A LABORATORY DEMONSTRATION FOR LEARNING
ABOUT MAST CELL DEGRANULATION
Jose´ Corado and Antonio Eblen-Zajjur
Fisiologı´a y Biofı´sica , Fa culta d de Ciencia s de la Sa lud, Universida d de Ca ra bobo, El Triga l 2002, Va lencia , Venezuela
A
simple experimental model of cell degranulation was implemented that exposed mast cells obtained from Sprague-Dawley rats to saponin. The model is flexible, easy, and low cost, is not very time-consuming to run, and needs a minimum of laboratory resources. It has been used for the last three years in our undergraduate medical physiology courses and has replaced the classic utilization of slides and drawings. AM. J. PHYSIOL. 276 (ADV. PHYSIOL. EDUC. 21): S19–S22, 1999.Key words:granule secretion; physiology education
Mast cells are considered critical effector cells in IgE-dependent host responses to parasites and allergic diseases and probably play a central role in normal homeostasis (5, 7). These cells are scattered in connec-tive tissues, around venules, in bronchial lumen, in bronchial and gastrointestinal mucosa, and in skin exposed to the external environment (7). Immuno-logic stimuli such as IgE cross-linking induced by antigens or by antibodies to IgE-bound membrane FceRI (2), as well as the a-anaphylotoxins C3a and
C5a, or nonimmunologic stimuli such as substance P, bacterial peptides, mannitol, morphine, and saponin (3) activate mast cells, which are capable of generat-ing and releasgenerat-ing an important number of potent mediators. Some are preformed and stored in the cytoplasmic granules (histamine, serine proteases, proteoglycans, carboxypeptidase A); others are synthe-sized after cell activation, including lipid mediators, platelet-activating factor, and cytokines such as inter-leukin (IL)-1, IL-3, IL-4, IL-5, IL-6, tumor necrosis factor-a (1, 3), and granulocyte/monocyte
colony-stimulating factor (8, 9). These mediators induce diverse effects in tissue inflammation, tissue remodel-ing, and immunity (4, 6).
The release of mediators is the consequence of a complex process called mast cell degranulation (MCD),
and it has considerable physiological and clinical importance, particularly in inflammatory processes and hypersensibility reaction type I. The clinical relevance of this process is evidenced frequently by the fact that a sudden, explosive systemic degranula-tion can result in a fatal vascular collapse (4, 6). Therefore, like many other physiological processes, an understanding of MCD is very important for the medical or biology student. However, experimental models of the degranulation process are rarely imple-mented for physiology courses. Generally, drawings, slides, or microphotographs of mast cells are pre-sented for undergraduate courses, whereas, in a few cases, the basophil/mast cell degranulation tests, in which released histamine is determined by radioenzy-matic assay, are used for postgraduate immunology or biology courses. This method is not suitable for an all-purpose physiology laboratory due to its high-cost equipment requirements.
We developed an experimental technique of study for MCD by using rat mast cells, saponin (as stimulator), and a light microscope with or without connection to a video camera. This technique is simple and low cost and allows direct observation of the degranulation process, improving its comprehension by the students.
I N N O V A T I O N S A N D I D E A S
1043 - 4046 / 99 – $5.00 – COPYRIGHTr1999 THEAMERICAN PHYSIOLOGICAL SOCIETY
TECHNICAL PROCEDURES
One male Sprague-Dawley rat (300-g body wt) was anesthetized with thiopental sodium (60 mg/kg ip). The level of anesthesia was verified by the absence of flexor, withdrawal, and corneal reflexes. Mast cells were obtained as follows: 10 ml of Ringer solution were injected into the peritoneal cavity of the rat with the use of a 10-ml plastic syringe with a 21-gauge needle. The needle was retired, and a light abdominal massage was applied. Fifteen minutes later, a second abdominal puncture was performed to obtain the remaining peritoneal liquid (,2 ml). The rat was then allowed to recover with body temperature control in an individual cage.
The cell density of the obtained peritoneal liquid was low and mainly constituted by deformed erythrocytes and some mononuclear cells. Five to ten microliters of this liquid were taken without centrifugation and deposed on a glass coverslip. The slide was then observed with a light microscope connected to a video camera (CDD-TR506, Sony) directly attached to the optical tube (340 final magnification). The
video signal could be connected to one or more television monitors and/or recorded on a conven-tional videocassette recorder (VCR). One mast cell was identified according to its morphological charac-teristics. However, this preliminary recognition re-quires confirmation by a physiological response test as follow: 5 µl from a solution (50 µg/ml in Ringer) of saponin (Sigma Chemical, St. Louis, MO) were added, with the use of a micropipette, to the border of the coverslip and allowed to diffuse until the cell could be observed. The time was recorded during the entire procedure by the time counter from the video camera and the VCR.
The experimental procedure took,20–30 min, includ-ing the weighinclud-ing, anesthetizinclud-ing, peritoneal aspiration of the rat, and the microscopic observation of cell degranulation (60–120 s). The selection of the mast cell was functionally confirmed by the degranulation, which occurred in 60–70% of the trials. If the degranu-lation response was not obtained, the procedure was restarted from the remaining peritoneal fluid, and this took another 60–120 s.
PROCEDURAL NOTES
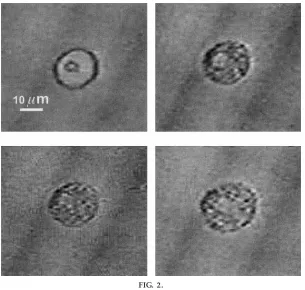
The presentation of the session started with the indication of some morphological, physiological, bio-chemical, and immunologic features of mast cells with the use of slides, drawings, and/or posters as summa-rized in Fig. 1. Deep discussion in interactive form was realized with the students, who had previously read from a selected bibliography. Thereafter, the experi-ment was started as described inTECHNICAL PROCEDURES. In our case, one microscope setup with a video camera connected to two television monitors (27-in. screen) was used to show cell events to 35 students. This setup clearly showed morphological changes during MCD. In Fig. 2,top left, the cell is shown in a nonactivated state, as characterized by a clearly de-fined membrane surface and homogeneous intracyto-plasmic density. The addition of saponin (50 µg/ml) induced membrane and cytoplasmic changes. Figure 2,top right, presents these changes 15 s after saponin
FIG. 1.
General scheme of antigen-specific mast cell degranulation. Ag, antigens; IgE, immunoglobulin E; TNF-a, tumor necr osis
factor-a; GM-CSF, granulocyte/ monocyte colony-stimulating
factor; PAF, platelet-activating factor.
I N N O V A T I O N S A N D I D E A S
application. A slight increase of cell diameter and a great increase in the cytoplasmic granular optical density were observed. The changes were enhanced after 30 s of saponin application (Fig. 2,bottom left). Finally, dramatic changes took place when, after 40 s of saponin application, the degranulation process made the cell membrane completely anfractuous, with an additional increase in cell volume (Fig. 2, bottom right). These procedures could be repeated two or three times with the initially obtained perito-neal liquid because of the short degranulation time (60–120 s).
CONCLUSIONS
The technique described in the present paper is very flexible and can be adapted according to laboratory equipment resources. For example, if one microscope is available, then an individual workstation can be
implemented for direct visualization. If the laboratory has one video camera, it can be connected to more than one television monitor with the use of video splitters. The use of the video camera has the advan-tage of video recording, which allows the use of replay, off-line video analysis, precision timing, and maintenance of a video library.
This simple and low-cost demonstration has repeat-edly proven its ability to illustrate not only basic but also more advanced concepts of the MCD process. For the last three years, it has been part of our regular practical session of medical physiology education, and it can also be used in the preliminary testing of agonist-antagonist interaction in the MCD process.
We thank Dr. Oscar Malpica and Dr. Sioly Mora de Orta for critical reading of the manuscript.
FIG. 2.
Morphological changes during mast cell degranulation induced by sapo-nin. Mast cell was obtained fr om peritoneal liquid of Sprague-Dawley rat.
Top left: nonactivated mast cell;top r ight: mast cell 15 s after activation with saponin (5 µl of solution of 50 µg/ ml saponin in Ringer);bottom left: mast cell 30 s after activation;bottom r ight: 40 s after activation.
I N N O V A T I O N S A N D I D E A S
This study was partially supported by Consejo de Desarrollo Cientı´fico, Humanı´stico y Tecnolo´gico, Universidad de Carabobo, Venezuela.
Address for reprints and other correspondence: A. Eblen-Zajjur, Fisiologı´a y Biofı´sica, Facultad de Ciencias de la Salud, Universidad de Carabobo, PO Box 3798, El Trigal 2002, Valencia, Venezuela (E-mail: [email protected]).
Received 3 April 1998; accepted in final form 6 January 1999.
Refer ences
1. Abbas, A. K., A. H. Lichtman, and J. S. Pober.Im m unologı´a celula r y m olecula r (2nd ed.). New York: Interamericana McGraw-Hill, 1995, p. 284–292.
2. Beaven, M. A., and H. Metzger. Signal transduction by Fc receptor: the FceRI case.Im m unol. Toda y14: 222–226, 1993.
3. Galli, S. J.New concepts about the mast cell.N. Engl. J. Med. 328: 257–265, 1993.
4. Har dman, J. G., A. G. Gilman, and L. E. Limbir d. The Pha rm a cologica l Ba sis of Thera peutics (9th ed.). New York: McGraw-Hill, 1995, p.659–661.
5. Pauwels, R., and M. Van Der Straeten. Drugs that modify mast-cell function: their mode of action. In:The Ma st Cell: Its Role in Hea lth a nd Disea se, edited by J. Pepys and A. M. Edwards. Davos, Switzerland: Pitman Medical, 1979, p. 61–75. 6. Rich, R. R., T. A. Fleisher, and B. D. Schwartz (Editors).
Clinica l Im m unology: Principles a nd Pra ctice (1st ed.). St. Louis, MO: Mosby Yearbook, 1996, vol. I, p. 408–430.
7. Roitt, I.Essentia l Im m unology(9th ed.). Oxford, UK: Blackwell Science, 1997, p. 330–331.
8. Sell, S., I. Berkower, and E. E. Max .Im m unology, Im m unopa -thology a nd Im m unity(5th ed.). Stamford, CT: Appleton and Lange, 1996, p. 217–218.
9. Walsh, L., G. Trincheri, H. A. Waldor f, D. A. Whitaker, and G. F. Murphy. Human dermal mast cells contain and release TNF-a, which induces endothelial-leukocyte adhesion
mol-ecule-1.Proc. Na tl. Aca d. Sci. USA88: 4220–4224, 1991.
I N N O V A T I O N S A N D I D E A S