PAPER
1SUBTOPIC
NUMBER OF QUESTIONS
2011 2012 2013 2AM 201 5 2016 2017
7 '1 Acids and Bases z z z 1
7 .2 f he Strength of Acids and Alkalis Z 1 1 1 1
7.3 Concentrations of Acids and Alkalis 1 1 1
7.4 Neutralisation 1 z 1 1 1 2
@
Acids
and
Bases
SPM 2013 Question 18
I
Which statement explains why water is needed toshow the acidic properties
ofan
acid?Penyataan manakah menerangkan mengapa
air
diperlukan untuk menunjukkan sifut keasidan bagi
suatu asid?
A
Neutralisation of acid occurs in water Peneutralan asid berlaku dqlamair
B
Ionisation of acid occurs in water Pengionan asid berlaku dqlamair
C
Water dissolves the acidAir
melarutkan asidD
Water oxidises the acidAir
mengoksidakan asidSPM 2013 Question 30
2
Diagram
l0
showstwo
beakers,P
andQ
thatcontain
granulated limestones,CaCO,
and pH paper respectively in dilute ethanoic acid.Rajah
l0
menunjukkandua
bikar
P
dan
Qyang
mengandungi ketulanbatu
kapur, CaCO,dan kertas pH masing-masing dalam asid etanoik
cair.
Dilute ethanoic acid Asid etanoik cair Granulated limestones Ketulan butu kapur
pH paper Kenas pH Diagram l0
Rajah 1O
Which observation is correct? Pemerhatian yang manakah betul?
P
a
Gas bubbles released Gelembung gas terbebas
pH
value:
InilaipH--
|Gas bubbles released Gelembung gas lerbebas
pH
value:4
nilaipH:4
Solution tums cloudy Larutan menjadi keruh
pH
value:4
nilai
pH:4
No change Tiada perubahan pHvalue:
InilaipH:
I SPM 2014 Question 53
Which statement is correct about acid?Pernyataan manakah yang betul tentang asid?
A
Acid
solution conducts electric current Larutan asid mengkonduksi arus elektrikB
Strong acid ionizes partially in water Asid kuat mengion separa dalamair
C
Weakacid
produceshigh
concentrationof
hydrogen ions
Asid
lemah
menghasilkan kepekatan ionhidrogen yang tinggi
D
The presence ofwater enables acid to producehydroxide ions
Kehadiran
air
membolehkan
asid menghas ilkan ion hidroksidaA
B
C
D
ffi
ANALYSIS OF THE 2011_ 2017 SPM PAPERS
)r
l---+
L---J
SPM 2011 Quesfion
fi
4
Which substance neutralises a solution with the pH value of 4?Bahan
manakthdengan nilai pH
4'l
A
Distilled waterAir
sulingmeneulrelkqn
suqtu
lat'utanB
Sodium chloride Natrium klorida SPM 2015 Quesaon 105
Which substance is an alkali? Bahan manakah adalah suatu qlkali?A
Zinc oxide Zink oksidaB
Magnesium oxide Magnesium oksidaC
Potassium hydroxide Kalium hidroksidqD
Aluminium hydroxide Aluminium hidroksida SPt|' 2015 Question 316
Table 2 shows the pH valuesoftwo
solutionswith
the same concentration.Jadual 2 menunjukkan
nilai
pH bqgi dualar
tandengan kepekatan yang sama.
Solution
Larutqn
pHa
2 R 6 Table 2 Jadual2Which stal€ment explains the differences in the pH values?
Pmyataan manakah yang menerangkan perbezaan
antarq nilai pH itu?
A
Q ionizes partially whereas R ionizes completelyQ
mengionsepara
manakalaR
mengion lengkapB
The concentrationof
hydrogen ionsin Q
islower than R
Kepekotan
ion
hidrogendalam
Q
adalah lebih rendah daripada RC
The number of mole of hydrogen ions in Q is less than RBilongon mol ion hidrogen
dalan
Q adalah kurang daripada RD
The degree of ionizationofQ
is higher than RDarjah pengionan
Q
adalah lebih
tinggi daripada RC
Sodium hydroxide Natrium hidroksidaD
Dilute ethanoic acid Asid elanoikcair
SPM 2015 Question 33
7
A substance has the following characteristics. Suatu bahan mempunlaiciri-ciri
befikut.. Turns moist blue litmus paper to red
Menukar kertas litmus biru lembap ke merah
.
Sour tasteRasa masam
.
Gas bubbles
releasedwhen reacts with
magnesiumGelembung gas terbebas apabila bertindak
balas dengan magnesium
What is the molecular formula
ofthe
substance? Apakah formula molekul bohanin?
A
CrH6B
CTH,OH SPM 2016 QuestionI
8
Which substance is a diprotic acid?Bahan mqnqkuh adalah satu asid diprotik?
A
Sulphuricacid C
Phosphoric acid Asid sulfurikB
Ethanoic acid Asid etanoikc
c,H5cooH
D
CTH5COOCTH5 As idfosforik
D
Hydrochloric acid Asid hidroHorik SPM 2016 Question 359
Compound X produces a solution with a pH value less than 7 when it is dissolved in water.What is compound X?
Sebation
X
menghasilkan suatular
tan dengan nilai pH kurang daripada 7 apabila dilanttkan ke dalamair
Apakah sebatianX?
A
Sodiumoxide C
Ammonium chloride Natriumoksidq
Ammonium HoridaB
Magnesiumoxide
D
Hydrogen chlorideMagnesium
oksida
Hidrogen klorida SPM 2017 Question 1310
Which pair ofacids is classified correctly? Pasangan asid yang manakah dikelaskan denganbetull
Monoprotic
acid Asid monobesDiprotic
acid Asid dwibes Ethanoic acid Asid etanoik Hydrochloric acid Asid hidrcklonk Hydrochloric acid Asid hidroklorik Ethanoic acid Asid etanoik Sulphuric acid Asid sulfurikNitric
acid Asidnitrik
Nitric
acid Asidnitrik
Sulphuric acid Asid sulfurik Dc
@
Acids
Alkalis
SPM 2011 Question 1
1l
Which
of
the following
is
correct about weakalkaline solution?
Antara yang berikut, yang manakah betul tentang
larutan alkali lemah?
A
Have pH valueof
13 Mempunyainilai pH
13B
Partially ionised in water Mengion separa dalamair
C
Concentration of the solution is low Kepekatan larutan adalah rendahD
Solution does not react with acidLarutan tidak bertindqk balas dengan asid SPM 2011 Question 43
12
Which
acid
contains
the
highest number
of
hydrogen ions?
Asid
manakahyang
mengandungi bilangan ion hidrogen yang paling tinggnA
25 cm3of I
moldmr
nitric acid25 cm3 asid
nitrik I
moldmr
B
25 cm3of I
moldmr
ethanoic acid25 cm3 qsid etanoik
I
moldmr
C
25 cm3of I
moldmr
sulphuric acid25 cm3 asid sulfurik
I
moldmr
D
25 cm3of I
moldmr
hydrochloric acid 25 cm3 asidhidroklor*
I
moldmr
SPM 2012 Question 1913
Which pair
showspH
value andthe
degreeof
dissociation for sulphuric acid?
Pasangan manakah yang menunjukknn nilai
pH
dan darjah penceraian bagi asid sulfurikyang betul?pHvalue
NiluipH
Degree of dissociation Darjah penceraian 2 High IIinggi
2 Low I Rendah 6 Low / Rendah 6HighlTinggi
SPM 2014 Question 4714
Which
solution containsthe
greatest numberof
hydrogen ions?
Laratan manakah mengandungi paling banyak ion
hidrogen?
A
0.3 dm3 of 2.0 moldmr
sulphuric acid 0.3 dm3 asid sulfurik 2.0 mol dm-3B
0.4 dm3 of 2.0 mol dm-3 nitric acid 0.4 dm3 asidnitrik2.0
moldmr
A
B C D
0.5 dm3 asid hidrcklorik 2.0 mol dma
D
0.6 dm3 of 2.0 moldmr
ethanoic acid0.6 dmr asid etanoik2.0 mol
dmr
SPM 2016 Question 1415
Whichof
thefollowing
is correct about a strongalkali?
Antara yang berikut, yang manakah betul tentang
sustu alkeli kuat?
A
Shows purple colour in universal indicator Menunjukkan warna ungu di dalam penunjuk universalB
Has high concentration ofhydrogen ion Mempunyai kepekatanion
hidrogen yang tinggiC
Ionises partially in water Mengion separa dalamair
D
Tastes sour Rasa masam SPM 2017 Question 1816
Diagram 3 shows an organ in the human digestivesystem.
Rajah
3
menunjukkan satu organ dalam sistempencernaqn manusia.
Gastric juice containing hydrochloric acid Jus gastrik yang
mengandungi asi.d
hidrcHorik Diagram 3
Rajah 3
What is the pH value of gastric juice? Apakah
nilai
pH bagijus gastrik?A2
B5
c7
D14
@
Concentrations
of
Acids
and
Alkalis
SPM 2012 Question 22
17
What
is
the
numberof
moles
in
100
cm3of
1.5 mol dm-3 of nitric acid?
Apakah bilangan mol dalam 100 cm3 asid
nitrik
1.5 mol dmr?A
0.015 molB
0.100 molC
0.150 molD
1.500 mol Food Makanan=l
=
aal Ie
E
SPM 2013 Question 34
18
Table 4 shows the concentration of hydrogen ionsin hydrochloric acid and sulphuric acid.
Jadual
4
menunjukkan kepekatanion
hidrogen dalam asid hidroklorik dan asid sulfurik.Acid Asid Concentration
of
hydrogen ions (mol dm-3) Kepekatan ion hidrogen (mol dm-3) 0. I mol dmr hydrochloric acid Asid hidroklorik 0.1 moldmr
0.10.1
mol
dmr
sulphuric acidAsid
sulfurik0.l
moldm 30.2
Iable 4 Jadual 4
Why
is
the
concentrationof
hydrogenions
in sulphuric acid higher than in hydrochloric acid? Mengapakahkepekatan
ion
hidrogen
dalamasid sulfurik lebih tinggi daripada
dolam asid hidroklorik?A
Sulphuric acid is denserAsid sulfurik lebih tumpat
B
Sulphuric acid is more soluble in water Asid sulfurik lebih mudah larut dalamair
C
Sulphuric acid is a skonger acid Asid sulfurik ialah asid yang lebih kuatD
Sulphuric acid is a diprotic acid Asid sulfurik ialah asid diprotik SPM 2015 Question 1319
Which of thefollowing
is the correct sequence in preparation of a solution by dilution method? Antara yong berikut, yang manakah urutan yangbetul dalam
penyediaansatu larutan
melalui kaedah pencairan?I
Transfer the solution into a volumetric flaskPindahkan
larutan
ke
dalam
kelalang volumetrikII
Calculate the volume of solution to be dilutedKira
isi padu larutan yang hendak dicairkanIII
Use a pipette to obtain the volume of solutionneeded
Guna pipet untuk memperoleh isi padu larutan yang diperlukan
Add distilled water to the required volume
of
solution
Tambah
air
suling
kepada isipadu
larutan yang dikehendakiII, I, tII, IV
II,
I,IV III
II,III, I, IV
II,III,
IV
I
@
Neutralisation
SPM 2011 Question 4720
The
following
chemical equation shows
thereaction between sulphuric
acid and
sodium hydroxide.ftrSOo(afl + 2NaOH(aq)
t
NarSOo(ae)+2HrO(l) What is the molarityof
sulphuric acid used when100 cm3 ofthe acid neutralises 0.04 mol of sodium hydroxide?
Persatnaan
kimia berikut
menunjukkan tindakbalas antara
asid
sulfurik
dengan
natriumhidroksida.
HrSOn(at) +
2NaOH(at)
t
NarSOn (aft) + 2HrO(ce)Berapakah
kemolaran
asid
sulfurik
yang digunakan apabila 100 cm3 asid itu meneutralkan0.04 mol natrium hidroksida?
A
0.02 mol dm3
C
0.20 mol dm-3B
0.08 moldmr
D
0.80 moldmj
SPM 2012 Question 46
2l
Apiece of sodium metal is put into a beaker whichcontains 30 cm3 of water to form a solution.
Which of the following can react with the solution?
Sedikit logam natrium
dimasukkanke
dalam sebuah bikar yang mengandungi 30 cm3air
untukmembentuk suatu larutan.
Antarayang berihtt, yang manakah boleh bertindak
balas dengan larutan inf?
A
Aqueous ammonia Ammonia akueusB
Potassium carbonate solution Larut an kalium kar b o natC
Lithium hydrogen carbonate solution Larutan litium hidrogen karbonatD
Hydrogen chloride solution Larutan hidrogen kloridaIV
A
B
c
22
Diagram 10 shows the apparatus set-upfor
theneutralisation reaction between a strons acid and a strong alkali.
Rajah
l0
menunjukkan susunan radas bagi tindakbalas peneutralan antara asid lant dan alknli kuat.
Sulphuric acid Asid sulfurik
50.0 cm3 of 0.1 mol dm-3 sodium hydroxide solution 50.0 cm3 larutan natrium hidroksida 0.1 mol dm 3
Diagram 10 Raiah
l0
25.0 cm3
of
sulphuric acid neutralises 50.0 cm3of
0.1 mol dmJ sodium hydroxide solution.What is the molarity of the sulphuric acid? 25.0 cm3 asid sulfurik asid meneutralkan 50.0 cm3
larutan natrium hidrol<sida 0.1 mol dmr. Apakoh kemolaran asid sulfurik''!
A
0.10 moldmr
B
0.15 moldmr
C
0.20 moldmr
D
0.40 mol dm-lSPM 2013 Question 47
23
The following
equation representsthe
reaction between hydrochloric acid and calcium hydroxidesolution.
Persamaan berikut mewakili tindak balas anlara
asid hidroklorik dan larutan kalsium hidrol<sida'
2HCl +
Ca(OH),
---, CaClr+ 2H2O 20:0 cm3of
0.1 mol
dmr
hydrochloricacid
isneutralised
by
50.0
cm3of
calcium
hydroxidesolution.
What
is
the molarity
of
the
calcium hydroxidesolution?
20.0 cm3 as i d hidrokl o r ik 0. I mol dmr di neutr a lkan
oleh 50.0 cm3 larutan kalsium hidrol<sida'
B erapakah kemolaran I arutan kals ium hidroks ida
itu2
A
0.020 moldmr
B
0.040 moldmr
C
0.125 moldmr
D
0.250 moldmr
24
TableI
shows the pH value when a certain volume of 1.0 mol dmr hydrochloric acid is added to 25 cmi of 1.0 mol dm 3 sodium hydroxide solution.Jqdual
I
menunjukkan nilaipH apabila suaa bi padu tertentu asid hi&oklorik 1 .0 mol dm 3 ditcrmbah kepada25
cfr
larutan natrium hifutksida 1.0 moldm'.
Volume of hydrochloric acid (cm3)Isi nqdu ssid hidroklorik (cm3)
pH value
NiluipH
0l0
25 40 14 11 IJX
2 Table 1 Jadual 1What is the value of X?
Apakah
nilaiX?
A t2
c7
B 9
D4
SPM 2015 Question 41
25
Diagram l0 shows an aquarium filled with freshwater.The
pH
valueof
waterin
the aquarium hasto
be maintained to make it suitable for the aquatic life. Rajahl0
menunjukkan sebuah akuariumyangdiisi
dengan
air
tawar Nilai pH
air
dalam akuarium ituperlu
dikekalkan untuk menjadikannya sesuaiuntuk hidupan akuatik.
Diagram 10 Raiah 10
The pH value of the water is found to be too low and unsuitable for many aquatic lifes.
Which is the most suitable method to raise the pH level of water in the aquarium.
Nilai pH
air
didapati terlalu
rendahdan
tidak sesuai untuk kebanyakan hidupan akuatik.Kaedah
manakahyang
paling
sesuai
untuk meningkatkan aras pHair
dalam akuariumitu/
A
Dissolve baking soda powder into the water Larutkan serbuk penaik ke dalamair
B
Flow carbon dioxide gas into the water Alirkan gas karbon dioksida ke dalamair
C
Dissolve table salts into the water Larutkan garam ke dalamair
D
Add vinegar into the waterSPM 2016 Question 46
26
Diagram 15 shows the situation that Ameng has been experiencing for a long time.Rajah 15 menunjukkan situasi dialami oleh Ameng untuk sekian lama.
Diagram 15 Rajah 15
He has problem to carry out agricultural activities. Which substance can be used to solve his problem?
Dia
telah menghadapi masalah untuk menjalani aktiviti pertanian.Apakah bahan
yang
boleh digunakan
untuk meny e le s a i ka n m as al ahny a ?A
Table salt Garam biasaB
Wood ash Abu kayuC
Rice husk Sekampadi
D
Lake waterAir
tasik SPM 2016 Questiont9
27
Which
sodium hydroxide solution
neutralisesl0
cm3 of 0.5 moldmr
sulphuric acid?Larutan natrium
hidrol<sida manakah
yangmeneutralkan 10 cm3 asid sulfurik 0.5 mol dm-3? Volume (cm3)
Isi pada (cm3)
Concentration (mol dm'3)
Kepekatan (mol dma)
5 0.5
l0
0.5l0
1.0 20 1.0 D SPM 2017 Question 2728
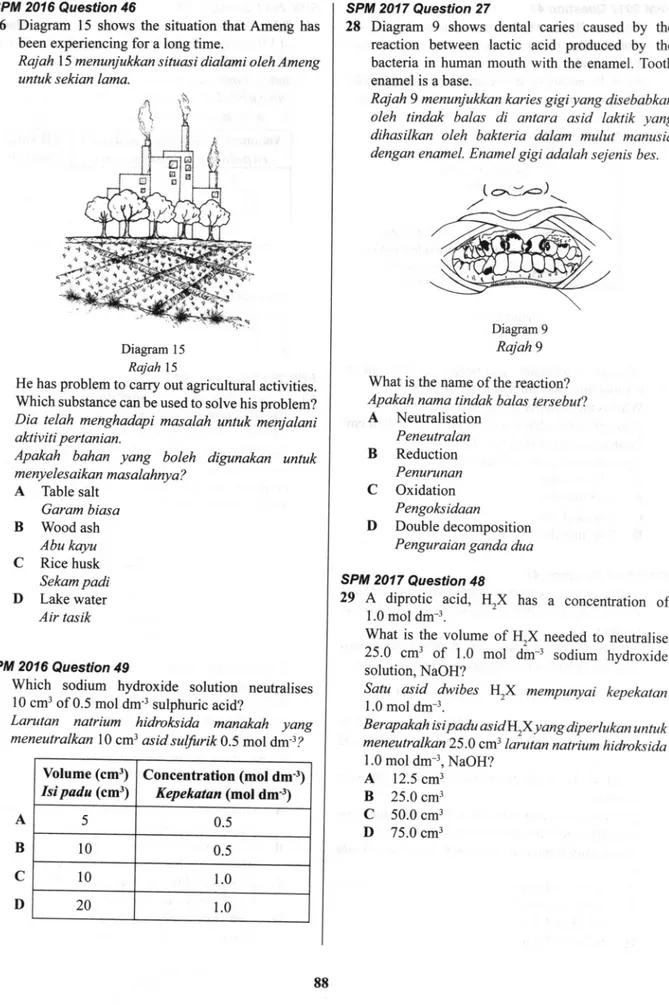
Diagram9
shows dental caries causedby
thereaction
betweenlactic acid
producedby
thebacteria
in
human mouthwith
the enamel. Tooth enamel is a base.Rajah 9 menunjukkan karies gigi yang disebabkan
oleh tindak balas
di
antara
asid
laktik
yangdihasilkan oleh bakteria dalam mulut
manusia dengan enamel. Enamel gigi adalah sejenis bes.Diagram 9
Rajah9 What is the name of the reaction?
Apakah nama tindak balas tersebut?
A
Neutralisation PeneutralanB
Reduction PenurunanC
Oxidation PengoksidaanD
Double decomposition Penguraian ganda duoSPM 2017 Question 48
29
A
diprotic acid,
HrX
has
a
concentrationof
1.0 mol dm-3.
What is the volume
of H,X
neededto
neutralise25.0
cm3of
1.0
mol
dmr
sodium
hydroxide solution. NaOH?Satu
asid
dwibes
HrX
mempunyai kepekatan 1.0 mol dm-3.Berapakah isi padu asidHrXyang diperlukan untuk
meneutralkan 25.0 cm3 larutan natrium hidrot<sida 1.0 mol dm-3, NaOH?