Transposon mutagenesis facilitates gene discovery by tagging genes for cloning. New genomics projects are now cataloging transposon insertion sites to define all maize genes. Once identified, transposon insertions are ‘hot spots’ for generating new alleles that are useful in functional studies.
Addresses
Department of Biological Sciences, 385 Serra Mall, Stanford University, Stanford, California 94305-5020, USA; e-mail: [email protected]
Current Opinion in Plant Biology2000, 3:103–107
1369-5266/00/$ — see front matter © 2000 Elsevier Science Ltd. All rights reserved.
Abbreviations bp base pairs
MTM Maize Targeted Mutagenesis
NSF National Science Foundation
TIR terminal inverted repeat
TUSC Trait Utility System for Corn
Introduction
The analysis of mutant phenotypes yields an understand-ing of gene function in a whole-organism context. Consequently, the generation, evaluation, maintenance and distribution of seed containing verified mutations or seed with populations of mutations suitable for high-throughput screening are essential in the modern genetic analysis of maize and other plants.
Saturation mutagenesis of maize genes involves two ambi-tious goals: to define all of the genes and to find the phenotype of every individual gene. This review will out-line how transposon mutagenesis can be used to achieve both goals. Transposons are already the primary tool for tagging and cloning maize genes. Studies so far demon-strate that specific transposons are well suited for either global mutagenesis and gene discovery or multiple rounds of mutagenesis at a defined target gene [1]. New genomics approaches, employing strategies for screening by PCR and for plasmid rescue, are now providing indexed collec-tions of mutacollec-tions and the sequences flanking transposon insertion sites. Many researchers will soon identify trans-poson-generated mutants in specific genes after querying a database rather than searching a corn field.
Insertional mutagenesis across the genome:
primer on key transposon properties
The Ac/Ds and MuDR/Mu maize transposons have been used in most recent mutagenesis experiments [1–3]. In stocks with the transcriptionally active regulatory elements
Ac or MuDR, family members with essential transposase-binding sites in the terminal inverted repeats (TIRs) are mobilized to create new insertion mutations. During a gene-tagging experiment, transposon activity is conveniently
monitored with a reporter allele that has a phenotype that beomes visible after the excision of a transposon (Figure 1a). Saturation mutagenesis of all genes requires a robust muta-genized population. An estimate of the distinct units (genes, for example) and the confidence level of the coverage are used to calculate the population size required to provide one insertion per unit, assuming random mutagenesis (Table 1).
Ac/Ds are ‘cut and paste’ transposons (Figure 1b). New insertions can occur throughout the life cycle: somatic insertions that occur in the apical meristem can be appar-ent in the shoot and can be transmitted to the next generation [3]. Most newly identified mutations, however, are recovered in single progeny, indicating that, in practice, insertions in gametes are the main source of new muta-tions. Germinal revertants are readily recovered (at a frequency of 10–1 to 10–2), indicative of late somatic or gametophytic excision events. These germinal revertants often differ in sequence [4], and are a rich source of allelic diversity for functional analysis [5•,6•], as they have been over evolutionary time [7]. Because only a few mobile
Ac/Ds exist in a mutagenic line and these elements insert preferentially into sites that are closely linked to the origi-nal element locations [3], Ac/Ds is an inefficient global mutagen (with a forward mutation frequency of 10–6[1]) but can be highly efficient regionally. There are also maize transposons with a high insertion preference for particular genes [8]: these are a restricted tool but one that is highly useful for those studying the target(s).
Ac/Ds elements spaced at 10–20 cM intervals across the recombination map would facilitate intensive mutagenesis of the entire maize genome [9•]. Because mobile Ac/Dscopy number is low, confirmation that a particular transposon insert is the cause of a mutant phenotype is easily obtained [3]. There are several strategies for exploiting Ac/Ds in maize that depend on enriching for transposed copies, which may be linked or unlinked to the original location [3] (Figure 2).
Saturation mutagenesis using maize transposons
Virginia Walbot
Table 1
Population size estimates for insertional mutagenesis to achieve at least one mutant per gene at a specific coverage.
Gene estimate Probability (%) Population size (n)
30,000 95 ~91,000
50,000 95 ~150,000
50,000 99 ~230,000
70,000 95 ~214,000
These strategies have also been implemented in transgenic dicots expressing maize transposons [10–15]; with genetical-ly engineered Ac/Ds, gene and enhancer trap transposons have also been developed [10–12].
MuDR/Mu transposons are active late in tissue develop-ment, resulting in tiny somatic sectors from ‘cut and paste’ events. MuDR/Mu undergo duplicative transposition in pre-meiotic and gametophytic cells (Figure 1c); conse-quently, few germinal revertants exist (excision frequency <10–5
,
which is less than a new germinal insertion) [1]. Mutator stocks typically contain several copies of MuDRand many Mu elements [16], resulting in a high forward mutation frequency (~10–4, range 10–3–10–5 in any gene [1,16]). When Muelement copy number is low, the muta-tion frequency is similar to that of Ac/Ds [17]. Because epigenetic silencing is stochastic and frequent, somatic instability (Figure 1a) and unmethylated MuTIRs should be verified before mutagenesis [2].
Muinsertions occur preferentially into low-copy DNA; there is no apparent local bias [16]. Recent analyses of hundreds of somatic (M Raizada, V Walbot, unpublished data) and germinal Muelement insertions (K Edwards, personal com-munication) confirm insertion into or near exon-like sequences. Although high copy numbers make MuDR/Mu
efficient mutagens, the resulting multiple mutant genes com-plicate the assignment of phenotypes to specific genes [2].
Transposon tagging in maize is much more efficient than would be predicted from the large genome size, ~2.3 × 109 base pairs (bp) [18]. More than half of the maize genome, however, consists of inactive retrotransposons found in complex arrays; these create the spacers containing 20–200 thousand base pairs (kbp) between maize genes
[19]; consequently, insertions would be expected to affect only one gene. With short introns, maize genes are as com-pact as those of Arabidopsis [20]. Thus, transcription, recombination [21] and DNA transposon insertion are all highly biased for the genes, and for these important process-es absolute genome size is of relatively little importance.
DNA sequencing strategies for genomic DNA
next to transposon insertions: methods used
to analyze transposons in a single plant
Initial applications of transposon tagging in maize relied on correlating the inheritance of a plant phenotype with a band on a DNA hybridization blot. Particularly for the multi-copy Mu elements, demonstrating tight linkage required some luck in restriction enzyme choice and in hybridization probes to resolve specific Muelements (Mu1,
Mu2,Mu3,Mu8 and MuDR); the MuTIR probe detects too many bands to be useful. Proof that the correct gene was cloned after recovery of the transposon-tagged genomic fragment requires the analysis of independent mutants, or a complementation test. Given the difficulty of maize transformation, complementation has so far been useful only for traits that can be assayed in transient expression assays with protoplasts or tissues amenable to particle gun bombardment [22].
Several techniques for amplifying and sequencing genomic DNA next to transposon ends are amenable to genomics approaches. PCR primers anchored on transposons are ‘read out’ into genomic DNA [23,24,25••] with a wide choice of strategies for priming from the flanking genomic DNA of unknown or specified sequence (Figure 2) [26••]. Initial methods relied on the separation of the PCR products by size, with manual recovery and analysis of bands that segre-gated with a phenotype. Nowadays, simplifications include
104 Genome studies and molecular genetics
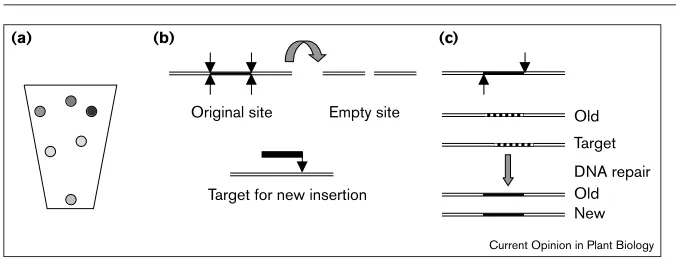
Figure 1
Transposon properties. (a) Somatic excision of a transposon from a gene of the anthocyanin pathway can result in spots of pigmentation in cell lineages with a functional allele. This visible phenotype demonstrates somatic mutability and transposon activity. A range of phenotypes is observed, because revertant alleles differ in sequence and function. (b) Cut and paste transposition. The
transposase liberates a double-stranded transposon from the original site by executing four strand breaks (arrowed at top left). Transposase creates staggered nicks at the target site (8 bp apart) and inserts the double-stranded transposon into a new site. The host chromosome is repaired (with modest changes) at the old site, and an 8 bp host sequence duplication is created by DNA
synthesis at the insertion site during repair synthesis. If timed to conicide with
chromosome replication, Ac/Dscopy number can increase. It is often observed that plant sectors or kernels on an ear differ in Accopy number, in twin sectors. If the transposon is excised from an old site after its DNA replication and is inserted into a new site before DNA replication, one daughter cell receives two copies of Ac(old site and new site) and one daughter cell receives one copy (new site). (c) Replicative transposition. Transposase makes two single-strand nicks next to the 3′ends of the transposon in the original site. In the model, transposase makes two staggered nicks in the target (9 bp apart for Muelements). A single strand (dotted line) is, in effect, transferred to a new site. Massive DNA replication is required to make both the old and new site double-stranded. A host sequence duplication is also created at the new insertion site.
Original site Empty site
Target for new insertion
Old
Old Target DNA repair New
Current Opinion in Plant Biology
shotgun cloning combined with high-throughput sequenc-ing. Selective PCR primer strategies can examine a subset of transposon family members or a subset of insertion sites (with primers spanning the joint with genomic DNA). Because transposon insertions result in characteristic host sequence duplications (9 bp for Mu[16], 8 bp for Ac/Ds[3]), compiling the continuous DNA sequence at any given insertion site uses the duplication for matching up the left and right genomic sequences.
‘Panhandle’ PCR, in which transposon TIRs form intramolecular duplexes in single-stranded DNA [26••] (Figure 2), is the foundation for a new National Science Foundation (NSF)-funded genomics project that aims to recover and sequence maize genomic DNA adjacent to transposed Ac[9•]. Plasmid rescue is an alternative strate-gy that is much simpler to use in practice but requires more effort to set up. Transgenic plants with transposons engineered to contain a bacterial plasmid allow the selec-tive cloning of transposon insertion sites after transformation of Escherichia coli with total genomic DNA; only plant DNA with a bacterial origin of replication and an antibiotic resistance marker is maintained in the trans-formed bacteria. This approach was pioneered by using
Ac/Ds-based elements introduced into dicots via
Agrobacterium-mediated transformation [27]; now RescueMu
plasmids based on Mu1 are being used to clone maize genes [28•].
Indexed mutant collections and parallel
searches for transposon insertions
Traditional genetics starts with the mutant individual and seeks the gene, whereas genomics methods start with the DNA sequence and search for the phenotypes. The first genomics approach, TUSC (Trait Utility System for Corn),
was developed by Pioneer Hi-Bred International; it is available to academic researchers who sign material trans-fer agreements to receive the seeds containing putative ‘hits’ to user-supplied sequence motifs. TUSC contains DNA samples and progeny seed from ~44,000 Mutator plants that probably contain >106 independent Mu inser-tions. DNA samples, from single plants or post-preparation pools, are screened for Muinsertions into a target gene or motif by using a PCR primer that reads out of Mu TIRs and a target-specific primer. All mobile Muelements share a very highly conserved sequence in the terminal 25 bp of the TIRs [16]; consequently, a single ‘read out’ primer suf-fices. TUSC has been used successfully by both Pioneer Hi-Bred International [29] and by academic researchers [30,31]. One drawback of a PCR strategy with a high cycle number is that both germinal and somatic insertions are found; ~10–20% genuine germinal insertions are verified among candidate insertions from TUSC screening [28•].
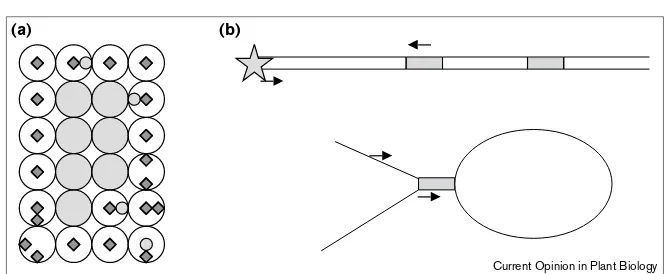
A convenient field organization into rows and columns for collecting pooled plant (and hence DNA) samples was implemented at about the same time as TUSC [32]; this method overcomes the identification of ‘false positive’ somatic insertions. DNA samples representing pooled leaf punches from a row of plants and separate sets of leaf punches from each column of plants are screened by PCR; two successful reactions define the row and the column and hence a specific plant that has the germinal insertion muta-tion (Figure 3). The Maize Targeted Mutagenesis (MTM) project, funded in the NSF plant genomics program, is now offering screening of a Mutator collection of ~50,000 plants and providing seed for the phenotypic evaluation of ‘hits’ [33•]. Project personnel are evaluating plant phenotypes at the kernel, seedling and adult plant stages to build a data-base of maize mutant phenotypes. To use MTM services, Figure 2
Identifying transposon insertions.
(a)Enriching for transposed Ac/Ds.In this diagram, kernels with excision sectors are white with spots of gray. Early somatic excisions that restore gene function result in a sector of gray kernels. By selecting seeds in a sector of kernels with a functionally revertant allele at the reporter gene (gray circles) investigators identify a somatic excision event from the original site; these kernels should contain the same transposed Acat a new location in the genome. Because Acshows a negative dosage effect on the frequency of somatic excision, kernels with fewer spots probably contain both the original donor site and a new transposed Ac[3]. (b) The amplification of sequences next to
transposons typically uses a ‘read out’ primer (arrows) complementary to the TIRs and a strategy to allow priming from the contiguous genomic DNA (star) such as a restriction site overhang ligated to an adapter. In ‘panhandle’
PCR [26••], TIRs form intramolecular
duplexes in single-stranded DNA; the flanking gene is in the pan and the transposon is the handle. Several restriction digestion and ligation steps are required to achieve this structure [9•,26••]. One restriction enzyme site within the pan structure represents the
artificial joining of the genomic DNA flanking the left and right ends of the transposon. Primers within the TIR sequence are used to amplify around the circle of genomic DNA; the second primer is in the transposon body to amplify back in the other direction when first-strand extension is completed.
Current Opinion in Plant Biology
investigators supply a gene sequence with intron–exon annotation; after receiving seed, investigators are obligated to contribute phenotypic analysis to the database.
In both TUSC and the row/column pooling method, DNA samples are finite. The NSF-funded Maize Gene Discovery Project uses RescueMuplasmid rescue to create immortalized collections of insertion sites in E. coli[28•]. Tagging fields with 2304 transgenic individuals are orga-nized into 48 rows and 48 columns, generating 96 separate libraries of RescueMuplasmids for each field. Users receive a 96-well plate containing 48 row and 48 column libraries and design their own PCR strategy to identify insertions; germinal events are present in one row and one column (Figure 3). To obtain seed, users submit 1 kb of DNA sequence next to the Mu insertion site of the RescueMu
plasmid of interest. Concomitantly, the Maize Gene Discovery Project will sequence row libraries to 95% cov-erage. Within a few years, users could first search the web site database [28•] for insertions into genes of interest and then perform PCR or hybridization on column libraries to ascertain which plant has the mutation.
Saturation mutagenesis within a gene
One mutation, no matter how instructive, is rarely suffi-cient to lead to a comprehensive understanding of a gene’s function. Most transposon insertions into exons or introns are ‘knockouts’ of gene function; alternative splicing events that use sequences in the ends of the transposon can result in modest expression in a few cases [1]. Transposon insertions, unlike most mutations induced by physical agents or Agrobacteriuminsertions, are ‘hot spots’ for secondary mutations. Of greatest current use in maize are Ac/Dsand Spm/Enmutants, from which germinal rever-tants are readily recovered; these typically contain one or a
few base changes in addition to the host sequence dupli-cation and, more rarely, larger deletions, ‘filler DNA additions’ or rearrangements. Of particular utility is the preference of Ac/Ds for local transposition; an Ac/Ds that transposes nearby can readily transpose back to the target gene, providing many new types of mutant allele [34,35]. For example, the promoter can be saturated with inser-tions at different sites followed by selection for minor alterations that affect regulation, or the requirements for splicing can be explored from insertion sites in or near the conserved intron motifs.
An important feature of all maize transposons is that they are somatically unstable in the presence of the transposase-encoding element. Consequently, somatic tissue is a mosaic of mutant and revertant cells of many different phenotypes. To produce somatic tissue of a single pheno-type, revertants can be selected, but not all of these are ‘knockout’ alleles. Alternatively for Ac/Ds, individuals lack-ing Ac can be recovered to stop somatic excisions. This strategy is virtually impossible for multi-copy MuDRlines; however, epigenetic silencing of transposons can occur spontaneously and is frequent in Mutator lines [16]. In the case of Mu1 insertions in promoters, methylation after silencing can activate a ‘read out’ promoter in the TIRs, in effect restoring gene expression [36]. Stabilization of a fully mutant phenotype is possible by selecting for dele-tions, which occur with about 10–2 frequency from the ends of Mu1elements [37].
Conclusions
Transposon-induced phenotypes have long provided geneticists with beautiful materials and insights into gene expression, development and chromosome mechanics. Cloned transposons have facilitated gene dis-covery and cloning. In the genomics era, maize transposons have emerged as the premier method for gene discovery and sequencing, as well as the phenotyp-ic analysis of gene expression in a whole-organism context. Transposons allow simultaneous effort in both phases of genomics, gene discovery and functional stud-ies. Transposons are more efficient than a sequential approach to gene sequencing followed by the design of tools to study gene expression.
Acknowledgements
I thank Michelle Facette for her comments on the manuscript. Research on Mutator and preparation of this article were supported by grants from the NSF (Maize Gene Discovery, DNA Sequencing and Phenotypic Analysis plant genomics grant 9872657) and the National Institutes of Health (GM49681).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Walbot V: Strategies for mutagenesis and gene cloning using transposon tagging and T-DNA insertional mutagenesis.
Annu Rev Plant Physiol 1992, 43:49-82. 106 Genome studies and molecular genetics
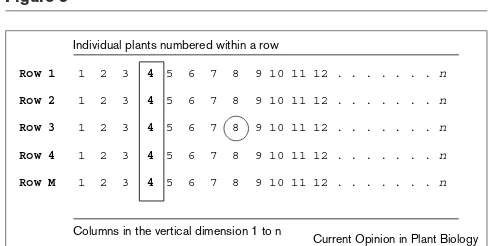
Figure 3
Row and column pooling strategies for high-throughput transposon insertion site screening. Plant samples such as leaf punches are collected from every plant in a row (individuals 1 to nin the horizontal direction). A single DNA sample is prepared from each row for PCR screening or plasmid rescue. Similarly, plant samples are collected from every column, the vertical direction of the grid; in this example column 4 is in bold and boxed. Positive PCR results from a row and a column define a specific plant with the germinal insertion of interest, for example, row 3 and column 8 (circled). Somatic insertions are amplified only in a row or only in a column in Mutator grids because somatic excisions and insertions occur mainly late in development.
Individual plants numbered within a row
Row 1 1 2 3 4 5 6 7 8 9 10 11 12 . . . n
Row 2 1 2 3 4 5 6 7 8 9 10 11 12 . . . n
Row 3 1 2 3 4 5 6 7 8 9 10 11 12 . . . n
Row 4 1 2 3 4 5 6 7 8 9 10 11 12 . . . n
Row M 1 2 3 4 5 6 7 8 9 10 11 12 . . . n
Columns in the vertical dimension 1 to n
2. Chomet PS: Transposon tagging with Mutator.In The Maize Handbook. Edited by Freeling M, Walbot V. New York: Springer, 1996:243-248.
3. Dellaporta SL, Moreno MA: Gene tagging with Ac/Dselements in maize.In The Maize Handbook. Edited by Freeling M, Walbot V. New York: Springer, 1996:219-239.
4. Nordborg M, Walbot V: Estimating allelic diversity generated by excision of different transposon types. Theor Appl Genet 1995,
90:771-775.
5. Martienssen RA: Functional genomics: probing plant gene function
• and expression with transposons.Proc Natl Acad Sci USA1998,
95:2021-2026.
A review of the ways in which transposons are currently used to analyze genes. 6. Maes T, De Keukeleire P, Gerats T: Plant tagnology. Trends Plant Sci
• 1999,4:90-96.
A review of amplification and detection strategies for multi-copy insertion elements.
7. Kidwell MG, Lisch D: Transposable elements as sources of variation in animals and plants. Proc Natl Acad Sci USA1997,
94:7704-7711.
8. Walker EL, Eggleston WB, Demopulos D, Kermicle J, Dellaporta SL:
Insertions of a novel class of transposable elements with a strong target site preference at the R-locus of maize.Genetics1997,
146:681-693.
9. Dooner H, Yan X. Plant genome project in the Dooner lab: Ac
• as a gene-searching engine.URL http://mbclserver.rutgers.edu/ ~xyan/waksman/PGRPpage.html
The website for the Ac/Ds-tagging genomics project; a long-term goal is to develop lines with Acelements spaced across the maize genome. 10. Nussaume L, Harrison K, Klimyuk V, Martienssen R, Sundaresan V,
Jones JDG: Analysis of splice donor and acceptor site function in a transposable gene trap derived from the maize element Activator.Mol Gen Genet1995, 249:91-101.
11. Springer PS, McCombie WR, Sundaresan V, Martienssen RA: Gene trap tagging of Prolifera, an essential MCM2-3-5-like gene in
Arabidopsis. Science1995, 268:877-880.
12. Sundaresan V, Springer P, Volpe T, Haward S, Jones JDG, Dean C, Ma H, Martienssen R: Patterns of gene-action in plant development revealed by enhancer trap and gene trap transposable elements.
Genes Dev1995, 9:1797-1810.
13. Souer E, Quattrochio F, de Vetten N, Mol J, Koes R: A general method to isolate genes tabbed by a high copy number transposable element.Plant J1995, 7:677-685.
14. Wisman E, Cardon GH, Fransz P, Saedler H: The behaviour of the autonomous maize transposable element En/Spm in Arabidopsis thaliana allows efficient mutagenesis.Plant Mol Biol1998,
6:989-999.
15. Wisman E, Hartmann U, Sagasser M, Baumann E, Palme K, Hahlbrock K, Saedler H, Weisshaar B: Knock-out mutants from an En-1 mutagenized Arabidopsis thalianapopulation generate phenylpropanoid biosynthesis phenotypes. Proc Natl Acad Sci USA 1998, 95:12432-12437.
16. Bennetzen JL: The Mutator transposable element system of maize.
Curr Top Microbiol Immunol1996, 204:195-229.
17. Gutiérrez-Nava, M, Warren CA, León P, Walbot V: Transcriptionally active MuDR, the regulatory element of the mutator transposable element family of Zea mays, is present in some accessions of the Mexican land race Zapalote chico.Genetics1998, 149:329-346. 18. Gale MD, Devos KM: Comparative genetics in the grasses.
Proc Natl Acad Sci USA1998, 95:1971-1974.
19. SanMiguel P, Tikhonov A, Jin Y-K, Motchoulskaia N, Zakharov D, Melake-Berhan A, Springer PS, Edwards KJ, Lee M, Avramova Z, Bennetzen J: Nested retrotransposons in the intergenic regions of the maize genome.Science1996, 274:765-768.
20. Brendel V, Carle-Urioste JC, Walbot V: Intron recognition in plants.
In A Look Beyond Transcription. Edited by Bailey-Serres J, Gallie DR. Rockville, MD: American Society of Plant Physiologists, 1998:20-28.
21. Dooner HK, Martinez-Ferez IM: Recombination occurs uniformly within the bronze gene, a meiotic recombination hotspot in the maize genome.Plant Cell1997, 9:1633-1646.
22. Nash J, Luehrsen KR, Walbot V: Bronze-2gene of maize: reconstruction of a wild-type allele and analysis of transcription and splicing.Plant Cell1990, 2:1039-1049.
23. Koes R, Sour E, van Houwelinger A, Mur L, Spelt C, Quattrochio F, Wing J, Oppendijk B, Ahmed SA, Maes T et al.: Targeted gene inactivation in petunia by PCR based selection of transposon insertion mutants. Proc Natl Acad Sci USA 1995,92:8149-8153. 24. Frey M, Chomet P, Glawischnig E, Stettner C, Grun S, Winklmair A,
Eisenreich W, Bacher A, Meeley RB, Briggs SP: Analysis of a chemical plant defense mechanism in grasses. Science 1997,
277:696-699.
25. Van den Broeck D, Maes T, Saur M, Zethof J, De Keukeleire P,
•• D’Hauw M, Van Montagu M, Gerats T: Transposon display identifies individual transposable elements in high copy number lines.
Plant J1998, 13:121-129.
The authors describe a PCR strategy for amplifying and analyzing genomic segments flanking transposon insertion sites. The strategy allows users to design flexible recovery systems (see also [24,26••]).
26. Hui, EK-W, Wang P-C, Lo SJ: Strategies for cloning unknown
•• cellular flanking DNA sequences from foeign integrants.
Cell Mol Life Sci1998, 54:1403-1411.
This recent review discusses all of the implemented strategies for PCR amplification of genomic segments flanking transposon insertion sites. 27. Chasan R: Ac tagging moves beyond maize. Plant Cell1993,
5:361-363.
28. Walbot V, Hake S, Freeling M, Chandler VL, Galbraith D, Larkins BA,
• Schmidt R, Smith L, Sachs MM, Brendel V: Maize gene discovery, DNA sequencing and phenotypic analysis.URL
http://www.zmdb.iastate.edu/zmdb/nsf_grant.html
This website includes the data and procedures for a maize expressed sequence tag project and the RescueMugene discovery project; it also includes new tools for annotating genes trained to maize sequences.
29. Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP: Cloning and characterization of the maize
An1gene.Plant Cell 1995, 7:75-84.
30. Das L, Martienssen R: Site-selected transposon mutagenesis at the hcf106 locus in maize.Plant Cell 1995, 7:287-294. 31. Hu GS, Yalpani N, Briggs SP, Johal GS: A porphyrin pathway
impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize. Plant Cell 1998,
10:1095-1105.
32. Chuck G, Meeley RB, Hake S: The control of maize spiklet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev 1998, 12:1145-1154.
33. Martienssen R, Freeling M, Alexander D, Stein L. Maize Targeted
• Mutagenesis Database.URL http://mtm.cshl.org
A database of phenotypic information on large populations of mutator plants. This website includes instructions on how to request PCR screens for inser-tions in specific motifs.
34. Athma P, Grotewold E, Peterson T: Insertional mutagenesis of the maize P-gene by intragenic transposition of Ac.Genetics1992,
131:199-209.
35. Moreno MA, Chen J, Greenblatt I, Dellaporta SL: Reconstitutional mutagenesis of the maize P-gene by short-range Ac
transpositions. Genetics1992, 131:939-956.
36. Barkan A, Maritenssen RA: Inactivation of maize transposon Mu
suppresses a mutant phenotype by activating an outward-reading promoter near the end of Mu1. Proc Natl Acad Sci USA1991,
88:3502-3506.
37. Levy AA, Walbot V: Molecular analysis of the loss of somatic instability in the bz2::mu1allele of maize.Mol Gen Genet1991,
229:147-151.
38. Gaut BS, Doebley JF: DNA-sequence evidence for the segmental allotetraploid origin of maize. Proc Natl Acad Sci USA 1997,