Brain Research 888 (2001) 330–335
www.elsevier.com / locate / bres
Short communication
¨
Phrenic motoneurons receive monosynaptic inputs from the Kolliker–
Fuse nucleus: a light- and electron-microscopic study in the rat
*
Shigefumi Yokota, Toshiko Tsumori, Katsuhiko Ono, Yukihiko Yasui
Department of Anatomy(2nd Division), Shimane Medical University, Izumo 693-8501, Japan
Accepted 10 October 2000
Abstract
The parabrachial complex, which plays an important role in respiratory regulation, has been reported to send projection fibers to the phrenic nucleus, but the synaptic organization between the parabrachial fibers and the phrenic motoneurons has not been examined. Using
¨
anterograde and retrograde tracing methods, we found in the rat that the parabrachial fibers originating mainly from the Kolliker–Fuse nucleus (KF) terminated not only within the phrenic nucleus but also on the radial dendritic bundles of the phrenic motoneurons. It was further revealed that the KF fibers made asymmetrical synapses predominantly with dendrites and partly with somata of the phrenic motoneurons. These data suggest that output signals from the KF may exert excitatory influence directly upon the phrenic motoneurons.
2001 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Respiratory regulation
¨
Keywords: Kolliker–Fuse nucleus; Phrenic nucleus; Parabrachial complex; Respiration; Rat
The parabrachial complex has been well known to be groups, such as the rostral ventrolateral respiratory group
¨ ¨
the relay site of visceral sensory information from the (rVRG) and Botzinger Complex (BotC) [2,13]. Direct nucleus of the solitary tract to the forebrain regions projections to the phrenic nucleus or phrenic motoneurons including the dorsal thalamus, hypothalamus, amygdala from the DRG [4,5,7,20], from the rVRG [4–7,20,24] and
¨
and insular cortex, to the brainstem regions including the from the BotC [4,5,7,20] have been revealed by various nucleus of the solitary tract and ventrolateral medulla, and neuroanatomical studies. The phrenic nucleus has also to the intermediolateral nucleus of the spinal cord; these been reported to receive projection fibers from the parab-target regions are involved in autonomic regulation, such rachial complex by retrograde tract tracing studies as cardiovascular and respiratory control (for review, see [17,18,20,21], by a transneuronal viral tracing study [4] [22]). Furthermore, recent studies [2,3,13,16] have demon- and by an anterograde tract tracing study [12]. It is, strated that electrical or chemical stimulation of distinct therefore, suggested that the direct projections from the populations of parabrachial neurons produces different parabrachial complex to the phrenic nucleus, probably to respiratory responses. Although the pathways responsible phrenic motoneurons are also responsible for some respira-for the execution of these respiratory responses have not tory effect. However, there have been no studies to yet been identified, it is assumed that the parabrachial examine the synapses between the parabrachial fibers and complex may influence breathing through its descending the phrenic motoneurons. In this communication, we first projections to the nucleus of the solitary tract containing show that the parabrachio-phrenic projections originate
¨
the dorsal respiratory group (DRG) and / or through those primarily from the Kolliker–Fuse nucleus (KF), and then to the ventrolateral medulla containing several respiratory examine the synaptic organization of projections from the
KF to phrenic motoneurons.
The experiments were performed in male Wistar rats
*Corresponding author. Tel.: 181-853-20-2106; fax: 1
81-853-20-weighing 230 to 340 g. The operative procedures were
2105.
E-mail address: [email protected] (Y. Yasui). performed under general anesthesia with intraperitoneal
injection of chloral hydrate (350 mg / kg). In eight rats, a solution composed of 4% paraformaldehyde and 15% FluoroGold (FG, Fluorochrome) was injected into the glycerin in the same buffer for 6 h, and then immersed ventral horn in C4 to C5 segments of the spinal cord. In overnight in a cold solution of 20% sucrose in the same each rat, a single injection was made iontophoretically buffer. Subsequently, serial transverse sections of 50 mm through a glass micropipette filled with a 5% solution of thickness were cut on a freezing microtome. The sections FG dissolved in saline under direct vision; the center of were washed in PBS, incubated in PBS containing 0.2% injection was located 0.8 mm laterally to the midline and Triton X-100 for 3 h, and then incubated in PBS con-2.2 mm below the surface of the spinal cord. The driving taining avidin–biotin–peroxidase complex diluted at 1:100 current (5mA, 200 ms, 2 Hz) was delivered for a period of for 1 h. After washing again in PBS, the sections were 10 min. The rats surviving 7 to 10 days after operation incubated in 25 ml of 0.1 M PB containing 10 mg DAB, 5 were deeply reanesthetized and perfused transcardially mg nickel ammonium sulfate and 10 ml of 30% H O .2 2
with 100 ml saline, followed by 500 ml of a solution BDA-labeled axons were visualized as deep blue to black composed of 4% paraformaldehyde and 0.25% glutaral- reaction products. After washing in PBS, the sections were dehyde in 0.1 M phosphate buffer (pH 7.3; PB) and then incubated overnight in PBS containing 3% normal rabbit with 150 ml of 10% sucrose in the same buffer. After serum, 0.2% Triton X-100 and goat anti-CTb (List Biol. perfusion, the brains and cervical spinal cords were Labs) diluted at 1:10,000. Subsequently, the sections were removed and saturated with a cold solution of 20% sucrose washed in PBS, incubated in PBS containing biotinylated in the same buffer, and serial transverse sections of the rabbit anti-goat IgG (Vector) diluted at 1:200 for 3 h, brainstem and cervical spinal cord were cut at 50 mm washed again in PBS, and then incubated in PBS con-thickness on a freezing microtome. The sections were taining avidin–biotin–peroxidase complex diluted at 1:100 washed in phosphate-buffered saline (pH 7.3; PBS), for 1 h. After washing in PBS, the sections were incubated incubated overnight in PBS containing 3% normal goat in 25 ml of 0.1 M PB containing 10 mg DAB and 10ml of serum, 0.2% Triton X-100 and rabbit anti-FG (Chemicon) 30% H O . CTb-labeled neurons were visualized as brown2 2
diluted at 1:3000. Subsequently, the sections were washed reaction products.
in PBS, incubated in PBS containing biotinylated goat The specimens, where a good overlap of CTb-labeled anti-rabbit IgG (Vector Labs) diluted at 1:200 for 3 h, neurons and BDA-labeled axon terminals was found, were washed in PBS, and then incubated in PBS containing cut out from C4 to C5 segments of the spinal cord and avidin–biotin–peroxidase complex (Vector Labs) diluted at collected in cacodylate buffer (pH 7.3) for the transmission 1:100 for 1 h. After washing in PBS, the sections were electron microscope experiments. They were postfixed incubated in 25 ml of 0.1 M PB containing 10 mg with a solution of 1% osmium tetroxide in the same buffer diaminobenzidine (DAB) and 10 ml of 30% H O . FG-2 2 for 1 h at room temperature. After washing in the buffer, labeled neurons in the parabrachial and KF nuclei were the specimens were stained en bloc with 1% uranyl acetate counted in every third section and their total numbers and in 70% ethanol for 1 h at room temperature, rapidly proportions were quantified. dehydrated through a graded series of dilutions of ethanol, In 15 rats, cholera toxin B subunit (CTb, List Biol. cleared in propylene oxide and then embedded flat in Labs) was applied to the phrenic nerve and biotinylated Epon. Subsequently, thin sections were cut on an ultra-dextranamine (BDA, Molecular Probes) was injected microtome, collected on the collodion-coated grids, and stereotaxically into the KF on the same side. In each rat, a stained with lead citrate. Finally, the sections were ex-ventral midline incision was made in the neck, and then the amined under an electron microscope (JEOL, JEM right phrenic nerve was isolated from surrounding tissue 1200EX).
332 S. Yokota et al. / Brain Research 888 (2001) 330 –335
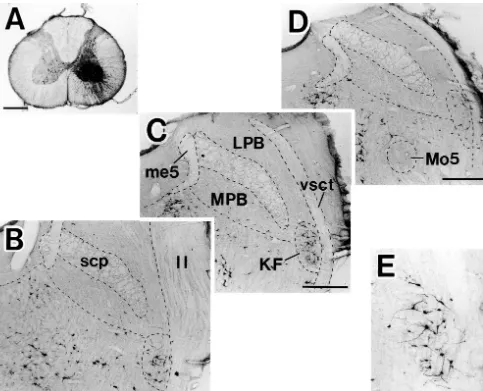
Fig. 1. The site of FluoroGold injection into the ventral horn in C4 segment of the spinal cord (A), and resulting retrograde labeling in the parabrachial region (B–D, rostral to caudal) and in the KF (E). The labeled KF neurons in C are shown in E at higher magnification. Note that many labeled neurons are seen in the rostral two-thirds of the KF (B, C). ll, lateral leminiscus; LPB, lateral parabrachial nucleus; me5, mesencephalic trigeminal tract; Mo5, motor trigeminal nucleus; MPB, medial parabrachial nucleus; scp, superior cerebellar peduncle; vsct, ventral spinocerebellar tract. Scale bars51 mm in A, 500
mm in B–D, 250mm in E.
Yokota
et
al
.
/
Brain
Research
888
(2001
)
330
–
335
333
334 S. Yokota et al. / Brain Research 888 (2001) 330 –335
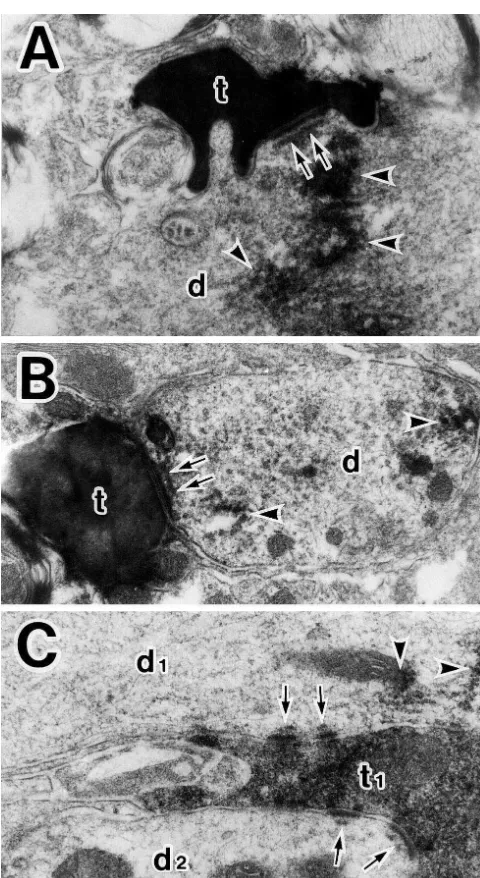
which were labeled with CTb; single BDA-labeled termi- observed in the active zone of the labeled axo-dendritic nals were occasionally found to form synaptic connections synapses.
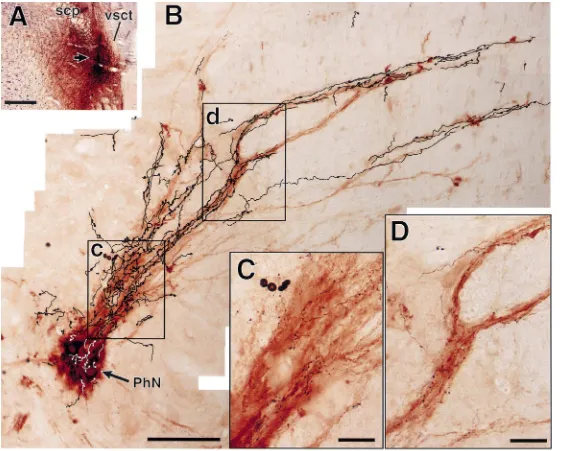
with different dendritic profiles (Fig. 3). On the other The present retrograde tracing study indicates that the hand, there were a few BDA-labeled terminals synapsing parabrachio-phrenic projections originate mainly from the upon somata of CTb-labeled phrenic motoneurons. As far KF, in accordance with the findings of a transneuronal as identified, the labeled terminal boutons formed viral tracing study [4]. Although there have been no studies asymmetrical synapses, although it was difficult to identify with anterograde tracers to indicate the existence of the the type of synaptic vesicles present because the density of parabrachio-phrenic projections of the rat, Holstege and the reaction product within the labeled boutons was heavy. Kuypers [12] demonstrated anterograde labeling within the Moreover, subsynaptic dense bodies [15,19] were often phrenic nucleus of the cat after tritiated leucine injection into the dorsolateral pontine tegmentum including the lateral part of the parabrachial nucleus and the KF. By means of a combination of anterograde and retrograde tracing techniques, we also showed that neurons in the KF sent their axons to the phrenic nucleus and further indi-cated that en passant varicosities of the axons were distributed along the radial dendritic bundles of phrenic motoneurons. The similar distribution pattern of afferent fibers to phrenic motoneurons has been shown in those
¨ from the rVRG and BotC [5].
It has been revealed that electrical or chemical stimula-tion of the parabrachial complex of the rat results in various types of respiratory responses which are dependent on the site of stimulation [2,13]. With respect to glutamate injection into the KF, Lara et al. [13] reported that it caused an expiratory facilitatory response. On the other hand, Chamberlin and Saper [2] indicated that a most intense inspiratory facilitatory response followed the in-jection into the KF region near the ventrolateral tip of superior cerebellar peduncle at mid to rostral levels of the nucleus, although hyperpnea or decrease in respiratory rate was produced by the injection into far rostral and midcaud-al areas or into the most latermidcaud-al and ventrmidcaud-al boundaries of the nucleus, respectively. The reason why the findings of Lara et al. are discrepant from those of Chamberlin and Saper is not clear, but both authors have emphasized that actions of KF outputs at the ventrolateral medulla and / or at the nucleus of the solitary tract are responsible for these respiratory effects. In fact, the KF has been known to send projection fibers to these lower brainstem regions [9,11]. Furthermore, DRG neurons in the nucleus of the solitary tract [8] and rVRG neurons in the ventrolateral medulla [6]
¨
or BotC neurons in the ventrolateral medulla [14,23,25] are considered to provide excitatory or inhibitory drive to phrenic motoneurons, respectively (see also [1] for re-view); these respiration-related areas have been revealed to project directly to the phrenic nucleus or phrenic motoneurons, as mentioned at the beginning. It is, there-fore, likely that an inspiratory facilitatory response follow-ing the stimulation of certain sites in the KF is caused by
Fig. 3. BDA-labeled axon terminals (t) making asymmetrical synapses KF outputs reaching phrenic motoneurons via the DRG
(arrows) with dendritic profiles (d) of phrenic motoneurons labeled with and / or rVRG. Another possible pathway responsible for CTb (A, B), and a BDA-labeled terminal bouton (t1) in asymmetrical this effect is the direct one from the KF to the phrenic contacts (arrows) with two different dendritic profiles (d1, d2), one of
motoneurons revealed here, in which asymmetrical
synap-which is labeled with CTb (C). Arrowheads indicate the reaction deposits.
tic connections are made between the KF fibers and the
Note that subsynaptic dense bodies in the synaptic active zone are
[10] J.V. Furicchia, H.G. Goshgarian, Dendritic organization of phrenic
the rVRG have also been demonstrated to form
asymmetri-motoneurons in the adult rat, Exp. Neurol. 96 (1987) 621–634.
cal axo-somatic and axo-dendritic synapses on phrenic
[11] H. Herbert, M.M. Moga, C.B. Saper, Connections of the
parabrachi-motoneurons [6], although the type of synapses between
al nucleus with the nucleus of the solitary tract and the medullary
the DRG fibers and the phrenic motoneurons remains reticular formation in the rat, J. Comp. Neurol. 293 (1990) 540–580. unknown. [12] G. Holstege, H.G.J.M. Kuypers, The anatomy of brainstem
path-ways to the spinal cord in cat. A labeled amino acid tracting study, Prog. Brain Res. 75 (1982) 145–175.
[13] J.P. Lara, M.J. Parkes, L. Silva-Carvhalo, P. Izzo, M.S. Dawid-Acknowledgements
Milner, K.M. Spyer, Cardiovascular and respiratory effects of stimulation of cell bodies of the parabrachial nuclei in the
anaesthet-The authors are grateful for the photographic help of Mr ized rat, J. Physiol. (1994) 321–329.
Makoto Oshita and Mr Tsunao Yoneyama and the support [14] E.G. Merrill, L. Fedorko, Monosynaptic inhibition of phrenic of Drs Ryuzo Fujimoto, Kazuya Fujita, Yasuichi motoneurons: a long descending projection from Botzinger neurons,¨
J. Neurosci. 4 (1984) 2350–2353.
Munenaga, Takashi Sakurai, Yayoi Sakurai, Hiromi
[15] M. Milhaud, G.D. Pappas, Post-synaptic bodies in the habenula and
Taguchi and Katsumi Tanaka.
interpeduncular nuclei of the cat, J. Cell Biol. 30 (1966) 437–441. [16] D. Mutolo, F. Bongianni, M. Carfi, T. Pantaleo, Respiratory responses to chemical stimulation of the parabrachial nuclear
References complex in the rabbit, Brain Res. (1998) 182–186.
[17] T. Onai, M. Miura, Projections of supraspinal structures to the [1] W.W. Blessing, The Lower Brainstem and Bodily Homeostasis, phrenic motor nucleus in cats studied by a horseradish peroxidase
Oxford University Press, New York, 1997. microinjection method, J. Auton. Nerv. Syst. 16 (1986) 61–77. [2] N.L. Chamberlin, C.B. Saper, Topographic organization of respira- [18] T. Onai, M. Saji, M. Miura, Projections of supraspinal structures to
tory responses to glutamate microstimulation of the parabrachial the phrenic motor nucleus in rats studied by a horseradish per-nucleus in the rat, J. Neurosci. 14 (1994) 6500–6510. oxidase microinjection method, J. Auton. Nerv. Syst. 21 (1987) [3] T.E. Dick, M.C. Bellingham, D.W. Richter, Pontine respiratory 233–240.
neurons in anesthetized cats, Brain Res. 636 (1994) 259–269. [19] A. Peters, S.L. Palay, H. deF Webster, Fine Structure of the Nervous [4] E.G. Dobbins, J.L. Feldman, Brainstem network controlling de- System, 3rd Edition, Oxford University Press, New York, 1991.
scending drive to phrenic motoneurons in rat, J. Comp. Neurol. 347 ´˜
[20] F. Portillo, P.A. Nunez-Abades, Distribution of bulbospinal neurons (1994) 64–86.
supplying bilateral innervation to the phrenic nucleus in the rat, [5] H.H. Ellenberger, J.L. Feldman, Monosynaptic transmission of
Brain Res. 583 (1992) 349–355. respiratory drive to phrenic motoneurons from brainstem
bulbospi-[21] G.C. Rikard-Bell, E.K. Bystrzycka, B.S. Nail, Brainstem projections nal neurons in rats, J. Comp. Neurol. 269 (1988) 47–57.
to the phrenic nucleus: a HRP study in the cat, Brain Res. Bull. 12 [6] H.H. Ellenberger, J.L. Feldman, H.G. Goshgarian, Ventral
respirato-(1984) 469–477. ry group projections to phrenic motoneurons: electron microscopic
[22] C.B. Saper, Central autonomic system, in: G. Paxinos (Ed.), The Rat evidence for monosynaptic connections, J. Comp. Neurol. 302
Nervous System, 2nd Edition, Academic Press, San Diego, 1995, (1990) 707–714.
pp. 107–135. [7] H.H. Ellenberger, P.L. Vera, J.R. Haselton, C.L. Haselton, N.
¨
[23] G.-F. Tian, J.H. Peever, J. Duffin, Botzinger-complex expiratory Schneiderman, Brainstem projections to the phrenic nucleus: an
neurons monosynaptically inhibit phrenic motoneurons on the anterograde and retrograde HRP study in the rabbit, Brain Res. Bull.
decerebrate rat, Exp. Brain Res. 122 (1998) 149–156. 24 (1990) 163–174.
[24] H. Yamada, K. Ezure, M. Manabe, Efferent projections of inspirat-[8] L. Fedorko, E.G. Merrill, J. Lipski, Two descending medullary
ory neurons of the ventral respiratory group. A dual labeling study inspiratory pathways to phrenic motoneurons, Neurosci. Lett. 43
in the rat, Brain Res. 455 (1988) 283–294. (1983) 285–291.
[9] C.E. Fulwiler, C.B. Saper, Subnuclear organization of the efferent [25] W.-Z. Zhang, H.H. Ellenberger, J.L. Feldman, Immunohistochemical connections of the parabrachial nucleus in the rat, Brain Res. Rev. 7 labeling of monoaminergic and GABA-ergic terminals in phrenic