www.elsevier.com / locate / bres
Short communication
Cortical cholinergic activity is related to the novelty of the stimulus
*
´
´
´
Marıa Isabel Miranda, Leticia Ramırez-Lugo, Federico Bermudez-Rattoni
´ ´ ´ ´
Departamento de Neurociencias, Instituto de Fisiologıa Celular, Universidad Nacional Autonoma de Mexico, 04510 Mexico D.F., Mexico Accepted 25 July 2000
Abstract
A number of studies have related cholinergic activity to the mediation of learning and memory. However, the acetylcholine (ACh) participation has been recently implicated in the early stages of memory formation but not during retrieval. The aim of the present study is to evaluate ACh release in the insular cortex (IC) during presentation of different taste stimuli and during their re-exposition by means of the free-moving microdialysis technique. We evaluated the changes in ACh release when a novel taste, saccharin or quinine was presented to the rat and after several presentations of saccharin. Unilateral microdialysis was performed in the IC 1 h before and 1 h after the presentation of: (1) a familiar stimulus (water), (2) a novel taste (quinine), (3) another novel taste (saccharin), (4) a second presentation, (5) a third presentation, and (6) a fourth presentation of saccharin. The volume consumed by the animals was registered as a behavioral parameter. The ACh levels from the microdialysis fractions were analyzed by an HPLC–ED system. Biochemical results showed a significant increment in the cortical ACh release induced by a novel stimulus compared with the release observed during the presentation of a familiar stimulus. The ACh release observed after several presentations of the stimuli decreased to the same levels as those produced by the familiar taste, indicating an inverse relationship between familiarity and cortical ACh release. These results suggest that the cholinergic system plays an important role in the identification and characterization of different kinds of stimuli. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Acetylcholine; Rat free-moving microdialysis; Gustatory cortex; Cholinergic basal forebrain
The acetylcholine (ACh) is released in the rat neocortex the mnemonic gustatory representation [6]. This cortex is in response to a variety of behavioral and environmental an important region for the acquisition, consolidation and conditions, including wakefulness, motor activity [10] retrieval of conditioned taste aversion (CTA) task, a robust restraint- or handling-induced stress [25] and by exposure and important behavioral model widely used for studying to auditory, visual and gustatory stimulation [14,31]. In the neurobiology of learning processes [12]. It has been this regard, a modulatory role for ACh in the activity of reported that cholinergic mechanisms are involved in CTA cortical neurons has been suggested by observations from formation [16,21]. For instance, it has been shown that electrophysiological and biochemical studies [2,10]. In ACh release in the IC is induced by the simple presenta-addition to these findings, which demonstrate differences tion of taste stimuli and this release is influenced by in ACh release related to different states of arousal, other behavioral rejection of aversive taste stimuli [31]. More-evidence suggests that associative conditioning may also over, when muscarinic antagonists were microinjected modify ACh release in the neocortex [1,13,24,28]. Thus, it bilaterally into IC shortly before the exposure of the rat to has been demonstrated that a significant enhancement of a novel taste, the treatment blocked the CTA [4,21]. cortical ACh release is associated with appetitive con- Additionally, it has been demonstrated that cortical micro-ditioning procedures [13]. Moreover, training for acquisi- injections of muscarinic antagonists before the first expo-tion of tactile discriminaexpo-tion performance causes a specific sure of saccharin enhanced the novelty of the taste when it enhancement in ACh release in the somatosensory cortex is presented for second time, in an incidental learning
[7]. situation [21]. Incidental learning is when animals are
The insular gustatory cortex (IC) has been involved in pre-exposed to a new taste stimulus before acquisition of CTA, leading to an attenuation of the acquisition of the conditioned aversion, this process is also called latent *Corresponding author. Tel.:15-25-622-5626; fax:15-25-622-5607.
´
E-mail address: [email protected] (F. Bermudez-Rattoni). inhibition [21].
Recently, we demonstrated that tetrodotoxin (TTX)- guide cannula was kept in place with three skull screws blockage of the nucleus basalis magnocellularis (NBM), and dental acrylic cement.
the most important cholinergic afferent to the cortex, Two or three days after surgery, all the animals were impairs acquisition but not retrieval of CTA [19]. Since deprived of water for 24 h and then habituated to the lesions or pharmacological blockade of cortical cholinergic microdialysis chamber once a day during 45 min trials, and activity disrupt only acquisition of CTA, the release of to drink water from a graded bottle during 15 min for 5 ACh seems to be related to the early stages of taste days, or until a stable water consumption baseline was
memory formation. reached. The rats were then separated into 6 groups, and
Taken together, these data suggest that in the IC, the each one received one microdialysis session during the cholinergic system plays a role in encoding the representa- presentation of a determined taste (see Table 1).
tion of gustatory stimuli. However, little is known of the During day nine, the novel saccharin (0.1%; SAC-1, actual role of cortical ACh in non-associative learning or n56), quinine (0.02%, QUIN, n53), and familiar, water incidental learning of gustatory stimuli. Therefore, the (WATER, n54); second (SAC-2, n55); third (SAC-3, principal goal of the present experiment was to measure n54) and fourth (SAC-4, n53) presentations of saccharin extracellular ACh release in the insular cortex of free- groups were subjected to microdialysis during consump-moving rats, using the microdialysis technique, during tion of a given stimulus (see Table 1A and C). The familiar presentation of novel, semi-familiar and familiar gustatory groups were given a saccharin solution inside the mi-stimulus. A preliminary report of this study has been crodialysis chamber, one, two or three days before the day presented in abstract form [20]. of microdialysis session. Liquid consumption was recorded Twenty-five male Wistar rats weighing 275–325 g at the during all presentations of water, saccharin or quinine. time of surgery were used. The animals were housed under One-way ANOVA followed by post-hoc multiple com-a 12-h light–dcom-ark cycle, with food com-and wcom-ater com-ad libitum, parisons were performed for all groups on the consumption except during behavioral tests. All the animals were and ACh levels obtained during microdialysis day. anaesthetized with pentobarbital (50 mg / kg) and were Dialysis was started by connecting the probe inlet implanted in the right IC (AP511.2 mm, L515.5 mm (dialysis probes CMA / 12 from CMA / Microdialysis, with from Bregma; V524.5 mm from dura) (Paxinos and a 3 mm total length of membrane) to the micro-infusion Watson, 1986) with one microdialysis guide cannula pump system (CMA / Microdialysis), which perfused the (CMA / 12) using standard stereotaxic procedures. The probe continuously at a rate of 2 ml / min with Ringer
Table 1
solution (118 mM NaCl, 4.7 mM KCl, and 2.5 mM CaCl )2
during the entire microdialysis procedure. To achieve detectable amounts of acetylcholine in the dialysate, we added 10 mM neostigmine bromide (Sigma) to the Ringer solution. Once the probe was connected to the guide cannula, the first 45 min sampling was discarded, and 7 samples were collected every 15 min (30ml / sample) and immediately frozen at 2808C and later analysed by high performance liquid chromatography and electrochemical detection (HPLC–EC).
The general microdialysis procedure in all groups was as follows (see Table 1C): During the first 1 h 15 min, the rats were left in the microdialysis chamber without any stimulus. The graded bottle with the gustatory stimuli (water, saccharin or quinine according to the group, see
Table 1A) was placed in the microdialysis chamber during Fig. 1. Mean volume of saccharin consumption (6S.E.M.) during presentation of various taste stimuli. *P,0.05 vs. WATER and SAC-1. the next 15 min; after this, the microdialysis continued for
&
P,0.05 vs. SAC-2. 1 h more.
The collected samples were assayed for ACh content by HPLC (Beckman) with electrochemical detection (BAS).
The samples were injected into a polymeric reversed phase 0.05). Post hoc analysis revealed that the groups which column (BAS); ACh and choline were then converted into received saccharin three or four times (SAC-3 and SAC-4) hydrogen peroxide and betaine in a post-column enzyme showed significant increment in consumption when com-reactor containing immobilized acetylcholinesterase and pared with the water consumption group (P’s,0.05). As choline oxidase (BAS). The hydrogen peroxide was de- expected, the rats presented a saccharin preference, be-tected electrochemically by a platinum electrode set at 500 cause there were significant increments in saccharin con-mV (vs. Ag /AgCl). The mobile phase consisted of a 50 sumption during the third (SAC-3), as compared to the mM sodium phosphate buffer (pH 8.5) and Kathon reagent first (SAC-1) presentation of taste (P,0.05). Moreover, (BAS). The detection limit was approximately 0.1 pmol. we found significant increased consumption during the After microdialysis, the rats were deeply anaesthetized fourth presentation (SAC-4) as compared with the first with pentobarbital and perfused transcardially with a 4% presentation of saccharin (SAC-1) or quinine (QUIN) solution of paraformaldehyde in phosphate buffer (0.15 M, (P’s,0.01), and with the second presentation of saccharin pH 7.4). The brains were placed overnight in paraformal- (SAC-2) (P,0.05) (Fig. 1).
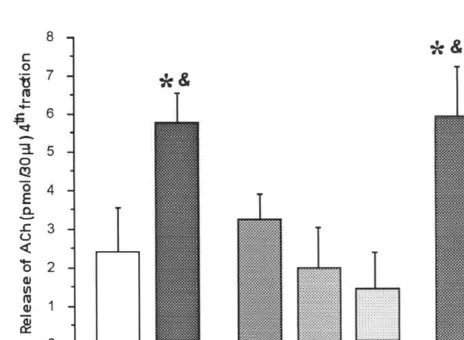
dehyde and then transferred to a 20% buffered sucrose Fig. 2 shows the release of cortical ACh during gustat-solution and stored at 48C until they were cut. Coronal ory stimulus consumption. ANOVA was performed with sections (50mm thick) were taken through the areas of the the mean of the fourth microdialysis sample (which was probe. The slide sections were stained by the cresyl violet
procedure. Histological examinations revealed that the localization of the guide cannulae and probes was within the IC in all groups (Table 1B). Although the probes invaded a small part of the parietal cortex, we assumed that the major ACh release measured comes from the gustatory insular cortex [18,19].
The drinking scores are shown in Fig. 1. The basal volume consumption (water) for all groups was approxi-mately 10 ml. The water consumption score during the microdialysis assay was similar to that observed during the previous basal water consumption and the consumption observed in previous experiments. In the same way, the first consumption of the different tastes, saccharin and quinine (in a low concentration that did not produce a neophopic response), observed during the microdialysis
Fig. 2. Mean extracellular levels of ACh in the IC during the fourth assay was similar to the water consumption (see Fig. 1).
fraction of microdialysis, just after the drinking of different tastes. Values
Simple ANOVA was carried out for all groups. On the day &
the only one that showed significant differences between were not significant ACh increases in the parietal cortex in groups), obtained immediately after the consumption frac- response to water or saccharine consumption, as has been tion due to the delay in transporting the perfusate (see also reported previously [31].
Table 1C). Simple ANOVA was carried out for all groups. The incidental learning observed in this experiment and The analysis showed significant differences among groups habituation are elementary paradigms of associative and (F(5,19)54.043, P,0.01) during the release of ACh. Post non-associative learning that reflect a preference response hoc analysis showed significant increments in cortical ACh to a learned inconsequential stimulus due to familiarity, release between the group that received the first presenta- and involves several behavioral processes, such as re-tion of saccharin (SAC-1) and quinine (QUIN) compared sponses to novelty, including arousal, emotion and stress-with the water consumption group (P’s,0.01). Additional- related factors and, on the other hand, a diminished ly, we found a significant increment in ACh release during response, which required learning-related processes and consumption of the first saccharin presentation (SAC-1) recognition or recall [8,9,23]. In this regard, open field compared with the release during the second (SAC-2), exploration and its habituation is another behavioral model third (SAC-3) and fourth (SAC-4) presentations of sac- closely related to cholinergic activity of the hippocampus. charin (P’s,0.05). Similarly, we observed significant Recent results [32] have shown that exposure of rats to a differences between ACh release during quinine (QUIN) novel environment led to increased extracellular levels of consumption, and during the third (SAC-3) and fourth hippocampal ACh which were positively correlated to (SAC-4) saccharin presentations (P’s,0.05). exploratory behavior. When re-exposing the same animals In addition, we measured ACh release in the parietal once more to the same environment, the exploratory cortex as a control site (AP22.5 mm, L 5.7, V 2.0 mm behavior decreased, but cholinergic activation remained with reference to Bregma), during consumption of water high. The apparent contradictory result with ours may (n55) and saccharin (n54) following the same procedure depend on the paradigm used and in the differences of as for the IC. Histological examinations revealed that the cholinergic activation involved in the stages of attention location of the guide cannula and probes were within the and learning, and the different cerebral regions related to somatosensorial region of frontoparietal cortex (Paxinos that particular behavior [5,11,17,29].
and Watson, 1986) in all the animals (data not shown). The A number of studies have shown that the insular microdialysis results showed that the consumption of water gustatory neocortex is strongly involved in mnemonic increased by 11.5% the ACh release in the parietal cortex gustatory representation during the taste aversion when compared to its own baseline release. When the paradigm, where the animal acquires aversion to a taste stimulus was a novel taste (saccharin), the ACh release cue when it is followed by digestive malaise [6,15]. increases 7.7% as compared to the release induced by Furthermore, cholinergic activity in the IC was found to be water, and 88.8% of its own baseline release. Nevertheless, related to taste processing by in vitro studies that showed
3 1
statistical analysis showed there were no significant differ- [ H] acetylcholine release after K -stimulation [16], and ences in the ACh release between the base line and their more recently by in vivo studies with intraoral infusions of
th
consumption in fraction 4 in both groups. various taste stimuli that revealed an induction of ACh The results reported here demonstrate that ACh is release in the IC [31]. The ACh plays an important role released in the IC when the rats have free access to liquid only in the early stages of the CTA; for example, it has consumption. After consumption of a familiar gustatory been shown that shortly before the exposure of the rat to a stimulus, (water), a significant increase (145%) in ACh novel taste (or conditioned stimulus), the muscarinic release is observed when compared to its baseline release antagonist scopolamine blocks conditioned taste aversion, before the gustatory stimulation. However, when the and this antagonist has no effect when microinjected stimulus is a novel taste (saccharin or quinine), the ACh shortly after exposure or during recall [21].
NBM-mediated cholinergic cortical release may play an im- Acknowledgements
portant role in early stages of memory formation, but not
This research was supported by CONACyT 31842N. We during recall of aversive memories [19]. Additionally, the
acknowledge the assistance of Oreste Carbajal and cortical ACh decrements during the taste familiarity
ob-Federico Jandete and give thanks to Shaun Harris for his served in this report indicate an important correlation
´
text review, and to Yolanda Dıaz de Castro for preparing between cortical cholinergic activity and the novelty
the manuscript. codification of the taste.
This data agrees with previous reports that suggest an important cholinergic role during processing of the novel
References
stimulus representation [21,22]. It is well known that pre-exposure of the animal to the new taste stimulus
[1] E. Acquas, C. Wilson, H.C. Fibiger, Conditioned and unconditioned several times before acquisition of CTA attenuates
acquisi-stimuli increase frontal cortical and hippocampal acetylcholine tion of the conditioned aversion; this process is called
release: effects of novelty, habituation, and fear, J. Neurosci. 16 latent inhibition. Dudai and co-workers demonstrated that (1996) 3089–3096.
cortical micro-injections of scopolamine 20 min before [2] J.S. Bakin, N.M. Weinberger, Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis, Proc. pre-exposure to the novel taste disrupted latent inhibition,
Natl. Acad. Sci. USA 93 (1996) 11219–11224. proving that blockade of cholinergic activity in the IC
[3] K.A. Baskerville, N.R. Heaston, J.B. Schweitzer, P. Herron, Role of during the first presentation of a non-familiar taste inter- acetylcholine in experience dependent plasticity in the somato-fered with the representation of a novel taste [21]. In this sensory cortex of the rat, Soc. Neurosci. Abstr. 21 (1995) 123.
´ ´
regard, it has been proposed that ACh, as a neuro- [4] F. Bermudez-Rattoni, C. Ormsby, M.L. Escobar, E. Hernandez-Echeagaray, The role of the insular cortex in the acquisition and modulator, may facilitate cortical plasticity by signaling
long lasting memory for aversively motivated behaviors, in: J.L. the stimulus relevance during memory formation.
Addi-´ ´
McGaugh, F. Bermudez-Rattoni, R.A. Prado-Alcala (Eds.), Plastici-tionally, a rapid and marked enhancement of protein ty in the Central Nervous System: Learning and Memory, Lawrence tyrosine phosphorylation of a set of neuronal and synaptic Erlbaum Associates, Hillsdale, NJ, 1995, pp. 67–82.
proteins, including the n-methyl-d-aspartate receptor [5] A. Blokland, Acetylcholine: a neurotransmitter for learning and memory?, Brain Res. Rev. 21 (1996) 285–300.
subunit 2B (NR2B) has been shown in the IC, but not in
´
[6] J. Bures, F. Bermudez-Rattoni, T. Yamamoto, Conditioned Taste other brain areas, after presentation of a non-familiar taste
Aversion: Memory of a Special Kind, Oxford University Press, New during conditioned taste aversion training [27]. The protein York, 1998.
tyrosine phosphorylation enhancement produced by an [7] E.A. Butt, G. Testtyler, R.W. Dykes, Acetylcholine release in rat unfamiliar taste can be mimicked by microinjections of the frontal and somatosensory cortex is enhanced during tactile
dis-crimination learning, Psychobiology 25 (1997) 18–33. cholinergic agonist carbachol [26].
[8] A. Cerbone, A.G. Sadile, Behavioral habituation to spatial novelty: Cortical ACh is hypothesized to modulate the general
interference and noninterference studies, Neurosci. Biobehav. Rev. efficacy of the cortical processing of sensory or association 18 (1994) 497–518.
information. Specifically, cortical cholinergic inputs medi- [9] H. Dai, M. Krost, R.J. Carey, A new methodological approach to the ate the abilities of the subjects to detect and select stimuli study of habituation: the use of positive and negative behavioral
indices of habituation, J. Neurosci. Meth. 62 (1995) 169–174. [30]. Moreover, the role of cholinergic activity in
learning-[10] J. Day, G. Damsma, H.C. Fibiger, Cholinergic activity in the rat induced plasticity has been underlined by recent reports
hippocampus, cortex and striatum correlates with locomotor activity: [2,3,7]. Bakin and Weinberger (1996) demonstrated that an in vivo microdialysis study, Pharmacol. Biochem. Behav. 38 the convergence, in the auditory cortex, of acoustic fre- (1991) 723–729.
quency information and application of cortical ACh, [11] H.C. Fibiger, Cholinergic mechanisms in learning, memory an dementia: a review of recent evidence, Trends Neurosci. 14 (1991) induced receptive field facilitation similar to that produced
220–223. by behavioral learning, and these changes could be blocked
´ ´
[12] J. Garcıa, P.S. Lasiter, F. Bermudez-Rattoni, D.A. Deems, A general by muscarinic antagonists [2]. Taken in conjunction, the theory of aversion learning, in: M.S. Braveman, P. Bronstein (Eds.), data suggests that ACh selectively influences those cortical Experimental Assessments and Clinical Applications of Conditioned cells involved in early stages of memory formation, Food Aversions, Ann. N.Y. Acad. Sci., 1985, pp. 8–21.
[13] F.M. Inglis, J. Day, H.C. Fibiger, Enhanced acetylcholine release in leading to the morphological changes that incorporate the
hippocampus and cortex during the anticipation and consumption of context and the meaning of the stimulus; i.e., familiar vs.
a palatable meal, Neuroscience 62 (1994) 1049–1056.
non-familiar [33]. [14] F.M. Inglis, H.C. Fibiger, Increases in hippocampal and frontal In conclusion, the results of this research show that the cortical acetylcholine release associated with presentation of sensory great increase in the cortical ACh release produced by a stimuli, Neuroscience 66 (1995) 81–86.
[15] S.W. Kiefer, Neural mediation of conditioning food aversions, Ann. novel taste decreased in the next presentation of the same
NY Acad. Sci. 443 (1985) 100–109. stimulus, reaching similar ACh levels as those induced by
´ ´ ´ ´ ´
[16] J.C. Lopez-Garcıa, J. Fernandez-Ruız, F. Bermudez-Rattoni, R. a familiar stimulus i.e., water. This suggests that ACh has Tapia, Correlation between acetylcholine release and recovery of a role in the recognition or encoding of the novelty of the conditioned taste aversion induced by fetal neocortex grafts, Brain
[17] R.G. Lydon, Cholinergic neurons and memory: an historical per- restore in vivo acetylcholine release and respond to behavioral spective and overview of current research, in: T.W. Stone (Ed.), CNS activation, Neuroscience 55 (1993) 353–362.
Neurotransmitters and Neuromodulators: Acetylcholine, CRC, Boca [26] K. Rosenblum, D.E. Berman, S. Hasvi, Y. Dudai, Carbachol mimics ´
Raton, 1995, pp. 197–232. effects of sensory input on tyrosine phosphorilation in cortex, ´
[18] M.I. Miranda, F. Bermudez-Rattoni, Recovery of taste aversion NeuroReport 7 (1996) 1401–1404.
learning induced by fetal neocortex grafts: correlation with in vivo [27] K. Rosenblum, R. Schul, N. Meiri, Y.R. Hadari, Y. Zick, Y. Dudai, extracellular acetylcholine, Brain Res. 759 (1997) 141–148. Modulation of protein tyrosine phosphorylation in rat insular cortex
´
[19] M.I. Miranda, F. Bermudez-Rattoni, Reversible inactivation of the after conditioned taste aversion training, Proc. Natl. Acad. Sci. USA nucleus basalis magnocellularis induces disruption of cortical acetyl- 92 (1995) 1157–1161.
choline release and acquisition, but not retrieval, of aversive [28] M. Sarter, J.P. Bruno, Cognitive functions of cortical Acetylcholine: memories, Proc. Natl. Acad. Sci. USA 96 (1999) 6478–6482. lessons from studies on tran-synaptic modulation of activated efflux,
´ ´
[20] M.I. Miranda, L. Ramırez-Lugo, F. Bermudez-Rattoni, Micro- TINS 17 (1994) 217–221.
dialysis in free moving rats reveals differential release of acetyl- [29] M. Sarter, J.P. Bruno, B. Givens, H. Moore, J. McGaughy, K. choline during consumption of a familiar, novel, or aversive taste McMahon, Neuronal mechanisms mediating drug-induced cognition stimuli, Soc. Neurosci. Abstr. 76 (1998) 12. enhancement: cognitive activity as a necessary intervening variable, [21] C. Naor, Y. Dudai, Transient impairment of cholinergic function in Cogn. Brain Res. 3 (1996) 329–343.
the rat insular cortex disrupts the encoding of taste in conditioned [30] M. Sarter, J.P. Bruno, Cognitive functions of cortical acetylcholine: taste aversion, Behav. Brain Res. 79 (1996) 61. toward a unifying hypothesis, Brain Res. Rev. 23 (1997) 28–46. [22] M. Orsetti, F. Casamenti, G. Pepeu, Enhanced acetylcholine release [31] T. Shimura, T. Yamamoto, Aversive taste stimuli facilitate
extracel-in the hippocampus and cortex durextracel-ing acquisition of an operant lular acetylcholine release in the insular gustatory cortex of the rat: a behavior, Brain Res. 724 (1996) 89–96. microdialysis study, Brain Res. 679 (1995) 221–226.
[23] A. Platel, R.D. Porsolt, Habituation of exploratory activity in mice: a [32] C.M. Thiel, J.P. Huston, R.K.W. Schwarting, Hippocampal acetyl-screening test for memory enhancing drugs, Psychopharmacology choline and habituation learning, Neuroscience 85 (1998) 1253–
78 (1982) 346–352. 1262.