www.elsevier.nlrlocateraqua-online
Salinity–temperature and nutritional effects on the
setting rate of larvae of the tropical oyster,
ž
/
Crassostrea iredalei Faustino
M.N. Devakie
a,), A.B. Ali
ba
Fisheries Research Institute, Batu Maung, 11960 Penang, Malaysia
b
School of Biological Sciences, UniÕersiti Sains Malaysia, 11800 Penang, Malaysia Accepted 13 September 1999
Abstract
Ž .
The combined effects of salinity–temperature and feeding algal species and density on the
Ž .
setting rate of Crassostrea iredalei larvae were studied. Six salinities 5, 10, 15, 20, 25 and 30‰
Ž .
were tested at five temperatures 24, 27, 30, 33 and 368C . A separate experiment using various
Ž 3 y1.
algal densities 0, 25, 50, 75, 100 and 125=10 cells ml of Isochrysis galbana, Chaetoceros
calcitrans and mixtures of both was conducted. The optimum salinity–temperature conditions
Ž .
were 20‰ and 308C, which supported the highest mean percentage "S.D. larval settlement rate
Ž .
of 31.4"3.4%. The use of different species of microalgae had a significant effect P-0.05 on
Ž .
the rate of larval settlement. The highest settlement rate was recorded for mixed algae 11.3% ,
Ž . Ž .
but this was not significantly different from I. galbana 10.0% P)0.05 , irrespective of density. Ch. calcitrans produced 9.5% larval settlement rate, which also was not significantly
Ž .
different from I. galbana P)0.05 . The effect of algae densities, irrespective of species, was that the highest set of 24.5% occurred at 100=103cells mly1.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Salinity; Temperature; Nutrition; Oyster; Larvae; Setting; Crassostrea iredalei
1. Introduction
Intrinsic factors such as heredity and larval age, and extrinsic factors such as nutritional history, water parameter and the physical and chemical characteristics of the
Ž substrate have been reported to influence the settlement of most bivalve larvae
Had-)Corresponding author. Tel.:q60-4-6263-925; fax:q60-4-626-2210; e-mail: [email protected] 0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
.
field, 1984 . Among these, salinity and temperature represent two important ecological factors that influence the biology of the sensitive planktonic stages of estuarine organisms, such as the oyster larvae. Both these factors are easily measured, manipu-lated and controlled compared to most other factors.
Oysters of the genus Crassostrea in general are considered to be euryhaline
organ-Ž .
isms Quayle and Newkirk, 1989 . They have the ability to adapt well to temperature
Ž .
fluctuations Angell, 1986 . Although temperature changes within the tropical or sub-tropical zone may appear to have much less influence on the biology of bivalves, controlled laboratory experiments are still required to determine the effects of tempera-ture, including effects with other parameters such as salinity. According to Dekshenieks
Ž .
et al. 1993 , oyster larvae generally exhibit a wide tolerance of temperature fluctuations, but these may have serious effects on their physiology.
Researchers have studied the effects of temperature and salinity on the development Ž
and growth patterns of bivalve species in the wild and in the laboratory e.g., Paul, 1980; .
Tettelbach and Rhodes, 1981; Kalyanasundram and Ramamoorthi, 1986 . Studies in temperate countries have been conducted in the laboratory to examine the combined
Ž
effects of salinity and temperature on the larval settlement of C. gigas Lund, 1971;
. Ž
Henderson, 1983 ; the effects of salinity on settlement of C.Õirginica larvae Hidu and .
Haskin, 1971 ; and the effects of temperature on settlement of C. gigas larvae
Ž .
settlement Cooper and Shaw, 1984; Scholz et al., 1985 . In Malaysia, there is one
Ž .
recent study on the effects of salinity on C. belcheri settlement by Tan 1993 . The present study aimed at examining the combined effects of salinity and temperature on the settlement of larvae of the tropical oyster, C. iredalei, under hatchery conditions for the purpose of optimising spat production.
Currently, the use of live feed in the commercial production of bivalve larvae, such as oyster, scallop and clams, is reduced by the transfer of spat as soon as possible from the hatchery to the nursery. This is because of the operational costs involved in mass production of microalgae for the hatchery. To further decrease costs of microalgal production in oyster hatcheries, the larval stages are fed with microalgae until they reach the eyed stage. Immediately after this, they may be transported to be set elsewhere, which is referred to as ‘remote setting’. From then onwards, the eyed larvae will be either partially or, in some cases, exclusively fed on natural phytoplankton. Whether metamorphosing larvae need to be given a nutritional supplement at this stage, and in what quantity, are current issues, especially if the setting period is prolonged. In the
Ž . Ž
temperate Pacific oyster C. gigas , larval setting is allowed for 48 h Henderson, 1983; .
Supan, 1987; Jones and Jones, 1988 ; but for some tropical oysters, such as C. belcheri ŽTan, 1993 and C. iredalei Devakie et al., 1993 , observations indicate that larval. Ž . settlement is staggered over 96 h.
Several researchers have expressed differences in opinion concerning the need to feed
Ž .
metamorphosing bivalve larvae. Lipovsky 1991 found that it was not necessary to feed metamorphosing C. gigas larvae, probably because their velum tends to degenerate and they are unable to filter food particles during this period. However, Paynther et al. Ž1993 stressed that C.. Õirginica larvae are unable to metamorphose without food. A
Ž .
the settlement period allowed for Pacific oysters is about 2 days, it is possible that the Ž larvae can depend on their lipid reserves during settlement without feeding Elston,
.
1991 . In the case of some tropical oyster larvae, however, since the observed larval setting period is about 4 days, it is likely that the synchrony and rate of settlement during this duration will differ. There is need to determine appropriate species of microalgae and densities suitable for feeding. Thus, this study aimed to determine if the setting rate during this period can be maximised by feeding.
2. Materials and methods
2.1. Combined effects of salinity and temperature on oyster larÕae setting
Ž .
Oyster broodstock C. iredalei were procured from a nearby oyster farm at Batu Lintang, Kedah in Peninsular Malaysia. The spawning and larval production were
Ž .
carried out in the hatchery of the Fisheries Research Institute FRI , Penang. Eyed larvae )250mm in length, were used for the experiment.
Ž .
The experimental procedure was based on the methods used by Henderson 1983
Ž . Ž .
and Tan 1993 . Six levels of salinities 5, 10, 15, 20, 25 and 30‰ were used at five
Ž .
temperatures 24, 27, 30, 33, and 368C . The range of temperatures chosen was based on the water temperature monitored from the oyster setting tanks in the hatchery at the FRI. Between 1995 and 1996, the minimum and maximum water temperatures recorded were 25.58C and 358C, respectively. The salinities were obtained by diluting 1 mm cartridge filtered and ultraviolet treated seawater from the hatchery with distilled water. The experiment was conducted with four replicates of each treatment, with 1-l beakers
Ž .
containing the respective salinity levels placed in temperature controlled "0.58C water baths. One water bath was used for each temperature. The beakers were lined with
Ž .
colourless sheet of high density polyethylene HDPE of 1 mm thickness to avoid larvae setting on the beaker sides. A white tile measuring 5 cm2 was placed at the bottom of each beaker to serve as a substrate for the larvae to set upon. Every beaker was stocked
y1 Ž
with 1000 oyster larvae, i.e., an initial density of 1 larvae ml . Aeration slight .
bubbling was provided by means of an adjustable portable aerator. A complete water change was made on the second day, and the respective salinity and temperature levels were maintained.
Ž .
Mixed algae, Chaetoceros calcitrans and Isochrysis galbana 50:50 ratio , were fed
3 y1 Ž
at a rate of 70=10 cells ml feeding density maintained during the larval rearing .
period .
Ž .
Larval settlement rate % was assessed based on the number of larvae set on the tile and the plastic sheet. The tile and plastic sheet were rinsed very gently in a bucket of filtered seawater to remove any unset or dead larvae. Unset larvae were considered not competent after the 96-h period.
2.2. Effect of feed type and density on oyster larÕae setting
Ž .
I. galbana and Ch. calcitrans. Setting rates of oyster larvae were determined using
Ž .
individual species and mixed algae 50:50 ratio as feed. Six densities of microalgae
3 y1 Ž .
were used: 25, 50, 75, 100 and 125=10 cells ml and a control without feed for individual and mixed species. The algal stock solution was first diluted with distilled water to lower the salinity to 20‰ before determining the algal density with a Coulter Counter Model ZI. The various densities were then calculated based on the initial algal count in the stock.
Ž .
The experiment was carried out in 1-l beakers six replicates for each density lined
Ž .
with high density polyethylene sheet of 1 mm thickness at room temperature 30"18C .
Ž .
A white tile substrate was placed at the bottom of the beaker as in the earlier experiment. Initial larvae density in each beaker was 1 larvae mly1. The experiment was carried out for 4 days to allow larval settlement and a total water change was made after 48 h. A volume of water was removed daily from each beaker. This was equal to the volume of algal solution to be added. The volume of algae to be added daily to each beaker was based on the algal counts determined each day. Care was taken not to siphon out larvae while reducing the water volume of the beaker by fitting the siphon tube with
Ž .
a small nylon filter. Larval settlement rate % was determined as in the previous experiment.
2.3. Statistical analyses
A two-factor ANOVA was used to determine if interactions occurred between salinity and temperature effects, and between algal species and density effects. Larval sets from different treatments were assessed by One-way ANOVA, while comparisons of their
Ž .
means were conducted using the Tukey’s honesty significant differences HSD method.
Ž .
The Statistix 4.0 Program Analytical Software, 1992 was used for analysis. All
Ž .
Table 1
Ž .
Mean percentage settlement "S.D. of larvae of C. iredalei for combinations of five temperatures and six salinities
Ž .
Within columns, means with a common subscript do not differ significantly Tukey’s HSD, P)0.05 .
Ž .
Within rows, means with a common superscript do not differ significantly Tukey’s HSD, P)0.05 .
Ž .
percentage values were transformed to arcsine values prior to analysis to normalise the
Ž .
data, based on Snedecor and Cochran 1989 .
3. Results
3.1. Combined effects of salinity and temperature on larÕal setting
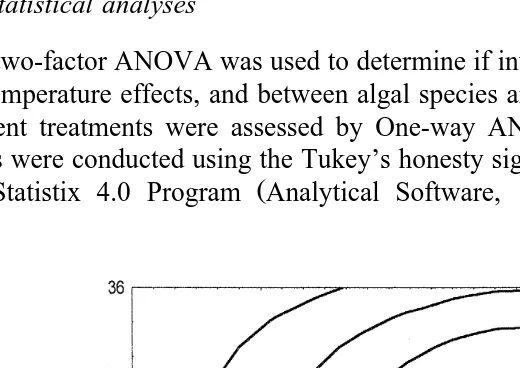
C. iredalei larvae were found to tolerate wide ranges of salinity and temperature within the limits selected for the experiment. Larval settlement was observed at all levels of salinity and temperature. Some larvae were still swimming or crawling, even after 96 h, while others were dead. At salinities below 15‰, however, the immediate response of most of the larvae was to settle at the bottom of the beaker before resuming swimming or crawling. Fig. 1 shows the rate of larval settlement in relation to temperature and salinity in the form of response–surface curves. Response–surface estimation was plotted by graphically transforming the standard deviation about the percentage mean settlement for each interaction of the variables shown in Table 1. At each point of variable interaction, a corresponding percentage response curve was connected to the positive standard deviation of the means at 5% contour intervals. The response–surface
Table 2
()
M.N.
De
Õ
akie,
A.B.
Ali
r
Aquaculture
184
2000
105
–
114
Table 3
Ž . Ž .
Mean percentage % settlement of larvae of C. iredalei obtained with different diets of microalgae and densities at room temperature 30"18C . Data for each
Ž . Ž .
treatment are means "S.D. . The means and standard error "S.E. of sample means are shown for all factor levels
Ž .
Note: Within columns, means with a common subscript do not differ significantly Tukey’s HSD, P)0.05 .
Ž .
Within rows, means with a common superscript do not differ significantly Tukey’s HSD, P)0.05 .
3 y1
Ž .
Microalgae Density 10 cells ml
0 25 50 75 100 125 Combined data of all densities
Ž .
for each diet "S.E.
dŽ . cŽ . cŽ . bŽ . aŽ . bŽ . Ž .
I. galbana 0.9 "0.3 b4.1 "1.5 b8.0 "2.3 b15.2 "2.4 a25.8 "5.1 a17.0 "1.8 10.0 "3.8
dŽ . c ,dŽ . bŽ . bŽ . aŽ . c ,dŽ . Ž .
Ch. calcitrans 0.9 "0.3 a ,b6.4 "1.7 a ,b11.1 "2.3 a ,b16.6 "3.9 a25.0 "4.1 b6.6 "0.8 9.5 "3.5
c b b a a b
Ž . Ž . Ž . Ž . Ž . Ž . Ž . Ž .
Mixed algae 50:50 0.9 "0.3 a9.1 "1.6 a13.1 "2.6 a21.0 "1.8 a23.1 "1.4 b9.5 "2.8 11.3 "3.4
Ž . Ž . Ž . Ž . Ž . Ž .
Combined data of all species 0.9 "0.0 6.3 "1.5 10.5 "1.5 17.4 "1.7 24.5 "0.8 10.5 "3.1
Ž .
Table 4
Analysis of variance of the effect of different diets and densities of microalgae on the settlement of larvae of
C. iredalei, using arcsine transformed percentage data
Source df ss F P
estimation used to investigate temperature–salinity interactions here should be consid-ered to provide estimations only and not precise set rates. From the contour, settlement rates of 15% and above are expected between salinities of 13–28‰ and temperatures above 248C to below 358C. Less than 5% settlement is expected for salinities below 8‰ and above 30‰, and at temperatures somewhat less than 248C and above 368C.
Ž .
The two-factor ANOVA Table 2 showed that variations in the larval settlement rate
Ž .
for salinity, temperature and their interaction were significantly different P-0.0001 . From the results, the best point of interaction for salinity and temperature was at 20‰
Ž .
and 308C, respectively, contributing the highest set rate "S.D. of 31.4"3.4%. There were average set rates of 14% to 24% for salinity and temperature between 15–25‰ and 27–338C, respectively.
3.2. Effect of feed type and density on larÕal setting
The highest set rates of 23.1–25.8% were obtained for 100=103 cells mly1,
Ž . 3 y1
irrespective of algal species Table 3 . Set rates at cell density of 125=10 cells ml ranged from 6.6% to 17.0%, while at lower cell densities of 25 to 50=103 cells mly1, set rate was less than 13%. The set rate obtained for the unfed control was negligible Ž0.9% . The best set rate, irrespective of algal density was obtained for mixed algae. Ž11.3% , although it was not significantly different P. Ž )0.05 from I. galbana 10.0% .. Ž .
Ž .
The two-factor ANOVA Table 4 showed that the variations in larval settlement rate Ž between algal species, density and their interactions were significantly different P
-. Ž .
0.05 . The use of Ch. calcitrans resulted in lower set rate 9.5% , but it was not
Ž .
significantly different P)0.05 from the settlement rate of larvae fed with I. galbana.
4. Discussion
Ž .
purpose of comparison except for an observation by Tan 1993 where setting was
Ž .
termed high rate not mentioned for salinities ranging from 18‰ to 24‰ at ‘‘room’’ temperature. The results of the present study more or less conformed to this observation.
Ž .
Another associated study by Tan 1993 on a related tropical species, C. belcheri, also
Ž .
showed that optimum sets of 35.8% and 38.6% not significantly different were Ž
obtained at salinities of 12‰ and 18‰, respectively, at ‘‘room’’ temperature level not .
given . Preliminary studies on these parameters in Thailand showed difference in optimal temperature condition for setting C. belcheri larvae: between 268C and 288C
Ž .
and salinity between 32‰ and 34‰ Sahavacharin, 1987 . However, subsequent studies have indicated that the same species could be induced to set by reducing salinity level
Ž .
from 32‰ to 15‰ Sahavacharin et al., 1988 .
Ž .
Lund 1971 showed that maximum settlement of larvae of the Pacific oyster, C.
gigas, occurred at a temperature of 308C within a salinity range of 22–34‰. A study by
Ž .
Henderson 1983 on C. gigas indicated that maximum set of 35–40% could be attained at a temperature of 308C and salinity of 30‰. Although high salinity is required for the larval settlement of Pacific oysters, the above-mentioned tropical oysters tend to withstand euryhaline conditions. However, both the Pacific and tropical oyster larvae
Ž .
require relatively higher temperature 308C for optimum setting.
The success of routine production of oyster larvae in the hatchery is basically dependent on the ability to manipulate and control the culture environment. One of the areas modified in relation to the biological conditions of the rearing site is conditioning of broodstock, which is expected to result in a viable larval production for culture in the
Ž .
hatchery Lipovsky, 1984 . Research has shown that the environmental factors including food, affecting the parent oysters are most important to the subsequent larval populations
Ž .
produced and their success of setting Creekman, 1977 . This probably explains the
Ž .
higher setting rate 14.3–31.4% observed at the salinity range of 15–25‰ and temperature range of 27–338C, because broodstocks were obtained from an environment Žat Batu Lintang, Kedah with similar salinity and temperature regimes. Upon collection,. the broodstock was spawned at salinity of 28–30‰ and at ambient temperature Ž30"28C , while larval rearing was at 20‰ at the same temperature condition..
The experiment on feed has shown that oyster larvae, when metamorphosing over a
Ž .
number of days, certainly need food irrespective of algal species , as observed from the results where settlement in unfed beakers was negligible compared to those being fed. However, algal density played a significant role in influencing settlement rate, as has
Ž .
been observed by Lund 1971 . Optimum settlement was observed at a density of 100=103 cells mly1 irrespective of feed species. Research on a temperate species
Ž .
indicated that this algal density supported good set rates. Lund 1971 , who worked with
Ž y1. 3 y1
C. gigas 1500 larvae ml found that using I. galbana at 100=10 cells ml
Ž .
resulted in a higher set rate of 7.8%. Subsequent study on C. gigas by Henderson 1983 Ž apparently showed that there were no significant influence of the algal densities 25, 50,
3 y1 .
75, and 100=10 cells ml and a control without feed used on the set rate. However, setting was seen to deteriorate at 100=103 cells mly1. Unfed larvae could have set
Ž .
From this study, it was observed that larval set increased as food density was raised to 100=103 cells mly1 but decreased as cell densities were increased further. High algal densities have been shown to result in the formation of biofilm layers that
Ž .
stimulate larval settlement Tritar et al., 1992; Parsons et al., 1993 . However, the effect can be detrimental at very dense algal densities where fouling debris is abundant ŽHenderson, 1983 . Schulte 1975 termed this situation as the critical cell density,. Ž . whereby the larval filtration rate is hindered if food concentrations exceed this level. This probably explains why set rates started to deteriorate at cell concentrations of 125=103cells mly1.
References
Analytical Software, 1992. An Interactive Statistical Analysis Program for Micro-computers. St. Paul, MN, 280 pp.
Angell, C.L., 1986. The Biology and Culture of Tropical Oysters. ICLARM Technical Report, No. 13. Manila, Philippines, 42 pp.
Baker, S.M., Mann, R., 1994. Feeding ability during settlement and metamorphosis in oyster Crassostrea Õirginica and effects of hypoxia on metamorphosis. Mar. Ecol.: Prog. Ser. 104, 91–99.
Cooper, K.L., Shaw, W.N., 1984. The effects of environmental factors on the ability of chemical cues to
Ž .
trigger settlement and metamorphosis to the Pacific oyster, Crassostrea gigas Thunberg . J. Shellfish Res.
Ž .
4 1984 , 85–86, Abstract.
Ž .
Creekman, L.L., 1977. The effects of conditioning the American oyster Crassostrea Õirginica with
Tetraselmis suecica and cornstarch on the growth, vigor and survival of its larvae. Master’s Thesis, Dept.
Mar. Science, Univ. Virginia, 58 pp.
Dekshenieks, M.M., Hofmann, E.E., Powell, E.N., 1993. Environmental effects on the growth and
develop-Ž .
ment of Eastern Oyster, CrassostreaÕirginica Gmelin, 1791 , larvae: a modeling study. J. Shellfish Res. 12, 241–254.
Devakie, N., Robert, H., Charles, L.A., 1993. Small-scale Oyster Culture on the West Coast of Peninsular Malaysia. BOBPrREPr63. GCPrRASr118rMUL. BOBP, Madras, India.
Elston, R., 1991. Shellfish Health Management and Maintenance. Record of Workshop on Remote Setting and Nursery Culture For Shellfish Growers in Olympia, WSG. Washington Sea Grant Program, Univ. Washington, Seattle, WA, 19th Feb. 1991, pp. 41–42.
Gallager, S.M., Mann, R., 1981. Use of lipid staining techniques for assaying condition in cultured bivalve larvae. J. Shellfish Res. 1, 69–73.
Hadfield, M.G., 1984. Settlement requirements of molluscan larvae: new data on chemical and genetic roles. Aquaculture 39, 283–298.
Henderson, B.A., 1983. Handling and Remote Setting Techniques for the Pacific oyster larvae, Crassostrea
gigas. Master’s thesis, Dept. Fisheries and Wildlife, Oregon State Univ. OR, 37 pp.
Hidu, H., Haskin, H., 1971. Setting of the American oyster related to environmental factors and larval behaviour. Proc. Natl. Shellfish. Assoc. 61, 35–49.
Jones, G.G., Jones, B.L., 1988. Advances in Remote Setting of Oyster Larvae. Aquaculture Assoc. of BC, Nanaimo, BC, 88 pp.
Kalyanasundram, M., Ramamoorthi, K., 1986. Temperature and salinity requirements for embryonic
develop-Ž .
ment of Saccostrea cucullata Born . Bull. Natl. Inst. Ocean. 19, 53–55.
Lipovsky, V.P., 1984. Oyster egg development as related to larval production in a commercial hatchery. Aquaculture 39, 229–235.
Ž .
Lund, D.S., 1971. Laboratory studies on setting of the Pacific Oyster Crassostrea gigas . Master’s thesis, Dept. Fisheries and Wildlife, Oregon State Univ., OR, 85 pp.
Parsons, G.J., Dadswell, M.J., Roff, J.C., 1993. Influence of biofilm on settlement of sea scallop, Placopecten
Ž .
magellanicus Gmelin, 1791 , in Passamaquoddy Bay, News Brunswick, Canada. J. Shellfish Res. 12,
279–283.
Paul, J.D., 1980. Salinity–temperature relationship in queen scallop Chlamys opercularis. Mar. Biol. 56, 295–300.
Paynther, K.T., Caudill, C., Meritt, D., Gallager, S., Walsh, D., 1993. Protein, carbohydrate and lipid levels associated with metamorphic success in larvae of eastern oyster, CrassostreaÕirginica. J. Shellfish Res. 12, 134.
Quayle, D.B., Newkirk, G.F., 1989. Farming Bivalve Molluscs: Methods for Study and Development. Advances in World Aquaculture, Vol. 1. The World Aquaculture Society In Assoc. with The International Development Research Centre. The World Aquaculture Soc., Baton Rouge, LA, 294 pp.
Sahavacharin, S., 1987. Methodology of induced spawning of oyster. Department of Fisheries, Bangkok, Thailand. Technical Paper, Contribution 2, 11 pp.
Sahavacharin, S., Chindanond, A., Amornjaruchit, S., Nugrang, J., Silapajarn, K., Chawivanskorn, V., Limsurt, S., Angell, C.L., McCoy, E.W., Mutarasint, K., Potaros, M., 1988. Hatchery techniques for
Ž .
tropical bivalve molluscs. In: McCoy, E.W., Chongpeepian, T. Eds. , Bivalve Culture Research in Thailand, Bivalve Culture Research in Thailand. ICLARM Tech. Report 19, pp. 19–30.
Scholz, A.J., Cooke, W.A., Cooper, K.L., Donaldson, J., 1985. Beach setting of eyed oyster larvae
Ž . Ž .
Crasssostrea gigas Thunberg in Puget Sound, Washington. J. Shellfish Res., p. 5 Abstract .
Schulte, E.H., 1975. Influence of algal concentration and temperature on the filtration rate of Mytilus edulis. Mar. Biol. 30, 331–341.
Snedecor, G.W., Cochran, W.G., 1989. Statistical Methods. 8th edn. Iowa State Univ. Press, Ames, IA, USA, pp. 289–290.
Supan, J., 1987. Using Remote Setting to produce Seed Oyster in Louisiana and the Gulf Coastal Region. Louisiana Sea Grant College Program. Louisiana State University, Baton Rouge, LA, 47 pp.
Tan, S.H., 1993. Aspects on Biology and Larval Development of Tropical Oyster, Crassostrea belcheri
ŽSowerby . Master’s thesis, School of Biological Sciences, Univ. Sc. Malaysia, 221 pp..
Tettelbach, S.T., Rhodes, E.W., 1981. Combined effects of temperature and salinity on embryos and larvae of the northern bay scallop, Argopecten irradians irradians. Mar. Biol., pp. 249–256.
Tritar, S., Prieur, D., Weiner, R., 1992. Effects of bacterial films on the settlement of the oysters, Crassostrea
Ž . Ž . Ž
gigas Thunberg, 1973 and Ostrea edulis Linnaeus, 1750 and the scallop Pecten maximus Linnaeus,
.