*Corresponding author. Tel.:#49-6151-163602; fax:#49-6151-166878. E-mail address:[email protected] (E. Wollenweber)
Biochemical Systematics and Ecology 28 (2000) 751}777
Lipophilic exudates of Pteridaceae
}
chemistry
and chemotaxonomy
Eckhard Wollenweber
!,
*
, Harald Schneider
"
!Institut fuer Botanik der Technischen Universitaet, Schnittspahnstrasse 3, D-64287 Darmstadt, Germany
"Department of Botany, Field Museum, Roosevelt Road at Lake Shore Drive, Chicago, IL 60605-2496, USA
Received 26 July 1999; accepted 27 September 1999
Abstract
A number of fern species, belonging to several genera of Pteridaceae, exhibit a more or less conspicuous farinose wax, which is mostly located on the lower leaf surface. Production of these waxes is often correlated with the presence of glandular trichomes. Particularly during the past two decades, a series of publications appeared on the chemical composition of these exudates. The major components were found to be#avonoids (chalcones, dihydrochalcones,#avanones, dihydro#avonols,#avones, #avonols), some of them with a complex substitution pattern, including esters and C-methyl derivatives, and even bis#avonoids. Diterpenoids and triter-penoids can also occur in such exudates. It is the purpose of this paper to survey the chemical composition of Pteridaceae exudates and their occurrence within the genera of the family. The chemotaxonomic signi"cance of the#avonoid aglycones at the generic, speci"c and popula-tional level is brie#y discussed.( 2000 Elsevier Science Ltd. All rights reserved.
Keywords:Pteridaceae; Farinose exudates; Flavonoid aglycones; Terpenoids
1. Introduction
More or less conspicuous white or yellow coatings are long known to occur on the lower leaf surfaces of a number of ferns, belonging to the genera Pityrogramma, Cheilanthes, Notholaena and others. In the pteridological literature, these coatings
were described as wax, cera, powder, farina, resins,`pseudostearoptenesa(cf. Wollen-weber, 1976a). It was found early that this material is excreted by glandular trichomes (De Bary et al., 1877). Already in 1906, Zopf isolated some components from the farinose exudates ofPityrogrammaspecies, but only in 1961 Nilsson (1961a,b)
identi-"ed these products as chalcones and dihydrochalcones.
A series of papers dealing with the composition of farinose fern exudates have since been published, in particular from the senior authors's laboratory; some papers of other authors appeared, too. Information on this subject is hence scattered in the phytochemical literature, a comprehensive survey is missing. Markham (1988), in a chapter on `Flavonoid distribution in lower plantsa, listed most of the results reported up to 1986, but his chapter covers both internal and external#avonoids. We deem it desirable, therefore, to present here a complete compilation of all#avonoids found to date in farinose (waxy) fern exudates and related epicuticular layers. In the present paper we list the #avonoid aglycones, we report their distribution in the relevant genera, and we discuss their chemotaxonomic implications. The terms wax and farina are used synonymous throughout the text. Many species also exhibit externally deposited terpenoids, which might have some importance as chemotaxonomic characters, as will be discussed for the relevant taxa.
2. Experimental
The#avonoid aglycones as well as the terpenoids in question are all more or less lipophilic, and they are deposited externally, on leaf surfaces. All these products can hence be recovered unambiguously by brie#y rinsing the fern material with acetone, sometimes with addition of toluene or methanol. Unless major components crystallize spontaneously, the concentrated extracts are subjected to column chromatography on silica and/or on polyamide and the components isolated are further puri"ed and identi"ed as reported e.g. in (Wollenweber et al., 1978b; Roitman et al., 1993). When the exudate constituents are known #avonoids, a small fragment of fern leaf is su$cient for thin-layer chromatographic identi"cation by direct comparison with markers. Terpenoids were also isolated by column chromatography, sometimes by
`#ash chromatographya. They are less suited for identi"cation by tlc comparisons, so normally spectroscopic studies are required. (For details see e.g. RuKedi et al., 1989; Appendino et al., 1992; Arriaga et al., 1996).
In some cases preservation treatment of herbarium specimen has eliminated the exudate material, so these are of no use for phytochemical analysis. Especially, most specimen collected in humid tropical regions lack farinose waxes, because they were treated with alcohol or similar"xatives before drying. (In only 6 out of 50 collections of Cheilanthes papuana from New Guinea and Moluccas Islands the farina is still present). Due to di!erent solubility of farina components, such preservation treatment may have changed the composition in some other specimens. These cases become evident, however, when specimen of various origins are compared and they are not considered for chemotaxonomic evaluation.
3. Results
3.1. Components of farinose waxes in Pteridaceae
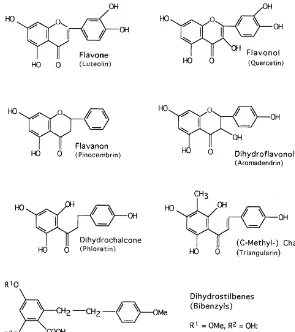
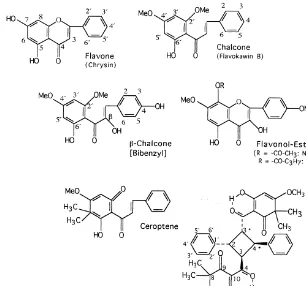
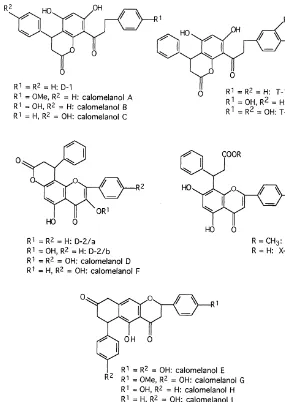
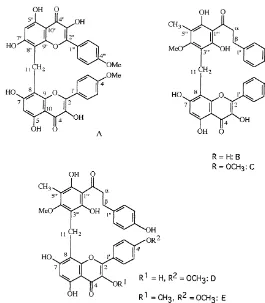
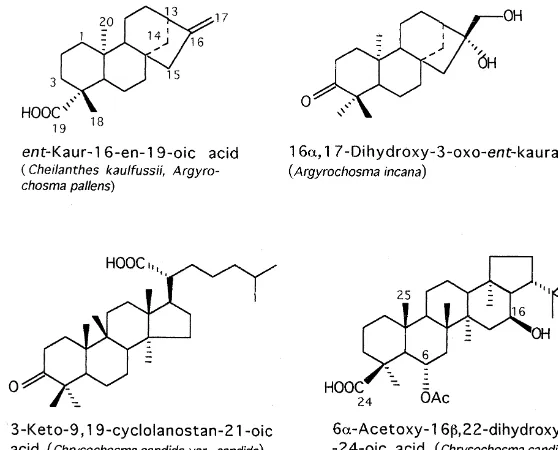
In this section we list in the"rst line all#avonoids so far reported as constituents of farinose fern exudates (Table 1). They are grouped by#avonoid classes, and within these classes they are sorted according to their substitution patterns as e.g. in Wollenweber, 1994. Structural formulae of representatives of the #avonoid classes concerned are given in Fig. 1. For standard numbering of#avones,#avonols, etc. on the one hand, and of chalcones and dihydrochalcones on the other hand, see Fig. 2. The latter also shows examples of ab-chalcone or bibenzyl and of#avonol-esters, as well as the structure of the chalcone-likePentagramma-product, ceroptene. In Fig. 3 the complex fern #avonoids reported so far are shown, and Fig. 4 shows the methylene-bis#avonoids found in Pentagramma.
For the sake of completeness we also mention two dihydrostilbenes or bibenzyls found in the exudate ofArgyrochosma species, namely 5-OH-3,4@ -diOMe-6-COOH-bibenzyl or `notholaenic acida, and 3-OH-5,4@-diOMe-6-COOH-bibenzyl or
`isonotholaenic acida. Their structures are included in Fig. 1.
The terpenoids so far encountered accumulated on the leaf surfaces of Pteridaceae comprise diterpenes and triterpenes. Some relevant structures are depicted on Fig. 5.
3.2. Morphological prerequisites for the production of farinose waxes
Farinose `waxesa in ferns are mainly the product of glandular trichomes with a stalk of 1}6 cells and one enlarged apical glandular cell (For a brief review of earlier descriptions see Wollenweber, 1978). These hairs are located on the lower surface in most taxa, such asArgyrochosma, Cheilanthes, Chrysochosma, Pityrogramma, Penta-gramma, but they may also occur on the upper leaf surface and even on the rachis, e.g. in Pentagramma pallida. The impression of a farinose or waxy coating on the leaf surface is caused by densely arranged glandular trichomes, whose glandular cells are covered with microcrystalline platelets, rods, etc., much like in farinose Primula
species (Barthlott and Wollenweber, 1981).
Flavonoid excreting hairs are restricted to the outer surface of the pseudoindusium inOnychium siliculosumand also inAdiantum poiretii. In farinose species ofCerosora
andPterozonium, glandular trichomes are only found in contact to sporangia. They are, therefore, classi"ed as paraphyses. Glandular paraphyses also occur in some non-farinose species of Pterozoniumand in the non-farinose closely related genera
AspleniopsisKUHN,AustrogrammeE. FOURN.,and TaenitisWILLD. ex SPRENGEL.
On the other hand, there also exist taxa that exhibit#avonoid exudates, although no glandular structures are observed. These species are discussed in Section 4. In this context we also want to mention that related phenolic exudates are also known for some fern taxa outside the Pteridaceae, e.g. in Grammitis SW. (grammitic acid, see
Wollenweber and Arriaga-Giner, 1991) and inDryopterisADANS. (see e.g. WideHn et al.,
1991; Wollenweber et al., 1998).
Table 1
Flavonoids found in fern exudates!
Chalcones
OH substitution ring A only substituted
methyl ethers, C-methyl derivatives, esters
1 2@,4@,6@-triOH
ring B also substituted
4@-Me, 2@-Me (cardamonin), 2@,4@-diMe (#avokawin B)
2 (2@,4@,6@,4@-tetraOH)
b-chalcones(bibenzyls)
4@-Me (neosakuranetin), 4@,4-diOMe
3 (2@,4@,6@,b,4-pentaOH)
methyl ethers, C-methyl derivatives, esters
1 (2@,4@,6@-triOH) 4@-Me,
OH substitution methyl ethers, C-methyl derivatives, esters
1 5,7-diOHPinocembrin pin-5-Me (alpinetin), 7-Me (pinostrobin), 5,7-diMe
2 5,7,4@-triOHNaringenin nar-7-Me (sakuranetin), -4@-Me (isosakur.), 7,4@-diMe
8 (5,6,7,8,4@-pentaOH) C-2@substituted
6,8-diMe, 6,7,8-triMe, 6,8,4@-triMe, 7,8,4@-triMe, 6,7,8,4@-tetraMe
9 (5,7,2@,4@-tetraOH) 2@-Me
methyl ethers, C-methyl derivatives, esters
1 (3,5,7,8,2@-pentaOH)
7,8-diMe
2 Ester (3,5,7,4@-tetraOHAromadendrin)
C-8 and C-2@substituted
arom-7-Me-3-Ac, 7-Me-3-But, 7-Me-4@-But
3 (3,5,7,8,2@-pentaOH) 7,8-diMe-2@-Ac
Flavones
OH substitution methyl ethers, C-methyl derivatives, esters
1 5,7-diOHChrysin
2 5,7,4@-triOHApigenin ap-7-Me (genkwanin), 4@-Me (acacetin), 7,4@-diMe
3 5,7,3@,4@-tetraOH Luteolin
C-2@substituted
lut-7-Me, 3@-Me (chrysoeriol), 4@-Me (diosmetin), 7,3@-diMe (velutin), 7,4@-diMe (pilloin)
4 (5,7,8,2@- tetraOH)
C-6 and C-8 substituted
7,8-diMe (skullkap#avone I)
5 (5,6,7,8,4@-pentaOH) 6,8-diMe (desmethoxysudachitin), 6,8,4@-triMe (nevadensin),
6,7,8-triMe (xanthomicrol)
Flavonols
OH substitution methyl ethers, C-methyl derivatives, esters
1 3,5,7-triOHGalangin gal-3-Me, 5-Me, 7-Me (izalpinin), 3,7-diMe, 5,7-diMe
2 3,5,7,4@-tetraOHKaempferol kae-3-Me (isokaempferid), 7-Me (rhamnocitrin), 4@-Me
Table 1*continued
3 3,5,7,3@,4@-pentaOHQuercetin C-6 substituted
qu-3,7-diMe, 3,3@-diMe, 3,7,3@-triMe (pachypodol), 3,7,4@-triMe (ayanin), 7,3@,4@-triMe, 3,7,3@,4@-tetra-Me (retusin)
4 (3,5,6,7,4@-penta-OH 6-Hydroxy-kaempferol) C-8 substituted
6-OH-kae-6,7,4@-triMe (mikanin)
5 3,5,7,8-tetraOH 8-Hydroxy-galangin 8-OH-gal-8-Me, 3,7-diMe
6 3,5,7,8,4@-pentaOHHerbacetin herb-7-Me (pollenitin), 8-Me (sexangularetin), 4@-Me, 3,7-diMe, 7,8-diMe, 3,4@-diMe, 8,4@-diMe (prudomestin), 3,8,4@-triMe, 7,8,4@-triMe (tambulin)
7 (3,5,7,8,3@,4@-hexaOH Gossypetin) C-6 and C-8 substituted
goss-7,4@-diMe
8 (3,5,6,7,8,4@-hexaOH)
C-2@substituted#C-8 substituted
3,6,8-triMe (sarothrin), 6,7,8-triMe
9 (3,5,7,8,2@-pentaOH) 3,7,8-triMe
10 (3,5,7,8,2@,3@-hexaOH) 3,7,8-triMe
11 (3,5,7,8,2@,4@-hexaOH) 7,8,4@-triMe, 3,7,8,2@-tetraMe, 3,7,8,2@,4@-pentaMe, 12 (3,5,7,8,2@,5@-hexaOH)
C-2@substituted#C-6 and C-8 substituted
3,7,8-triMe, 3,7,8,2@-tetraMe
13 (3,5,6,7,8,2@,4@-heptaOH) 3,6,7,8,tetraMe, 3,6,7,8,2@-pentaMe, 3,6,7,8,4@-pentaMe, 3,6,7,8,2@,4@-hexaMe Esters
C-8 substituted
14 (8-OH-Galangin) 8-OH-gal-7-Me-8-Ac, 7-Me-8-But
15 (Herbacetin) herb-7-Me-8-Ac, 7-Me-8-But, 7,4@-diMe-8-Ac, 7,4@-diMe-8-But
16 (Gossypetin)
C-8 and C-2@substituted
goss-7,4@-diMe-8-Ac, 7,4@-diMe-8-But, 3,7,3@-triMe-8-Ac
17 (3,5,7,8,2@-pentaOH) 7-Me-8-Ac
!For structural formulae and numbering system see Figs. 1 and 2, for complex#avonoids and methylene bi#vonoids see Figs. 3 and 4.
Fig. 1. Representatives of di!erent#avonoid classes. Notholaenic and isonotholaenic acid.
The colour of the waxes varies from snow white, yellowish white and golden yellow to orange (and even reddish-orange in Pterozonium). The greatest variability is observed inPentagramma triangularis. In species with scant wax production, the lower leaf surface appears rather greenish-white. Some species show a characteristic colour, while in others the colour may vary slightly from specimen to specimen. Yellow and orange are often constant and typical, but transitions are found between yellowish-white and greenish yellowish-white. Also in species exhibiting snow-yellowish-white waxes the composi-tion of the exudate is often, but not always, constant. A correlacomposi-tion between geographic range, aspect, height, edaphic requirements, the colour and chemistry of the ceraceous exudate has been reported forChrysochosma hookerisyn.Notholaena standleyi(Seigler and Wollenweber, 1983).
Fig. 2. Numbering of#avones,#avonols, etc., compared with numbering of chalcones & dihydrochalcones. Examples of a b-chalcone and of#avonol-esters. Structures of ceroptene and a-diceroptene.
The colour of the waxes is largely correlated with the chemical composition. Orange colour is normally caused by chalcones, and so is yellow colour in some cases. However, yellow colour may also point to the presence of#avonols, in particular of 8-O-substituted#avonols. White exudate material can be composed of dihydrochal-cones,#avanones, or#avones (and sometimes of diterpenes). Sometimes slight vari-ations of the farina tint (e.g. faint pink instead of chalky white) are observed, that cannot be attributed to the presence or absence of certain chemicals.
3.3. Distribution of farinose waxes in Pteridaceae, based on genus level
In the following we list all exudate#avonoids found in relevant genera, regardless of their occurrence in certain species. Within a given genus, the#avonoid patterns can be rather uniform (e.g.Pityrogramma), but they can also vary from species to species (e.g.
Chrysochosma), and also within a species (e.g. Cheilanthes argentea, Chrysochosma trichomanoides).
Fig. 3. Complex Flavonoids fromPityrogrammaspp.
Due to the extremely variable number of species concerned per genus as well as of
#avonoids encountered, the following inventory cannot be presented in a standard-ized manner. For genera comprising only few farinose species (and producing few
#avonoid aglycones), the#avonoids are cited in the text (e.g.Adiantum, Onychium), while for others they are compiled in tables (e.g. Pentagramma, Argyrochosma, Chrysochosma).
The classi"cation follows Tryon (1990) with the following exceptions.Pentagramma
has been accepted as an independent genus of the Cheilanthoideae (Schneider, 1996).
Fig. 4. Methylene bis#avonoids fromPityrogramma triangularisvar.triangularis.
are placed inChrysochosmaaccording to the reinterpretation of the type ofNotholaena
R. BR. (Pichi Sermolli, 1989). Taxa of doubtful hierarchical status such as Aleuritop-terishave been accepted as groups without taxonomic rank in the genusCheilanthes. The order of subfamilies and genera re#ects the diversity of farinose taxa.
1. Cheilanthoideae 1.1 CheilanthesSW.
Tryon (1990) has included the following taxa in Cheilanthes s.l., but they are monophyletic units with unknown phylogenetic relationships.
1.1.1. Aleuritopteris Group"Aleuritopteris FE&E* Cheilanthessubgen.
Aleuritop-terisFRASER-JENK.
This is a very diverse pantropical group which includes farinose species ( Aleuritop-terisser.AleuritopterisCHING) as well as non-farinose species (LeptolepidiumHSINGet
S.K. WU). The taxonomy and relationships of the group is poorly understood. The
Argenteagroup andNegripterisgroup may be close relatives. In farinose species, the
Fig. 5. Diterpenes and Triterpenes found in fern`waxesa(examples).
lower surface of the lamina is covered with mostly whitish, only rarely golden yellow waxes. Almost all species have been analyzed for their exudate composition:
Cheilanthes agetae(SAIKI) C.M. KUO,C. albidissimaFRASER-JENK.,C. ancepsBLANF.,
C. bicolor(ROXB. et GRIFF. ex FRASER-JENK.,C. bullosaKUNZE,C. chrysophyllaHOOK.,
C. dalhousiaeHOOK.,C. dubiaOPE,C. farinosa(FORSSK.) KAULF.,C. formosanaHAYATA,
C. griseaBLANF.,C. krameriFRANCH.ETSAV.,C. kuhniiMILDE,C. papuanaC.CHR.,C.
platychlamys (CHING) FRASER-JENK., C. pulveracea C.PRESL, C. subdimorpha B.K. NAYAR, C. welwitschii HOOK. ex BAKER. The following analyzed taxa are currently
plazed inAleuritopteris: A. afraPIC.SERM.,A. decursiva(FORSSK.) SAIKI,A.yavaSAIKI,
andA. stenochlamysCHING.
Components: The#avonoid aglycones encountered in this group are compiled in Table 2. The species show infraspeci"c as well as intraspeci"c di!erences in their farina composition. Many unpublished data are available; the results of detailed analysis will be published elsewhere. (Wollenweber, 1976b, c, 1977b, 1982a, 1997; Serizawa and Wollenweber, 1977; Scheele et al., 1987).
1.1.2. ArgenteaGroup"AleuritopterisFEHEseriesargenteaCHING.
The taxonomically poorly known group occurs from Korea, Japan, China, Taiwan, Indochina to northern Thailand, and in the Himalayas. White or yellow waxes cover the lower surface of the lamina. The non-farinose fernCheilanthes concolor(LANGSD. et
FISCH.) R.M.TRYONet A.F.TRYONresemblesCheilanthes argentea(GMEL.) FEHEin many
morphological features. The group includes about 16 species (Ching and Shing, 1990).
Table 2
Flavonoid aglycones found in`Cheilanthesa
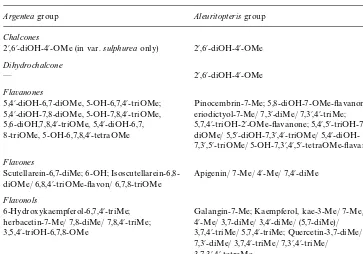
Argenteagroup Aleuritopterisgroup
5,4@-diOH-6,7-diOMe, 5-OH-6,7,4@-triOMe; 5,4@-diOH-7,8-diOMe, 5-OH-7,8,4@-triOMe, 5,6-diOH,7,8,4@-triOMe, 5,4@-diOH-6,7, 8-triOMe, 5-OH-6,7,8,4@-tetraOMe
Pinocembrin-7-Me; 5,8-diOH-7-OMe-#avanone; eriodictyol-7-Me/ 7,3@-diMe/ 7,3@,4@-triMe; 5,7,4@-triOH-2@-OMe-#avanone; 5,4@,5@-triOH-7,3@ -diOMe/ 5,5@-diOH-7,3@,4@-triOMe/ 5,4@ -diOH-7,3@,5@-triOMe/ 5-OH-7,3@,4@,5@-tetraOMe-#avanone Flavones
Scutellarein-6,7-diMe; 6-OH; Isoscutellarein-6,8-diOMe/ 6,8,4@-triOMe-#avon/ 6,7,8-triOMe
Apigenin/ 7-Me/ 4@-Me/ 7,4@-diMe 3,7,4@-triMe/ 5,7,4@-triMe; Quercetin-3,7-diMe/ 7,3@-diMe/ 3,7,4@-triMe/ 7,3@,4@-triMe/ 3,7,3@,4@-tetraMe
Only two species have been investigated chemically:Cheilanthes argentea(GMEL.) FEHE
andC. tamburii(HOOK.) CHING.C. argenteais part of a species complex, which need
further taxonomic studies.
Components: The analyzed specimens ofC. argenteashow a remarkable diversity of farina components. Their#avonoid patterns indicate the existence of two chemotypes (Wollenweber, 1982a; Wollenweber and Roitman, 1991). This result is also supported by the existence of two di!erent diterpenes in the farina: specimens from Japane and mainland Asia exhibit 3-hydroxy-anticopalic acid, while specimens from Taiwan exhibit anticopalic acid (Wollenweber et al., 1982a). The #avonoid aglycones are compiled in Table 2, compared with those of the Aleuritopteris group and the
Brandigeigroup. (Wollenweber et al., 1980).
1.1.3. Brandegei Group R.M.TRYONand A.F.TRYON
This group consists of"ve South American species (Tryon et Tryon, 1982). The farinose indumentum on the lower surface of the lamina is often yellow to orange. OnlyC. aurantiaca T. MOORE("Notholaena aurantiaca) andC. aurea BAKERhave
been studied chemically.
Components: Cheilanthes aurantiaca exhibits 2@,6@-diOH-4@-OMe-chalcone, while
C. aureaexhibits a so far unidenti"ed chalcone. (Wollenweber, 1977b, 1982a).
1Coll. ETS 36; Sodore Hot Springs, Awash-River Valley, Ethiopia, June 1970 [L].
2Cavalarie 57, Tinla, S.W. China, 1911 [K]; China, Yunnan, Maire, C. Bonati 6105 B [ZT]; Yunnan, Maire 6558 [US]; Yunnan, Rock 4879 [US].
1.1.4. NegripterisGroup"NegripterisPIC.SERM.
Pichi Sermolli (1946) has described this taxon as a distinct genus based on an atypical sporangium, but other characters indicate close relationships toAleuritopteris
group. The group is monotypic, the sole species N. scionana (CHIOV.) PIC.SERM.
occurring in East Africa and Arabia Whitish or slightly yellowish farina are found on the lower surfaces of the lamina.
Components: Fragments of several specimens have been studied for their exudate components,1but these could not be identi"ed. Thin layer chromatograms indicate a certain similarity to#avonoid patterns observed inCheilanthes argentea.
1.1.5. SinopterisGroup"SinopterisC. CHR.ETCHING
The group consists of only two species, endemic in China, with some unique characters. It may be closely related to theArgentea-group. OnlyCheilanthes albofusca
BAKERhas been examined chemically.2
Components: One sample exhibits Kae-7,4@-diMe#apig-7,4@-diMe, whereas three others exhibit luteolin-7,3@,4@-triMe. We do not dare to give any interpretation.
1.2. Argyrochosma(J.SM.) WINDHAM
Nearly all species of the genus exhibit a remarkable farinose indumentum. Most species have been analysed for their exudate chemistry, but publications are scattered. So far no comprehensive survey allowing comparisons has been published. Flavonoid data are available for A. chilensis (FEHE and REMY) Windham, A. dealbata (PURSH)
WINDHAM,A. delicatula(MAXON ETWEATH.) WINDHAM,A. fendleri(KUNZE) WINDHAM,
A. incana(C.PRESL) WINDHAM,A. limitanea(MAXON) WINDHAM,A. microphylla(METT. EXKUHN) WINDHAM,A. nivea(POIR.) WINDHAM,A. pallens(WEATH. in R.M. TRYON)
WINDHAM, A. palmeri (BAKER) WINDHAM, A. peninsularis (MAXON & WEATH.)
WINDHAM,A. pilifera(R.M. TRYON) WINDHAM. Since no material for analysis could be
obtained,A. formosa(LIEBM.) WINDHAM,A. jonesii(MAXON) Windham,A. lumholtzii
(MAXONand WEATH.) Windham andA. stuebeliana(HIERON.) WINDHAMhave not yet
been checked.Notholaena bryopodaMAXONlacks an epithet but it is a member of the
genus. (Windham, 1987).
Components: All#avonoids found in representatives of this genus are listed in Table 3.Note: The species di!er greatly in the composition of their farinose waxes. Several of them exhibit species-speci"c#avonoid patterns, some #avonoids even occur exclus-ively in one single species. The list of#avonoids found in the genus may insofar be somewhat misleading.
In this context we also brie#y report the occurrence of dihydrostilbenes (" biben-zyls, see Fig. 1) and terpenoids inArgyrochosma exudates.A. chilensis, A. dealbata,
Table 3
Flavonoid aglycones (and bibenzyls) found in`Notholaenaa
Argyrochosma Chrysochosma 4@-Me/ 7,4@-diMe; eriodictyol-7-Me/ 4@-Me/ 7,3@-diMe/7,4@-diMe/ luteolin-7,3@-diMe/ 7,4@-diMe
Apigenin/ 7-Me/ 4@-Me/ 7,4@-diMe; Scutellarein-6,7-diMe/ 6,7,4@-triMe; Luteolin/ 7-Me/ 3@-Me/ 4@-Me/ 7,3@-diMe/ 7,4@ -diMe; 5,2@-diOH-7,8-diOMe
Flavonols
Galangin-3-Me/ 5-Me/ 3,7-diMe; kaempferol/ 3-Me/ 7-Me/ 4@-Me/ 3,7-diMe/ 3,4@-diMe/ 7,4@-diMe/ 3,7,4@-triMe; quercetin-3,3@-diMe/ 7,3@-diMe/ 3,7,3@-triMe/ 3,7,4@-triMe/ 3,7,3@,4@-tetraMe
Galangin/ 3-Me/ 5-Me/ 7-Me/ 5,7-diMe; kaempferol/ 3-Me/ 7-Me/ 4@-Me/ 3,7-diMe/ 3,4@-diMe/ 7,4@-diMe/ 3,7,4@-triMe; herbacetin-7-Me/ 7,4@-diMe; quercetin-3-Me/ 7-Me/ 3,7-diMe/ 7,3@-diMe/ 3,7,4@-triMe; myricetin-3,7,3@,4@-tetraMe/ 3,7,3@,4@,5@-pentaMe; 5,2@-diOH-3,7,8-triOMe; 5,2@,3@ -triOH-3,7,8-triOMe; 3,5,2@-triOH-7,8,4@-triOMe/ 5,4@-diOH-3,7,8,2@ -tetraOMe/ 5-OH-3,7,8,2@,4@-pentaOMe; 5,2@,5@ -triOH-3,7,8-triOMe/ 5,5@-diOH-3,7,8,2@-tetraOMe; 5,2@,4@ -triOH-3,6,7,8-tetraOMe-#av., 5,4@-diOH-3,6,7,8,2@-pentaOMe/ 5,2@ -diOH-3,6,7,8,4@-pentaOMe/ 5-OH-3,6,7,8,2@,4@-hexa-OMe-#avonol. Flavonol-Esters
* 8-OH-gal-7-Me-8-Ac/ 8-But; 7-Me-8-Ac/ 8-But,
herb-7,4@-diMe-8-Ac/ -8-But; goss-7,4@-diMe-8-Ac/ 8-But, goss-3,7,3@-triMe-8-Ac; 3,5,2@-triOH-7-Me-8-OAc; 5,6,5@ -triOH-3,7,8-triMe-2@-OAc; 5-OH-3,7,2@,3@,4@-pentaMe-8-OAc; Dihydrostilbenes("bibenzyls)
notholaenic acid, isonotholaenic acid
A. limitaneavar.mexicanaproduces notholaenic acid (one specimen showed both isomers).
A. delicatula, A. incana, A. pallens, A. palmeri, A. peninsularisandA. piliferaproduce terpenoids instead (Wollenweber et al., 1993). Diterpenes fromA. pallensandA. incana
were reported by RuKedi et al. (1989). For examples of terpenoid structures see Fig. 5. (Roitman et al., 1992; Wollenweber, 1976c, 1977b, 1981, 1982a, 1984, 1989; Wollen-weber and Favre-Bonvin, 1979; WollenWollen-weber and Roitman, 1991; WollenWollen-weber et al., 1980,1993).
1.3. Chrysochosma(J.SM.) KUEMMERLE ("Notholaena SENSU R.M.TRYON non R.
BR.)
All species of the genus possess farina, except forC. ekmanii (MAXON) PIC.SERM.
Flavonoid data are available for C. aznis (METT.) PIC.SERM., C. aliena (MAXON)
PIC.SERM., C. ascherboniana (KLOTSCH) PIC.SERM., C. californica (D.C.EATON)
PIC.SERM., C. candida (M. MARTENS and GALEOTTI) KUEMMERLE var. candida, C.
candida (M. MARTENS and GALEOTTI) KUEMMERLE var. copelandii (C.C.HALL)
PIC.SERM., C. galapagensis (WEATH. and STENSON) PIC.SERM., C. galeotti (FEHE)
PIC.SERM.,C. grayi (DAVENP.) PIC.SERM., C. greggii (METT. EX KUHN) PIC.SERM.,C.
hookeri KUEMMERLE ("Notholaena standleyi MAXON), C. lemmonii (D.C. EATON)
PIC.SERM., C. neglecta (MAXON) PIC.SERM., C. rigida (DAVENP.) PIC.SERM., C. rosei
(MAXON) PIC.SERM., C. schawneri (E. FOURN.) PIC.SERM., C. sulphurea (CAV.)
KUEMMERLE,C. trichomanoides(L.) PIC.SERM.
Flavonoid data are listed in Table 3. The above comments to the#avonoid survey ofArgyrochosmaalso apply toChrysochosma. A survey on the triterpenes found inC. candida, C. grayii, C. greggii, C. neglecta, C. rigidaandC. schawneri(cyclolanostane, dammarane and hopane derivatives) is given in (Arriaga-Giner et al., 1997). For examples of some structures see Fig. 5.
(Arriaga-Giner and Wollenweber, 1986, Arriaga-Giner et al., 1987; Iinuma et al., 1986a; Jay et al., 1979a, b, 1981, 1982; Scheele et al., 1987; Seigler and Wollenweber, 1983; Wollenweber, 1976a,b,c, 1977a,b, 1982a, 1984, 1989; Wollenweber and GoHmez, 1979; Wollenweber and Roitman 1991; Wollenweber and Yatskievych, 1982; Wollenweber et al., 1978a,b, 1982b, 1988).
The comparative table of exudate#avonoids inArgyrochosmaand Chrysochosma
(Table 3) shows some noticeable features: Chalcones andb-chalcones are produced only in Argyrochosma, whereas Chrysochsoma produces often dihydrochalcones. Flavanone- and dihydro#avonol-esters as well as#avonol-esters are found only in
Chrysochosma.Further,Chrysochosmashows a greater variety of#avanones,#avones, and #avonols. Dihydrostilbens, on the other hand, were reported only from Ar-gyrochosma. These phytochemical results might underline the division of the previous genusNotholaenasensu R.M. Tryon into two distinct genera.
1.4. PentagrammaYATSK., WINDHAMand WOLLENWEBER (1990)
All species/subspecies of the genus exhibit remarkable yellow or whitish farina, mostly on the lower lamina surface (P. pallida, P. triangularis subsp. triangularis,
P. triangularis subsp.semipallida and P. triangularis subsp. maxonii), or a resinous exudate (P. triangularissubsp.viscosa). They have been analysed in extensive studies
by D.M. Smith (1980) and by Wollenweber et al. (Refs. see below).
Components: An extremely high number of exudate #avonoids is known from
Pentagramma,but many of these occur only in certain species or subspecies. It does not make sense, therefore, to list them for the genus in total. In the following we hence give the#avonoid composition for the species.
P. pallida(WEATH.) YATSK., WINDHAMand WOLLENWEBER: Major#avonoids of the
abundant white farina are: 5,7-diOH-6-CH
3-#avanone (strobopinin),
5,7-diOH-8-CH
3-#avanone (cryptostrobin), 5,7-diOH-6,8-di-CH3-#avanone
(desmethoxymat-teucinol). These C-methyl-#avanones are not produced by any other species, so they are quite characteristic for P. pallida. Minor #avonoids are pinocembrin-5-Me, pinocembrin-5,7-diMe, 7-OH-5-OMe-8-CH
3-#avanone, 2@,4@-diOH-6@
-OMe-chal-cone (carda-monin), 2@-OH-4@,6@-diOMe-chalcone (#avokawin B), 2,4@-diOH-6@ -OMe-3@-CH
3-chalcone, 2@-OH-4@,6@-diOMe-3@-CH3-chalcone (aurentiacin).
(Wollenweber et al., 1979a, 1981a,b; Markham et al., 1987).
P. triangularis (KAULF.) YATSKIEVYCH, WINDHAM and WOLLENWEBER subsp. tri-angularis * The major and species-speci"c product is ceroptene (Fig. 2). This chalcone-like compound exhibits brilliant yellow#uorescence and can, therefore, be detected directly on fern material viewed under UV
366. Minor products:a-diceropten
(trace constituent only), 2@-OH-4@,6@-diOMe-chalcone (#avokawin B), 2@,6@ ,4-triOH-4@-OMe-3@-CH
3-chalcone (triangularin), 5-OH-7-OMe-6-CH3-#avanone,
5-OH-7-OMe-8-CH
3-#avanone, 8-hydroxy-galangin, 5,8-diOH-3,7-diOMe-#avonol
(8-OH-gal-3,7-diMe), 3,5,7-triOH-8-CH
3-#avonol, 3,5,7-triOH-6,8-diCH3-#avonol,
5,7-diOH-3-OMe-8-CH
3-#avonol, 5,7-diOH-3-OMe-6,8-diCH3-#avonol,
3,5-diOH-7-OMe-8-CH
3-#avonol, 3,5-diOH-7-OMe-6,8-di CH3-#avonol,
3,5,8-triOH-7-OMe-6-CH
3-#avonol, 5,7,8-triOH-3-OMe-6-CH3-#avonol, 3,5,7-triOH-8-OMe-6-CH3
-#avonol (pityrogrammin), 5,8-diOH-3,7-diOMe-6-CH
3-#avonol. Two
methylene-bis-#avonoids (Fig. 4, structures A and B) were the"rst representatives of this group of substances found in nature (Roitman et al., 1993). Four further
methylene-bis-#avonoids were reported later (Fig. 4, str. C}F) (Iinuma et al., 1994, 1997).
(Dietz et al., 1981; Roitman et al., 1993; Vilain et al., 1987; Wollenweber, 1989; Wollenweber and Roitman, 1991; Wollenweber and Smith, 1981; Wollenweber et al., 1985; Yatskievych et al., 1990).
P. triangularis subsp. viscosa (NUTT. EX D. EATON) YATSKIEVYCH, WINDHAM and
WOLLENWEBERproduces 2@,6@,4@-triOH-4@-OMe-3@-CH
3-dihydrochalcone,
herbacetin-3,7-diMe, and 3,5,4@-triOH-7-OMe-6,8-diCH
3-#avonol. (Wollenweber et al., 1979b).
P. triangularis subsp. semipallida (J. HOWELL) YATSKIEVYCH, WINDHAM and
WOLLENWEBERexhibits kaempferol-3,4@-diMe as major product, along with traces of
gal-3-Me, gal-5-Me, gal-5,7-diMe, and kae-3-Me.
P. triangularissubsp.maxonii(WEATH.) YATSKIEVYCH, WINDHAMand WOLLENWEBER
exhibits galangin and traces of some so far unidenti"ed#avonoids (some of which might be identical with compounds found inP. triangularisvar.triangularis).
Wollenweber et al. in 1985 reported the#avonoid patterns of two collections that were not ascribed to distinct varieties or chemotypes. In addition to a series of
#avonoids reported above forP. triangularis, these collections exhibit 2@,6@-diOH-4@ -OMe-chalcone, 2@,6@-diOH-4@,4-diOMe-chalcone, 2@,6@-diOH-4@ -OMe-dihydrochal-cone, 2@,6@-diOH-4@,4-diOMe-dihydro-chalcone, pinocembrin-7-Me, 7-Me, kae-3,7-diMe, herbacetin-8,4@-diMe, herb-3,8,4@-triMe and 2@,6@,4-triOH-4@-OMe-3@,5@ -diCH
3-dihydrochalcone. } For a detailed discussion of #avonoid patterns in the
Pentagramma("Pityrogramma)triangulariscomplex see Smith (1980). (Wollenweber, 1979; Wollenweber and Dietz, 1980).
2. Taenitidoideae (C.PRESL) R.TRYON
2.1. PityrogrammaLINK
All 14 species possess farinose greenish-white, white or yellow waxes on the lower side of the sterile and fertile parts of the lamina. The composition of the farina was analysed for most of the species:P. argentea(WILLD.) DOMIN,P. aurantiaca(HIERON.)
C. CHR.,P. austroamericanaDOMIN,P. calomelanos(L.) LINK,P. chrysoconia(DESV.)
MAXON ex DOMIN, P. chrysophylla (SW.) LINK, P. dealbata (C.PRESL) R.M.TRYON,
P. dukei LELLINGER, P. ebenea (L.) PROCTOR, P. lehmannii (HIERON.) R.M. TRYON,
P. pulchella(T. MOORE) DOMIN,P. sulphurea(SW.) MAXON,P. tartarea(CAV.) MAXON,
P. trifoliata(L.) R.M.TRYON, P.WILLIAMSIIPROCTOR.
Components: 2@,6@-diOH-4@-OMe- and 2@,6@-diOH-4@,4-diOMe-chalcone, 2@,6@ -diOH-4@-OMe- and 2@,6@-diOH-4@,4-diOMe-dihydrochalcone (exceptional: 2@,6@ ,4-triOH-4@-OMe-ch and 2@,6@,4-triOH-4@-OMe-dhch); galangin, gal-7-diMe, kaem-pferol-3,7-diMe, apigenin-7-Me and ap-7,4@-diMe (Wollenweber, 1972, 1976a, 1977a, 1979, 1980; Hitz et al., 1982). Note: chalcones and dihydrochalcones are major products in all species, while#avones and#avonols are found as minor products in only a few species. The complex#avonoids D-1 and D-2a/ D2-b (Wagner et al., 1979; Donnelly et al., 1987; Iinuma et al., 1993) (see Fig. 3) are found inP. calomelanos, P. chrysoconia, P. dealbata, P. sulphureaandP. trifoliata. (Wollenweber and Dietz, 1980. Complex#avonoids X-1 and X-2 (Favre-Bonvin et al., 1980Iinuma et al., 1986b) were detected in individual plants ofP. austroamericana("P. calomelanosvar.aureoyava
[Hook] Weath. ex Bailey), P. sulphurea and P. tartarea (Wollenweber and Dietz, 1980). The structurally closely related`calomelanolsaA-J (Fig. 3) were reported from
P. calomelanos from Indonesia (Asai et al., 1992) [in fact, it might be P. aus-troamericana]. The complex#avonoids T-1, T-2, T-3 (Dietz et al., 1980) occur inP. sulphurea, P. trifoliataandP. williamsii(Wollenweber and Dietz, 1980).
2.2. PterozoniumFEHE
At least three out of 14 species possess glandular paraphyses which excrete a more or less obvious yellow or reddish-orange farina:P. brevifrons(A.C.SMITH) LELLINGER,
P. reniforme(MARTIUS) FEHE,P. scopulinumLELLINGER.
Components: P. brevifronsand P. scopulinumexhibit 2@,6@-diOH-4@
(`cera brunneo-rubraa, Lellinger, 1967) suggests that the latter species also excretes chalcone(s). (Wollenweber, 1979).
2.3. OnychiumKAULF
Only one out of some eight species, namely O. siliculosum (DESV.) C.CHR.
("Onychium auratumKAULF.), exhibits a yellow farinose exudate on the outer surface
of the pseudoindusia.
Components: The farina is composed of 2@,6@-diOH-4@-OM-chalcone and 2@,6@ -diOH-4@,5@-diOMe-chalcone.
(Ramakrishnan et al., 1974; Wollenweber, 1982b). The corresponding#avanones (pinostrobin and 5-OH-6,7-diOMe-#avanone, named onysilin), as reported by Wu et al. in 1981, were shown to be artifacts (Wollenweber, 1982b).
2.4. Cerosora(BAKER) DOMIN
Only one of the three species, namely C. chrysosora (BAKER) DOMIN, shows
a farinose indumentum (yellow). The glandular hairs are placed between the sporangia on the fertile lamina.
Componentsof the farina have not been analyzed, due to lack of material.
3. Adiantoideae(C.PRESL) R.M.TRYON
3.1. AdiantumL.
Farina is only known from Adiantum poiretii WIKSTR. var. sulphureum (KAULF.)
R.M.TRYON("A. sulphureumKAULF.) with a more or less dense yellow wax on the
outer surface of the pseudoindusia and sometimes on parts of the lower surface of the lamina. This variety is distinct fromvar. poretiionly in the presence of farinose waxes.
Specimens with scarce farina have been collected in Chile.A. poiretiiis widespread but scattered distributed from southern South America to Mexico, from (temperate and tropical) Africa to India. However, farinose plants (var.sulphureum) are only known from southern South America (Chile) and in parts of Africa (Kenya, Tanzania, Zimbabwe, South Africa). Detailed studies are needed to understand the evolution of farinose coatings in this species.
Components: In some plants of A. poiretii var. sulphureum, 2@,6@-diOH-4@ -OMe-chalcone and 2@,6@-diOH-4@-diOMe-dihydrochalcone are the major components, accompanied by galangin and gal-7-Me (izalpinin). Other plants exhibit 2@,4@,6@ -trihydroxy-chalone along with pinocembrin and naringenin-7-methyl ether (sakuranetin). The existence of two chemotypes should, therefore, be considered. No correlation is observed between the exudate#avonoid pattern and the geographic origin (Wollenweber, 1976b, 1979).
4. Non-farinose species
Externally deposited#avonoid aglycones are also found in some non-farinose ferns.
4.1. Platyzomatoideae(NAKAI) A.F.TRYON
4.1.1. PlatyzomaR.BR.
A monotypic genus, endemic in Australia (P. microphyllaR.BR.). This fern does not
really produce a farina:`Two- or three-celled, capitate glands are abundant on the pinnae, which usually have accumulations of yellowish exudate on the surface. Freshly collected specimens have a characteristic scent and, when pressed, leave an oily stain on paper.a(Wollenweber et al., 1987).
Components: Major #avonoids are 2@,6@-diOH-4@-OMe-chalcone and 2@,6@ -diOH-4@,5@-diOMe-chalcone. Minor components and trace constituents are pin-7-Me; gal-5-Me, gal-7-Me, gal-3,7-diMe, kae-7-Me, kae-3,7-diMe, kae-3,7,4@-triMe. (Wollen-weber and Roitman, 1991; Wollen(Wollen-weber et al., 1987).
4.2. Cheilanthoideae
Species of the genus Cheilanthes with glandular hairs (C. kaulfusii KUNZE, C.
micropterisSW.,C. pilosaGOLDM.,C. pruinataKAULF.,C.viscidaDAVENP.)
Cheilanthes kaulfussii.Components: Galangin, gal-3-Me, gal-3,7-diMe, kaempferol, kae-3,7-diMe, kae-3,7,4@-triMe.
(Scheele et al., 1987; Wollenweber, 1997). (a diterpen is reported in RuKedi et al., 1989).
Cheilanthes micropteris. One of the samples seen (Monberg, 1394) exhibts at least two #avonoid aglycones, but these could not be identi"ed, due to the paucity of material.
Cheilanthes pruinata. Components: Galangin and gal-3-Me (Wollenweber, 1998 unpubl.)
Cheilanthes viscida. Components: Apigenin, ap-7-Me, ap-4@-Me, ap-7,4@-diMe.
(Wollenweber, 1997).
4.3. Several species of the genusPellaea LINK lacking glandular trichomes have
been found to exhibit external#avonoid aglycones:p. andromedaei-folia(KAULF.) FEHE,
p. brachyptera(T. MOORE) baker,p. bridgesiiHOOK.,P. mucronata(D.C.EATON) d.C.
EATON,p. quadripinnata(FORSSK.) Prantl andp. truncata GOODING ("p.
longimuc-ronataHOOK.).
Components: Galangin, gal-3-Me, gal-7-Me; Kaempferol, kae-7-Me, kae-4@-Me, kae-3,4@-diMe, kae-3,7,4@-triMe; Quercetin-7-Me, qu-7,3@-diMe.
(Wollenweber, 1979 and unpublished data).
Table 4
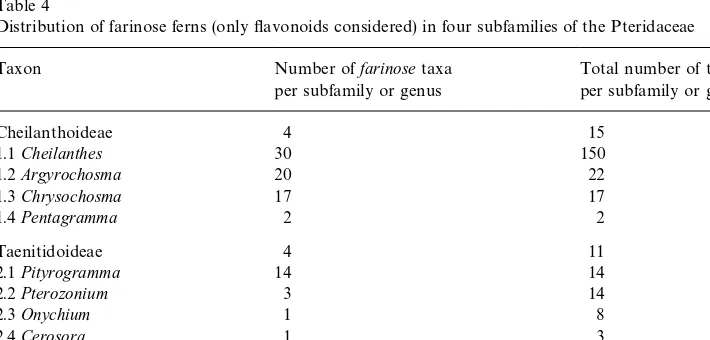
Distribution of farinose ferns (only#avonoids considered) in four subfamilies of the Pteridaceae
Taxon Number offarinosetaxa
per subfamily or genus
Total number of taxa per subfamily or genus
Cheilanthoideae 4 15
1.1Cheilanthes 30 150
1.2Argyrochosma 20 22
1.3Chrysochosma 17 17
1.4Pentagramma 2 2
Taenitidoideae 4 11
2.1Pityrogramma 14 14
2.2Pterozonium 3 14
2.3Onychium 1 8
2.4Cerosora 1 3
Adiantoideae 1 1
3.1Adiantum 1 150
4.4. Taenitidoideae
JamesoniaHOOK.ETGREV.
A tropical American genus of about 19 species which is closely related toEriosorus
FEHE. The leaves of both genera are usually densely pubescent and often the aglandular
hairs are mixed together with glandular ones. Tryon (1962) noted a vernicose or crustoce upper surface of the pinnae forJamesonia imbricatavar.glutinosa(KARST.)
A.F.TRYON and J. scammanae A.F.TRYON. Traces of #avonoids were recovered in
specimens of J. imbricata var. glutinosa, but it was not possible to identify the components. The lower surface of these species is densely covered with whitish hairs, and whitish crusts are only detectable on the upper side and parts of the rachis. It is not clear, however, if the observed traces of #avonoids are the products of the glandular hairs and/or part of the relatively thick cuticle.
3.4. Chemotaxonomy
Farinose waxes are found in about 80 pteridophyte species out of 14 genera of only one family, the Pteridaceae. The family is divied in six subfamilies with more than 800 species in about 35 genera. Farinose species are restricted to small fractions of its three subfamilies Adiantoideae, Cheilanthoideae, and Taenitidoideae (for a synopsis see Table 4). This scattered distribution suggests a polyphyletic origin of farinose waxes within the family and subfamilies. Although no phylogenetic analysis exists for Pteridaceae, proposed relationships (Tryon, 1990) indicate an independent evolution of genera with farinsoe waxes. This hypothesis is further supported by recent cladistic analysis of the subfamily Cheilanthoideae (Gastony and Rollo, 1995).
Farinose species often co-occur with non-farinose species in the same genus (e.g.,
Cerosora,Cheilanthessubgen.Aleuritopteris). Some genera such asPityrogrammaand
Pentagrammaare characterized by the (almost) general presence of farinose waxes, whereas in the neo-tropicalArgyrochosmaandChrysochosmaonly a few species lack such coatings. InAdiantum, only one out of more than 100 species exhibits a farinose exudate (Adiantum sulphureumvar.poiretii).
Within the genusCheilanthes, the provisionally segregated"ve groups may repres-ent monphyletic units. Non-farinose species are included e.g. in the Aleuritopteris
group, which otherwise mainly consist of farinose species. Previously, some authors separated the non-farinose taxa as genus Leptolepidium S.K. Wu, but there exists a close relationship to farinose species of the Aleuritopteris group (Fraser-Jenkins, 1992). Close a$nities of farinose and non-farinose species are also found inCerosora, in which two very closely related species di!er mainly in the presence (C. chrysosora) or absence (C. sumatrana HOLTTUM) of glandular paraphyses producing farinose
coatings. These patterns again suggest a polyphyletic origin of farina production, including the possibility of repeated loss and gain of this feature. Furthermore, the development of waxes may be suppressed or stimulated by genetic factors. This is indicated by the variability in the density of coatings in widespread ferns, such as
Pityrogramma calomelanos. Environmental factors, on the other hand, seem to play a secondary role, if any. They may only in#uence the amount of waxes produced (quantitative aspect), but not the chemical composition (qualitative aspect). No matter, whether e.g. a sample of a certainNotholaenaspecies is taken from a more than hundred years old herbarium specimen, or freshly collected in the Sonoran Desert, or clipped from a plant cultivated in a greenhouse in Europe, the#avonoid pattern is constant, at least in qualitative respect.
The presence of glandular hairs is a prerequisite for the development of farinose waxes. The suppression of trichome development results in the reduction or lack of farinose waxes. Examples are the species of the proposed genusLeptolepidum, which di!er from other species of Aleuritopteris only in the absence of trichomes on the lamina. In other cases, e.g. inPterozonium, hairs are present, but they lack a glandular apical cell, or the glandular cells do not exude waxes. Therefore the lack of farinose waxes can be the result of di!erent mutations (deletions). Some species ofCheilanthes, which do not produce a farinose coating, possess glandular hairs (e.g. Cheilanthes micropteris, C kaulfussii, C.viscida), thus indicating relationships to farinose species.
Analyses ofC. kaulfussiiandC.viscida(Scheele et al., 1987; Wollenweber, 1979) have
shown the presence of similar components in these glands. Such components are also found accumulated on the leaf surface of non-farinose species with thick cuticles in the genusPellaea(Wollenweber, 1979 and unpublished results), where they are probably excreted by unmodi"ed epidermal cells.
The colour of the waxes may be a useful character in the identi"cation of at least some species, e.g. Cheilanthes chrysophylla and C. welwitschii, but in other taxa infraspeci"c variation is also observed. The colour is determind by various factors such as the chemical composition, the microstructure and size of crystals of the quasi-crystalline material, and to a certain extent also to the density of exudates.
Table 5
Flavonoid aglycones in Pteridaceae, an overview of all genera with farina. Symbols:#"present,-"absent,?"unknown. Rare components are added as O-2,5"2- and/or 5-O-substituted#avonoids, C-6,8"6- and/or 8-C-methylated#avnoids, E"#avonoid esters, B"bi#avonoids. The components are found in one or more taxa of the genus/group, but mostly they are not present in all. Especially the rare components are often restricted to one or few species
Taxon Chalcones Dihydro-chalcones Flavanones Dihydro-#avonols Flavonols Flavones
Cheilanthoideae
1.1.1AleuritopterisGroup # # # ! # #
1.1.2ArgenteaGroup # ! # ! # #
1.1.3BrandegeiGroup # ! ! ! ! !
1.1.4NegripterisGroup ? ? ? ? ? ?
1.1.5SinopterisGroup ! ! ! ! # #
1.2Argyrochosma # # # ! # #
1.3Chrysochosma ! # #C-2,5 E E #C-2,5 E #C-2,5
1.4Pentagramma #C-6,8 #C-6,8 #C-6,8 ! #C-6,8 #C-6,8 B
Taenitidoideae
2.1Pityrogramma # # # ! # #
2.2Pterozonium # ! ! ! ! !
2.3Onychium # ! ! ! ! !
2.4Cerosora ? ? ? ? ? ?
Adiantoideae
3.1Adiantum # # # ! # !
772
E.
Wollenweber,
H.
Schneider
/
Biochemical
Systematics
and
Ecology
28
(2000)
751
}
Most of the farina components analyzed so far are derivates of the #avonoid biosynthetic pathway, with the occasional accumulation of terpenoids in addition. The composition of the farina may vary, as has already been exempli"ed in the description of the respective genera and units (survey see Table 5). It may be of interest that in many cases only chalcones constitute the #avonoid pattern. Similarly, other exudates consist only of widespread components, such as apigenin and galangin derivatives. Occasionally, rare components with a relative complex substitution pattern such as C-6,8-disubstituted #avones and #avonols,
#avonoid esters, etc occur. They seem to be restricted to the cheilanthoid genera Argyropchosma, Chrysochosma and Pentagramma in the New World, and to the SE Asian Cheilanthes argentea complex, a fact that may have taxonomic signi"cance. However, the chemical composition of the farinose waxes o!ers generally only little information with regard to intergeneric relationships (Table 5). This may be correlated with the scattered distribution of farina and exudate production within genera and the possibility of polyphyletic origin both of taxa and of farina production.
The chemical composition of the farina proved to be useful in populational studies, since some variability is observed, leading frequently to de"ning chemotypes (see Table 7.1 in Wollenweber, 1995). This does not exclude the usefulness of such patterns at the speci"c level. Some components, such as ceroptene, which is known exclusively fromPentagramma triangularisvar.triangularis., are de"nitively species-speci"c. The
#avonoid patterns often characterise closely related species, allowing for the recogni-tion of sister species in puzzling groups such as Aleuritopteris, Argyrochosma, and
Chrysochosma.
To conclude,#avonoid patterns may be signi"cant at the species and also at the populational level, whereas they proved to be of less value at the generic level. Terpenoids appear to be much less important as chemotaxanomic characters, due to their rare occurrence as well as to their di$cult analysis. It is assumed that the
#avonoid chemical composition may have a high value in taxonomic and phylogenetic studies of genera with frequent occurrence of farinose waxes.
Acknowledgements
E. W. wishes to thank all the keepers of herbaria who made fragments of specimens available, and Dr. Karin M. Valant-Vetschera (Vienna) for critical comments on the manuscript.
References
Appendino, G., Gariboldi, P., Wollenweber, E., Sironi, A., Molinari, H., 1992. Triterpenes from the frond exudate of the fernNotholaena greggii. Phytochemistry 31, 923}927.
Arriaga-Giner, F.J., Wollenweber, E., 1986. 6a-Acetoxy-16b, 22-dihydroxyhopan-24-oic acid, a triterpene from the fernNotholaena candidavar.copelandii. Phytochemistry 25, 719}721.
Arriaga-Giner, F.J., Iinuma, M., Tanaka, T., Mizuno, M., Scheele, C., Wollenweber, E., 1987. Novel #avonoids from the fernNotholaena sulphurea. Zeitschrift fuer Naturforschung 42c, 1063}1069. Arriaga, F.J., Rumbero, A., Wollenweber, E., 1996. Three further Dammarane Type Triterpenes from the
Frond Exudate of the FernNotholaena rigida. Zeitschrift fuer Naturforschung 51c, 750}752. Arriaga-Giner, F.J., Rumbero, A., Wollenweber, E., 1997. Two new epimeric diterpenes from the frond
exudate of the fern.Notholaena rigida. Zeitschrift fuer Naturforschung 52c, 292}294 ([Note error: The paper reports Triterpenes, not Diterpenes!].).
Asai, F., Iinuma, M., Tanaka, T., Takenaka, M., Mizuno, M., 1992. Five complex#avonoids in the farinose exudate ofPityrogramma calomelanos. Phytochemistry 31, 2487}2490.
Barthlott, W., Wollenweber, E., 1981. Zur Feinstruktur, Chemie und Taxonomischen Signi"kanz epi-cuticularer Wachse und aKhnlicher Sekrete. Trop. Subtrop. P#anzenwelt (Akademie Wissenschaften und der Literatur Mainz. 32, 35}97.
De Bary, A., 1877. Vergleichende Anatomie der Vegetationsorgane der Phanerogamen und Farne. W. Engelmann, Leipzig.
Dietz, V.H., Wollenweber, E., Favre-Bonvin, J., GoHmez, P., 1980. A novel class of complex#avonoids from the frond exudate ofPityrogramma trifoliata. Zeitschrift fuer Naturforschung 35, 36}40.
Dietz, V.H., Wollenweber, E., Favre-Bonvin, J., Smith, D.M., 1981. Two#avonoids from the frond exudate ofPityrogramma triangularisvar.triangularis. Phytochemistry 20, 1181}1182.
Donnelly, D.M.X., Fukuda, N., Wollenweber, E., Polonsky, J., PrangeH, T., 1987. A dihydrocinnamoyl neo#avonoid fromPityrogramma calomelanos. Phytochemistry 26, 1143}1145.
Favre-Bonvin, J., Jay, M., Wollenweber, E., Dietz, V.H., 1980. Deux Nouvelles Flavones d@un Exsudat de Fouge`re (Pityrogramma calomelanosvar.aureoyava). Phytochemistry 19, 2043}2045.
Fraser-Jenkins, C.R., 1992. The fern, allies of the Far West Himalaya: some additions and corrections. Botanica Helvetica 102, 143}157.
Gastony, G.J., Rollo, D.R., 1995. Phylogeny and generic circumscriptions of cheilanthoid ferns (Pteridaceae: Cheilanthoideae) inferred from rbcL nucleotide sequences. American Fern Journal 85, 341}360.
Hitz, C., Mann, K., Wollenweber, E., 1982. New#avonoids from the farina of Pityrogrammaspecies. Zeitschrift fuer Naturforschung 37, 337}339.
Iinuma, M., Tanaka, T., Mizuno, M., Mabry, T.J., Wollenweber, E., Favre-Bonvin, J., Voirin, B., Jay, M., 1986a. Revised structure of a#avonoid from the fernNotholaena aschenborniana. Phytochemistry 25, 1257}1258.
Iinuma, M., Hamada, K., Mizuno, M., Asai, F., Wollenweber, E., 1986b. Complex #avonoids from Pityrogrammafrond exudates: synthesis of two#avones with C-C-linked dihydrocinnamoyl substitu-ents. Zeitschrift fuer Naturforschung 41, 681}683.
Iinuma, M., Tanaka, T., Asai, F., Miyauchi, K.-I., Wollenweber, E., 1993. Spectral characters of a complex #avonoid isolated from the farinose exduate of Pityrogramma calomelanos. Phytochemistry 33, 1247}1248.
Iinuma, M., Tanaka, T., Suzuki, K., Lang, F.A., 1994. Two bi#avonoids in the farinose exudate of Pentagramm triangularis. Phytochemistry 35, 1043}1047.
Iinuma, M., Kakuto, Y., Tanida, N., Tanaka, T., Lang, F.A., 1997. Unusual bi#avonoids in the farinose exudate ofPentagramma triangularis. Phytochemistry 44, 705}710.
Jay, M., Favre-Bonvin, J., Wollenweber, E., 1979a. Dihydroxy-4@,5 tetrameHthoxy-2@,3,7,8#avone, et hy-droxy-5 pentameHthoxy-2@,3,4@,7,8#avone, deux nouveaux composeHs naturels isoleHs deNotholaena aznis (Pteridophyta). Canadian Journal of Chemistry 57, 1901}1902.
Jay, M., Wollenweber, E., Favre-Bonvin, J., 1979b. Novel 2@-substituted#avonols from the farinose exudate ofNotholaena aznis. Phytochemistry 18, 153}154.
Jay, M., Voirin, B., Viricel, M.-R., Wollenweber, E., 1981. A new natural#avone with tetra-substituted B-ring from the fern.Notholaena aschenborniana. Phytochemistry 20, 2307}2308.
Jay, M., Viricel, M.-R., Favre-Bonvin, J., Voirin, B., Wollenweber, E., 1982. New#avonol acetates from the frond exudate of the fernNotholaena aschenborniana. Zeitschrift fuer Naturforschung 37c, 721}723. Lellinger, D.B., 1967.Pterozonium(Filicales: Polypodiaceae). In: Maguire, B., et al. (Ed.), The Botany of the
Markham, K.R., 1988. Distribution of#avonoids in the lower plants and its evolutionary signi"cance. In: Harborne, J.B. (Ed.), The Flavonoids. Advances in Research since 1980. Chapman & Hall, London, pp. 427}468.
Markham, K.R., Wollenweber, E., Schilling, G., 1987. Structure revision for two C-methyl#avanones from Pityrogramma pallida. Journal of Plant and Physiology 131, 45}48.
Nilsson, M., 1961a. Dihydrochalcones from the Fronds of Pityrogramma chrysophyllavar. marginata. Domin. Acta Chemica Scandinavica 15, 154}158.
Nilsson, M., 1961b. Chalcones from the Fronds ofPityrogramma chrysophylla, var.heyderi. Acta Chemica Scandinavica 15, 211}212.
Pichi-Sermolli, R.E.G., 1946. Negripteridaceae e Negripteris, nuova famiglia e nuovo genere delleFilicales. Nuovo Gion. Bot. It. N.S. 53, 129}169.
Pichi Sermolli, R.E.G., 1989. Again on the typi"cation of the generic name ofNotholaenaR. Brown. Webbia 43, 301}310.
Ramakrishnan, G., Banerji, A., Chadha, M.S., 1974. Chalcones fromOnychium auratum. Phytochemistry 13, 2317}2318.
Roitman, J.N., Mann, K., Wollenweber, E., 1992. Two dibenzoylmethanes from the frond exudate of Notholaenaspecies. Phytochemistry 31, 985}987.
Roitman, J.N., Wong, R.Y., Wollenweber, E., 1993. Methylene bis#avonoids from frond exudate of Pentagramma tringularisssp.triangularis. Phytochemistry 34, 297}301.
RuKedi, P., Wollenweber, E., Marx, D., Scheele, C., 1989. Kaurane-type diterpenes from fern frond exudates. Zeitschrift fuer Naturforschung 44, 901}904.
Scheele, C., Wollenweber, E., Arriaga-Giner, F.J., 1987. New#avonoids from cheilanthoid ferns. Journal of Natural Production 50, 181}187.
Schneider, H., 1996. Vergleichende Wurzelanatomie der Farne. Dissertation, University of ZuKrich. Shaker, Aachen.
Seigler, D.S., Wollenweber, E., 1983. Chemical variation inNotholaena standleyi. American Journal of Botney 70, 790}798.
Serizawa, S., Wollenweber, E., 1977.Cheilanthes kuhnii var.brandtiiand the composition of its farina. American Fern Journal 67, 107}108.
Shing, K.H., 1990. Pteridophyta: Pteridaceae, Acrostichaeceae, Stenochlaenaceae, Sinopteridceae, Adian-tuaceae, Hemionitidaceae, Parkeriaceae (Ceratopteriadaceae). In: Shing, K.H. (Ed.), Flora Republicae Popularis Sinicae 3(1). Science Press, Beijing, pp. 1}306.
Smith, D.M., 1980. Flavonoid analysis of thePityrogramma triangulariscomplex. Bulletin of the Torrey Botanical Club 107, 134}145.
Tryon, A.F., 1962. A monograph of the fern genusJamesonia. Contrary Gray Herbelist 191, 109}197. Tryon, R.M., 1990. Pteridaceae. In: Kubitzki, K., Kramer, K.U., Green, P.S. (Eds.), Families and genera of
vascular plants, Pteridophytes and Gymnosperms, Vol. 1. Springer, Berlin, pp. 230}256.
Vilain, C., Hubert, A., Dupont, L., Markham, K.R., Wollenweber, E., 1987.a-Diceroptene: a new dimeric structure for Isoceroptene. Zeitschrift fuer Naturforschung 42c, 849}854.
Wagner, H., Seligmann, O., Chari, M.V., Wollenweber, E., Dietz, V.H., Donnelly, D.M.X., Meegan, M.J., O'Donnell, B., 1979. Strukturell neuartige 4-Phenyl-benzopyran-2-one ausPityrogramma calomelanos. Tetrahedron Letters 1979, 4269}4272.
WideHn, C.-J., WideHn, K., Vida, G., Reichstein, T., 1991. The phloroglucinols of theDryopteris villarii complex and some related ferns (Pteridophyta, Dryopteridaceae). Botanical Helvetica 101, 77}120.
Windham, M.D., 1987.Argyrochosma, a New Genus of Cheilanthoid Ferns. American Fern Journal 77, 37}41.
Wollenweber, E., 1972. Flavonols from the fronds ofPityrogramma chrysoconia. Phytochemistry 11, 427. Wollenweber, E., 1976a. Flavonoide Exkrete bei Goldfarnen und Silberfarnen. Zeitschrift fuer P#
anzen-physiologie 78, 344}349.
Wollenweber, E., 1976b. Flavonoid exudations in farinose ferns Phytochemistry 15, 2013.
Wollenweber, E., 1976c. Die Komponenten des Mehls bei Cheilanthes und Notholaena } ein chemotaxonomisches Merkmal?. Berichte der Deutscher Botanischen Gesellschaft 89, 243}246.
Wollenweber, E., 1977a. Die Zusammensetzung des Flavonoid-Mehls bei einigen Farnen. Zeitschrift fuer P#anzenphysiologie 85, 71}76.
Wollenweber, E., 1977b. Chalkone und Dihydrochalkone als Mehlbestandteile bei Farnen (Gattungen CheilanthesundNotholaena). Zeitschrift fuer Naturforschung 32, 1013}1014.
Wollenweber, E., 1978. The distribution and chemical constituents of the farinose exudates in gymnogram-moid ferns. American Fern Journal 68, 13}28.
Wollenweber, E., 1979. Einige Neufunde externer Flavonoide bei amerikanischen Farnen. Flora 168, 138}145.
Wollenweber, E., 1981. Unusual#avanones from a rare American fern. Zeitschrift fuer Naturforschung 36c, 604}606.
Wollenweber, E., 1982a. Flavonoid aglycones as constituents of epicuticular layers in ferns. In: Cutler, D.F., Alvin, K.L., Price, C.E. (Eds.), The Plant Cuticle, Linn. Soc. Symp. Series No. 10. Academic Press, London, pp. 215}224.
Wollenweber, E., 1982b. The occurrence of #avanones in the farinose exudate of the fern Onychium siliculosum. Phytochemistry 21, 1462}1464.
Wollenweber, E., 1984. Exudate #avonoids of Mexican ferns as chemotaxonomic markers. Rev. Latinoamer. Quim. 15, 3}11.
Wollenweber, E., 1989. Exudate#avonoids in ferns and their chemosystematic implication. Biochemistry and Systems Ecology 17, 141}144.
Wollenweber, E., 1994. Flavones and#avonols. In: Harborne, J.B. (Ed.), The Flavonoids}Advances in Research since 1986. Chapman and Hall, London, pp. 259}335.
Wollenweber, E., 1995. Chemie und Chemotaxonomie. In: Kramer, K.U., Schneller, J.J., Wollenweber, E., (Eds.), Farne und Farnverwandte., Thieme, Stuttgart, pp. 159-179.
Wollenweber, E., Arriaga}Giner, F.J., 1991. Grammitic acid, a long} chain substituted benzoic acid derivative of the fernGrammitis argyrata. Phytochemistry 30, 2307}2308.
Wollenweber, E., Dietz, V.H., 1980. Flavonoid patterns in the farina of goldenback ferns and silverback ferns. Biochemestry Systems Ecology 8, 21}33.
Wollenweber, E., Favre-Bonvin, J., 1979. Novel dihydrostilbene from fronds ofNotholaena dealbataand Notholaena limitanea. Phytochemistry 18, 1243}1244.
Wollenweber, E., GoHmez, P., 1979. Flavonoid patterns in the farina ofNotholaena aznis, a gymnogram-moid fern. Brenesia 16, 123}129.
Wollenweber, E., Roitman, J.R., 1991. New frond exudate#avonoids from cheilanthoid ferns. Zeitschrift fuer Naturforschung 46c, 325}330.
Wollenweber, E., Smith, D.M., 1981. The chemoidentity of the holotype of Pityrogramma triangularis. American Fern Journal 71, 120.
Wollenweber, E., Yatskievych, G., 1982. Flavonoid esters from the fern.Notholaena neglecta. J. Nat. Prod., Lloydia. 45, 216}219.
Wollenweber, E., Favre-Bonvin, J., Lebreton, P., 1978a. Ein Butyryl-Flavonol aus dem Mehl von Notholaena aznis. Phytochemistry 17, 1684}1685.
Wollenweber, E., Favre-Bonvin, J., Jay, M., 1978b. A novel type of#avonoids:#avonol esters from fern exudates. Zeitschrift fuer Naturforschung 33c, 831}835.
Wollenweber, E., Dietz, V.H., MacNeill, C.D., Schilling, G., 1979a. C-Methyl-#avanones as farina on the fronds ofPityrogramma pallida. Z. P#anzenphys. 94, 241}246.
Wollenweber, E., Dietz, V.H., Smith, D.M., Seigler, D.S., 1979b. A novel C-methylated dihydro-chalcone from Pityrogramma triangularis var. viscosa. Zeitschrift fuer Naturforschung 34c, 876}877.
Wollenweber, E., Dietz, V.H., Schillo, D., Schilling, G., 1980. Zeitschrift fuer Naturforschung 35c, 685. Wollenweber, E., Rehse, C., Dietz, V.H., 1981a. The occurrence of Aurentiacin and Flavokawin B on
Pityrogramma triangularisvar.pallidaandDidymocarpusspecies. Phytochemistry 20, 1167}1168. Wollenweber, E., Walter, J., Schilling, G., 1981b. New#avanones and chalcones from the farinose frond
exudate ofPityrogramma pallida. Zeitschrift Fuer P#anzenphysiologie 104, 161}168.
Wollenweber, E., RuKedi, P., Seigler, D.S., 1982a. Diterpenes ofCheilanthes argentea, a fern from Asia. Zeitschrift fuer Naturforschung 37c, 1283}1285.
Wollenweber, E., Smith, D.M., Reeves, T., 1982b. Flavonoid patterns and chemical races in the California Cloak-fern,Notholaena californica. In: Farkas, L., GaHbor, M., KaHllay, F., Wagner, H. (Eds.), Flavonoids and Bio#avonoids, 1981, Studies in Organic Chemistry, Vol. 11. Elsevier, Amsterdam, pp. 221}226. Wollenweber, E., Dietz, V.H., Schilling, G., Favre-Bonvin, J., Smith, D.M., 1985. Flavonoids from
chemo-types of the goldback fern.Pityrogramma triangularis. Phytochemistry 24, 965}971.
Wollenweber, E., Scheele, C., Tryon, A.F., 1987. Flavonoids and spores ofPlatyzoma microphyllum, an endemic fern of Australia. American Fern Journal 77, 28}32.
Wollenweber, E., Marx, D., Favre-Bonvin, J., Voirin, B., Kaouadji, M., 1988. 3-Methoxy#avones with uncommon B-ring substitution from two species ofNotholaena. Phytochemistry 27, 2673}2676. Wollenweber, E., DoKrr, M., Waton, H., Favre-Bonvin, J., 1993. Flavonoid aglycones and a dihydrostilbene
from the frond exudate ofNotholaena nivea. Phytochemistry 33, 611}612.
Wollenweber, E., Stevens, J.F., Ivancic, M., Deinzer, M.L., 1998. Acylphloroglucinols and #avonoid aglycones produced by external glands on the leaves of twoDryopterisferns andCurrania robertiana. Phytochemistry 48, 931}939.
Yatskievych, G., Windham, D.M., Wollenweber, E., 1990. A reconsideration of the genusPityrogramma Link (Adiantaceae) in the South-Western United States. American Fern Journal 80, 9}17.