)
3. Methane coupling to ethylene.
2CH
4+ O
2= C
2H
4+ 2H
2O(g) E= 0.748 V at 80 °C ; 0.755 V (500 °C)
4. Methane to methanol.
CH
4+ ½ O
2= CH
3OH(g) E= 0.565 V at 80 °C ; 0.453 V (500 °C)
Hydrogen/Oxygen FC.
H
2+ ½ O
2= H
2O (g) E= 1.17 V at ( 80 °C) ; 1.062 V (500 °C)
Methane /Oxygen FC
CH
4+ 2O
2= CO
2+2H
2O(g) E = 1.033 V at (80 °C) ; 1.037 V (500 °C)
Ethane to Ethylene FC :
C
2H
6+ 1/2O
2= C
2H
4+ H
2O
For performance data see Fig 4A on p 764Methane to Ethane-Ethylene FC
CH4 Conversion and Products Selectivity vs Temperature . (Max. 91% Selectivity for C2’s, at 23.7% Conversion of CH4 at 1273K ). Note: C2H4>> C2H6
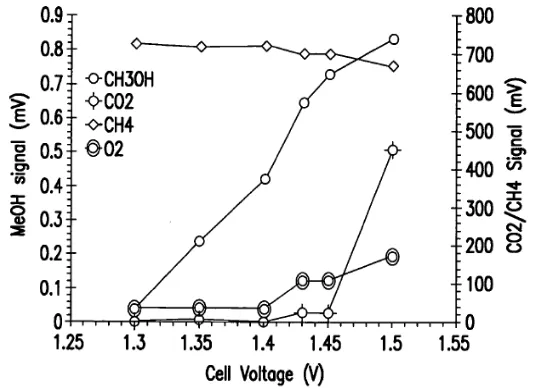
Electrolytically induced catalytic methane to methanol conversion
.
Methanol conc. vs anode (applied) potential using V2O5/SnO2 anode for wt % V2O5 ,at 100 C. Max: 61% current efficiency and 88%
selectivity for CH3OH B. Lee, T. Hibino, J. Catal. 2011, 279, 233
Electrode catalyst design: The Direct Methane FC
Net: CH4 +2O2 = CO2+ 2H2O(g) , E 1.033V at 80 C CH4+2H2O = CO2 + 8H+ +8e- 2O
2 + 8H+ + 8e- = 4H2O
Voltage-Current Polarization Curve for OMC tethered catalysts.
Key Novelty : Pt complex, ‘molecular’ CH4 activation catalyst ,tethered to an ordered mesoporous carbon (OMC) support.
M. Joglekar, V. Nguyen, S. Pylypenko, C. Ngo., Q. Li ,M.E. O’ Reilly, T.S. Gray, W.A Hubbard, T. B. Gunnoe, A. M. Herring, B.G. Trewyn J. Am. Chem. Soc. 2016,138 116
Figure: Schematic of cell design from Abstract
Electrochemical Promotion of Catalysis (EPOC)
or
Non-Faradaic
electrochemical modification of catalytic activity (NEMCA)
(a) Faradaic : Stoichiometric use of electron as a reactant, (a) (b)
Eg n= 12el / mole C
2H
4reacted
.
(b) Non-Faradaic: Non- stoichiometric use of electron,
with n potentially much larger.
(Ʌ
> 1,) often
Ʌ
> > 1
Oxide ion conducting YSZ disc, Pt catalyst-electrode coating.
Following C
2H
4Oxidation rate as a function of applied potential.
Faraday Efficiency,
Ʌ
=
∆r/r
e= 74,000
∆r/r
0= 25
Mechanism: (as for CO, CO
2reactants on Pt / YSZ solid el.)
Ethylene Oxidation rate
increases
of up to 25 X (∆r)with applied potential of ca. 0.6 V (375 C) K. Katsaounis J. Appl. Electrochem. 2010 40 885; C.G. Vayenas et al (2001)
in “Electrochemical activation of catalysis__” Kluwer/Plenum. Pub. Fig 1 p866 of Katsaounis
Non- Faradaic ”Electroreforming” of methane to syn. gas and H
2Steam-methane reforming (SMR)
,
current
practice:
CH
4+2H
2O ↔ CO + 3H2+H
2O Ni cat., then WGS to 4H
2+CO
2Endothermic React. Heat from external methane combustion.
Tubular metal alloy reactors, at ca. 30 atm. Pressure and ca. 800 °C
. Electroreforming:
CH4 Conv. vs T(K) for Pt/metal oxide cats. Products yield vs T(K) for conventional (left), Electroreforming Apparatus. Open : Conv. SMR : Dark : Electroreforming. and electroreforming (right), for which the E = 500 V -800 V for I= 3mA of Dramatic decrease in reaction temperature yield exceeds the calc. thermo. equilibrium. “ Dark current” ;Elect. Eff. 15-25 %
NEMCA Effect . Faradaic Efficiency, Ʌ (reacted Y. Sekine, et al, (Waseda Univ, Jpn) Catalysis Today, 2011, 116, 125, CH4/mole- electron)= 84 for Pt/Ce0.5Zr0.5O2 cat. Y. Sekine et al , Int. J. of Hydrogen Energy 2013, 38 ,3003 .
From Cat. Today 2011 Reactor Schematic p118
Electrical Discharges/Plasma for Methane Conversion
Methane oxidative coupling in corona discharge , +/- catalyst
Feed: CH4/O2 = 4 @ 100 sccm A. Marafee, C.Liu, G.Xu, R. Mallison ,L. Lobban
Ind.Eng.Chem.Res. 1997,36,632
From Kado Fig 1 p1378
Potential for: Hydrocarbons→ Chemicals via
Electrocatalysis
With Electric Power Co- Generation (All exothermic conversions)
With Electric Power Consumption (Exothermic or endothermic conv.)
Hydrocarbon/Air Fuel Cell Systems .
Reports for chem. products (currently) only from SOFC’s
Electrolyte : Polymer (PEM) Phosphoric Acid --- ? Solid Oxide, ceramic (SOFC) Molten Carbonate
Electrochemical Promotion of Catalysis (EPC) : Dramatic rate enhancement in
reduction, oxidation & isomerization reactions. “Triode” operation in SOFC’s. Potentially lowest power consumption . Issue: Practical reactor design
Electroreforming for endothermic CH4 to syn gas &H2 . Large rate increases and
improved conversion equilibria, at lower temps. Electricity use efficiency, only 15-25% Plasma processes for methane to syn gas. Key issue : High power usage.
(Faradaic processes)