Effects of background color on growth
performances and physiological responses of
scaled carp (
Cyprinus carpio L
.) reared in a
closed circulated system
S.E. Papoutsoglou
a,* , G. Mylonakis
a, H. Miliou
a,
N.P. Karakatsouli
a, S. Chadio
baLaboratory of Applied Hydrobiology,Faculty of Animal Production,Agricultural Uni6ersity of Athens, Iera Odos75,Votanikos118 55,Athens,Greece
bLaboratory of Animal Anatomy and Physiology,Faculty of Animal Production, Agricultural Uni6ersity of Athens,Iera Odos75,Votanikos118 55,Athens,Greece
Received 10 October 1999; accepted 14 March 2000
Abstract
Growth performances and physiological responses ofCyprinus carpioafter long-term (14 weeks) background color adaptation were investigated. Six groups of ten individuals each (initial body weight 116 g) were reared in black, green and white tanks (two replicate groups for each color). At the end of the experiment, blood (cortisol, glucose, haematocrit, cholesterol, triglycerides, total lipids, osmolality, electrolytes, pCO2, pH), liver (total lipids,
glycogen, hepatosomatic index) and growth (body weight, specific growth rate, food conver-sion ratio, condition factor, proximate carcass composition) parameters were determined. Plasma cortisol levels in white-adapted carp were significantly (PB0.05) lower than those in black, while in green-adapted fish did not differ significantly from those in both other counterparts. White-adapted carp showed the highest specific growth rate and the lowest food conversion ratio, whereas black-adapted fish exhibited the opposite pattern. In addi-tion, mean (%) increase of body weight in white-adapted carp was 4.66 and 3.58% higher than that in black- and green-adapted fish, respectively. Furthermore in white-adapted carp, blood pCO2and pH were significantly higher and lower, respectively, than those obtained in
black- and green-adapted fish. In black-adapted carp, liver total lipid levels were significantly lower, and plasma total lipid levels were significantly higher, than those in white- and
www.elsevier.nl/locate/aqua-online
* Corresponding author. Tel:+30-1-5294401; fax:+30-1-5294401. E-mail address:[email protected] (S.E. Papoutsoglou)
green-adapted fish. No significant variations were observed in the other parameters. It is concluded that different background colors may lead to different growth performances of scaled carp depending upon rearing conditions. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Background color; Growth; Physiology; Cortisol; Stress;Cyprinus carpio
1. Introduction
It has been shown that in some teleost species a notable background color adaptation occurs as a final result of defense mechanisms (Bond, 1996). Color modification can also occur during fish reproductive period (Baker, 1963; Moyle et al., 1996), as well as under stress conditions (Mazeaud et al., 1977; Papoutsoglou, 1998). It is well known that these color changes are due to aggregation and dispersion of pigment (neural and hormonal process) and to alterations in the amount of pigment and the number of chromatophores (hormonal process) (Bowley et al., 1983; Green and Baker, 1989; Sugimoto, 1993). Hormones involved are
melanocyte-stimulating hormone (a-MSH), melanin-concentrating hormone
(MCH), melatonin, and catecholamines (Filadelfi and Castrucci, 1994; Papout-soglou, 1998).
Studies in Oncorhynchus mykiss, Anguilla anguilla, Salmo trutta, Sarotherodon mossambicus, Pleuronectes americanus and Zacco temmincki have shown that enhanced release ofa-MSH is associated with skin darkening (dispersion of melanin
granules) on a black background, whereas high levels of circulating MCH are related to skin lightening (aggregation of melanin granules) on a white background (Van Eys and Peters, 1981; Iga and Takabatake, 1982; Baker et al., 1985; Pickering et al., 1986; Powell and Baker, 1988; Burton, 1993). It has also been found that under certain conditions,a-MSH stimulates the secretion of cortisol from interrenal
tissue (Lamers et al., 1992), while MCH depresses the release of corticotrophin-re-leasing bioactivity from hypothalamic tissue (Baker et al., 1985; Green et al., 1991). In addition to exhibiting changes in pigmentation, rainbow trout (O. mykiss) adapted to a black background, in response to externally induced stress, showed higher plasma corticotrophin and cortisol levels, lower plasma MCH levels and similar plasmaa-MSH levels compared to white-adapted counterparts (Gilham and
Baker, 1985; Green and Baker, 1991; Green et al., 1991). Also, Baker and Rance (1981), working with O. mykiss and A. anguilla in a noisy aquarium, have shown that plasma cortisol levels were higher in fish maintained in black tanks for 3 – 4 weeks compared to those in white-adapted counterparts. Furthermore, background color has been shown to influence the behaviour ofOreochromis niloticus (Fanta, 1995).
Despite the worldwide economic importance of carp (Cyprinus carpioL.) culture, which is carried out by the application of all known production systems (using earth ponds, plastic or concrete tanks, net cages, recirculated water systems), there is no published information related to the physiological responses of this species caused by different rearing background colors.
The present study has been designed to evaluate the effects of long-term adaptation to black, green and white background on growth performances and physiological responses of scaled carp under certain experimental conditions using a recirculated water system.
2. Materials and methods
2.1.Fish and experimental design
Fish acclimated to laboratory conditions for 18 months were used in the experiment. They were kept in pale-blue circular fiberglass tanks and fed twice a week ad libitum. Sixty fish with mean initial body weight of 116 g were randomly distributed in six black, white and green cylindrical tanks of 27-l capacity, at a density of ten individuals per tank (two replicate tanks for each color). The water used (water exchange rate: 5.9 times per hour) was tap water drawn from the 50-ton laboratory recirculating system (dechlorinated, filtered and UV-sterilized), provided with compressed air supply and its physicochemical parameters (means9S.E.M.)
were monitored daily (temperature=23.990.07°C, DO=6.090.03 ppm, pH=
7.2590.005, total ammonia=0.2490.006 ppm, NH3=2.390.1×10− stant photoperiod (8L:16D) was used throughout the experiment, which lasted 14 weeks. Light intensity just over the net cover of the tanks was approximately 75 – 80 lux. Fish were fed by hand a pelleted diet, once per day (1.5% of initial body weight), of the following composition: moisture 9.5%, crude protein 47%, crude fat 11.5%, crude fiber 2%, ash 11.5% and N.F.E. 18.5%. Feeding level was not adjusted during the experiment to prevent possible stress responses caused by the weighing procedures in fish.
2.2.Blood sampling and analytical methods
At the end of the experiment, all fish per tank were anaesthetized using 2-phenoxyethanol (0.0008 ml/g per l) and immobilized within 2 min. All fish groups were handled in the same way. Blood samples were taken individually from both the heart and ventral aorta by heparinized syringes. Blood from the heart was immediately used for haematocrit determination, as well as for electrolytes (Na+
, Cl−and K+), pCO
2and pH measurements (288-Blood Gas System, Ciba-Corning).
levels were measured by radioimmunoassay (RIA) using a commercially available kit (Coat-A-Count Cortisol, DPC) which has been previously validated for fish (Ainsworth et al., 1985). In the present study, the sensitivity of the assay was 0.2
mg/dl and intra- and interassay coefficient of variation was 3.2 and 6.5%,
respectively.
Liver total lipids (Folch et al., 1957), hepatosomatic index and liver glycogen (Montgomery, 1957) were also determined. Conventional techniques were used for specific growth rate (SGR), food conversion ratio (FCR) and condition factor determinations (Papoutsoglou and Tziha, 1996). In addition, all fish of each group were minced together, without viscera, lyophilized and used for proximate carcass analysis (six replicates for each group) according to Kjeldahl and Soxhlet methods. Comparisons of means were performed by one-way analysis of variance (ANOVA) and Duncan’s multiple range test (Sokal and Rohlf, 1995). Differences were considered significant at PB0.05. Where appropriate, data were log
trans-formed in order to obtain homogeneity of variance. Untranstrans-formed means9
S.E.M. are given. The data obtained for the replicate groups of each color were pooled, after testing the significance of differences between them in all parameters examined. For the parameters not individually estimated (SGR, FCR, carcass composition) the duplicated mean is presented.
3. Results
Skin color adaptation to background color was completed by the end of 2 weeks in all populations. Fish from black tanks obtained deeply darkened skin color, while fish from white tanks became distinctively pale. Carp maintained on green background held the color they initially had before transfer to the experimental tanks (grey-brownish). Not any obvious change of body color was further observed in all groups throughout the experiment.
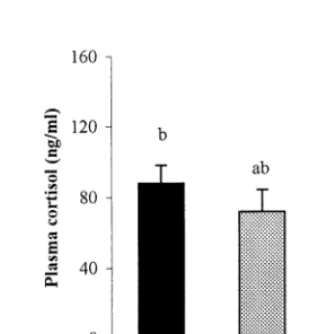
Plasma cortisol levels in white-adapted carp (43.05910.316 ng/ml) were signifi-cantly (PB0.05) lower than those in black-adapted fish (87.69910.352 ng/ml), whereas in green-adapted fish (71.58912.389 ng/ml) levels did not significantly differ from those in both other counterparts (Fig. 1). In addition, in white-adapted carp, pCO2and pH were significantly higher and lower, respectively, compared to
those observed in and green-adapted carp (Table 1). Furthermore, black-adapted fish exhibited the highest plasma total lipid levels, significantly different from those in both other counterparts. No significant differences were found in the other blood parameters among the fish adapted to three colored backgrounds.
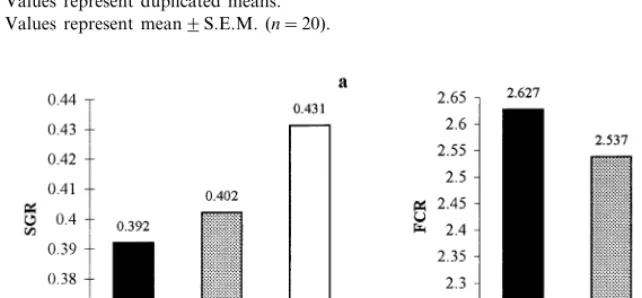
No statistically significant (P\0.05) differences for the values of final body weight, condition factor, hepatosomatic index and liver glycogen content and no important differences in values of the major carcass components were found among experimental fish groups (Table 2). Nevertheless, black-adapted carp showed the lowest liver total lipid levels, significantly different (PB0.05) from those observed in the other groups.
Fig. 1. Plasma cortisol levels (means9S.E.M.) after adaptation for 14 weeks on black (n=18), green (n=20) and white (n=20) backgrounds; means marked with different letter are significantly different (PB0.05).
Table 1
Effect of background adaptation for 14 weeks on blood parameters*
Background color
n White
Black n Green n
20 88.6698.209a
Glucose (mg/100ml) 70.6597.191a 17 86.7699.561a 16
3.4490.803a 15
pO2 (mmHg) 19 1.9490.262a 14 2.5290.768a
pCO2(mmHg) 9.3090.576a 19 8.9890.298a 14 13.0090.807b 15 19 7.4790.037b 14
pH 7.5490.033b 7.3690.041a 15
19 120.2891.430a 14 124.4591.038a 15 Na+(mmol/l) 121.4791.277a
2.8990.266a 13
K+(mmol/l) 15 3.3490.194a 11 2.2690.372a Cl−(mmol/l) 99.6891.311a 19 97.0091.637a 14 102.0091.195a 15
18 0.26290.002a 14
Osmolality (osmol/Kg) 0.25990.001a 0.26290.001a 15 20 41.9890.910a 20
Haematocrit (%) 41.3591.470a 42.1090.978a 20
19 132.0895.164a 20
140.5995.903a Triglycerides (mg/100ml) 148.4896.410a 20
Cholesterol (mg/100ml) 126.5196.451a 20 116.7795.029a 20 131.4493.936a 20 Total lipids (mg/100ml) 739.4937.62b 19 612.8924.39a 20 613.4924.15a 19 * Means with different superscript are significantly different (PB0.05). Values represent mean9
Table 2
Effect of background adaptation for 14 weeks on growth and liver parameters*
Background color
Carcass total lipid (% w.w.)† 5.841
3.114
Mean (%) increase of b.wt† 39.226
Initial body weight (g)‡ 118.897.71a 114.697.17a 114.696.87a Hepatosomatic index‡ 1.3390.093a 1.3590.080a
11.8590.636b 10.6790.523b
8.4990.420a Total liver lipids (% w.w.)‡
1.5090.231a
1.3290.196a 1.7290.246a Liver glycogen (% w.w.)‡
* Means with different superscript are significantly different (PB0.05). †Values represent duplicated means.
‡Values represent mean9S.E.M. (n=20).
Fig. 2. Effect of background adaptation for 14 weeks on: (a) specific growth rate (SGR) and (b) food conversion ratio (FCR); duplicated means.
4. Discussion
The lack of related data concerning specific physiological responses of carp caused by different background color adaptation, does not permit adequate com-parisons of the results obtained in the present work.
plasma cortisol levels. Previous data in rainbow troutOncorhynchus mykissshowed no significant differences in plasma cortisol levels between black- and white-adapted fish (Gilham and Baker, 1985; Green and Baker, 1991). However, in the present study and under the specific rearing conditions used, significant differences in plasma cortisol levels between black- and white-adapted carp were observed. Plasma cortisol levels in white-adapted carp were lower than those in black-adapted fish probably due to increased plasma MCH levels, which exert an hypothalamo-corticotrophic modulation (Green et al., 1991).
According to the present results neither plasma glucose nor liver glycogen levels could be related to those of plasma cortisol. Similarly, other studies (e.g. on
Ictalurus punctatus, O. mykiss, Hemitripterus americanus) have shown that chroni-cally elevated cortisol levels are not necessarily associated with alterations in glycogen stores or blood glucose levels (Davis et al., 1985; Andersen et al., 1991; Vijayan et al., 1996). Mommsen et al. (1999), reviewing the effects of cortisol on carbohydrate metabolism, referred that following exposure to increased cortisol levels, plasma glucose and liver glycogen can be increased, decreased or remain unaltered, depending on species, developmental stage and metabolic state of the animal.
Taking into consideration the data related to the total liver lipid and glycogen contents obtained in the present study, it could be assumed that the intermediary metabolism of carp is characterized by the use of lipids, mainly, for their energy requirements. This hypothesis is supported by the lowest total liver lipid levels observed in black-adapted fish, which also showed the highest plasma total lipid levels and cortisol as well. Catabolic action of cortisol on lipids metabolism, reducing total liver content and promoting plasma free fatty acids (FFA) metabolism, has been observed in a number of both fresh and seawater teleost species (Pickering and Pottinger, 1995; Sheridan, 1986).
The observed differences in the levels of blood pCO2 and pH may have been
due to an increased aerobic activity in white-adapted carp compared to that in black- and green-adapted fish. This increased aerobic activity may indicate an increase in oxygen consumption, although it was not measured in the present study. Besides, a possible increase in oxygen consumption by the white-adapted carp could be related to the highest SGR that they exhibited. Carter and Brafield (1992) have found significant positive correlations between oxygen
con-sumption and SGR in grass carp Ctenopharyngodon idella. Furthermore, Fanta
(1995) observed that Oreochromis niloticus on white background exhibited higher respiratory frequencies than on black, blue, green, yellow and red backgrounds. In conclusion, the results obtained showed that different background colors may lead to different growth performances of scaled carp, depending upon the specific experimental conditions used, which include biotic (e.g. stocking density, size, age, physiology, feeding habit, genetics) and abiotic (e.g. feeding pattern, illumination, temperature, water quality) factors.
Acknowledgements
We are grateful to Mr Th.V. Kyprianidis and Mr X. Vrettos for their assis-tance.
References
Ainsworth, A.J., Bowser, P.R., Beleau, M.H., 1985. Serum cortisol levels in channel catfish from production ponds. Prog. Fish Cult. 47, 176 – 181.
Andersen, D.E., Reid, S.D., Moon, T.W., Perry, S.F., 1991. Metabolic effects associated with chroni-cally elevated cortisol in rainbow trout (Oncorhynchus mykiss). Can. J. Fish Aquat. Sci. 48, 1811 – 1817.
Baker, B.I., 1963. Effect of adaptation to black and white backgrounds on the teleost pituitary. Nature 198, 404.
Baker, B.I., Rance, T.A., 1981. Differences in concentrations of plasma cortisol in the trout and the eel following adaptation to black or white backgrounds. J. Endocr. 89, 135 – 140.
Baker, B.I., Bird, D.J., Buckingham, J.C., 1985. Salmonid melanin-concentrating hormone inhibits corticotrophin release. J. Endocr. 106, R5 – R8.
Barton, B.A., Iwama, G.K., 1991. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Ann. Rev. Fish Dis., 3 – 26.
Bond, C.E., 1996. Biology of Fishes. Saunders College Publishing, Florida, p. 750. Bonga, S.E.W., 1997. The stress response in fish. Physiol. Rev. 77, 591 – 625.
Bowley, T.J., Rance, T.A., Baker, B.I., 1983. Measurement of immunoreactivea
-melanocyte-stimulat-ing hormone in the blood of rainbow trout kept under various conditions. J. Endocr. 97, 267 – 275.
Burton, D., 1993. The effects of background colouration and alpha – MSH treatment on melanophore frequency in winter flounder,Pleuronectes americanus. J. Comp. Physiol. 173A, 329 – 333.
Davis, K.B., Torrance, P., Parker, N.C., Suttle, M.A., 1985. Growth, body composition and hepatic tyrosine aminotransferase activity in cortisol-fed channel catfish,Ictalurus punctatusRafinesque. J. Fish Biol. 27, 177 – 184.
Fanta, E., 1995. Influence of background color on the behavior of the fish Oreochromis niloticus (Cichlidae). Arq. Biol. Technol. 38, 1237 – 1251.
Farbridge, K.J., Leatherland, J.F., 1992. Plasma growth hormone levels in fed and fasted rainbow trout (Oncorhynchus mykiss) are decreased following handling stress. Fish Physiol. Biochem. 10, 67 – 73.
Filadelfi, A.M.C., Castrucci, A.M.L., 1994. Melatonin desensitizing effects on the in vitro responses to MCH, alpha-MSH, isoproterenol and melatonin in pigment cells of a fish (S. marmoratus), a toad (B.ictericus), a frog (R. pipiens), and a lizard (A. carolinensis), exposed to varying photope-riodic regimens. Comp. Biochem. Physiol. 109A, 1027 – 1037.
Folch, J., Lees, M., Sloane-Stanley, G.A., 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497 – 509.
Foo, J.T.W., Lam, T.J., 1993. Serum cortisol response to handling stress and the effect of cortisol implantation on testosterone level in the tilapiaOreochromis mossambicus. Aquaculture 115, 145 – 158.
Gilham, I.D., Baker, B.I., 1985. A black background facilitates the response to stress in teleosts. J. Endocr. 105, 99 – 105.
Green, J.A., Baker, B.I., 1989. Influences of nerves and hormones on the control of trout and grass carp melanophores. Life Sci. 45, 1127 – 1132.
Green, J.A., Baker, B.I., 1991. The influence of repeated stress on the release of melanin-concentrat-ing hormone in the rainbow trout. J. Endocr. 128, 261 – 266.
Green, J.A., Baker, B.I., Kawauchi, H., 1991. The effects of rearing rainbow trout on black or white backgrounds on their secretion of melanin-concentrating hormone and their sensitivity to stress. J. Endocr. 128, 267 – 274.
Iga, T., Takabatake, I., 1982. Action of melanophore-stimulating hormone on melanophores of the cyprinid fishZacco temmincki. Comp. Biochem. Physiol. 73C, 51 – 55.
Lamers, A.E., Flik, G., Atsma, W., Wendelaar-Bonga, S.E., 1992. A role for di-acetyl a-melanocyte-stimulating hormone in the control of cortisol release in the teleostOreochromis mossambicus. J. Endocr. 135, 285 – 292.
Mazeaud, M.M., Mazeaud, F., Donaldson, M., 1977. Primary and secondary effects of stress in fish: Some new data with a general review. Trans. Am. Fish. Soc. 106, 201 – 212.
Montgomery, R., 1957. Determination of glycogen. Arch. Biochem. Biophys. 67, 378 – 386.
Mommsen, T.P., Vijayan, M.M., Moon, T.W., 1999. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 9, 211 – 268.
Moyle, P.B., Cech, J.J. Jr, 1996. Fishes: Introduction to Ichthyology. Prentice Hall, New Jersey, p. 608.
Papoutsoglou, S.E., 1998. Endocrinology of Fishes. Stamoulis Press, Athens, p. 599 (in Greek). Papoutsoglou, S.E., Tziha, G., 1996. Blue tilapia (Oreochromis aureus) growth rate in relation to
dissolved oxygen concentration under recirculated water conditions. Aquacult. Eng. 15, 181 – 192. Pickering, A.D., 1993. Growth and stress in fish production. Aquaculture 111, 51 – 63.
Pickering, A.D., Pottinger, T.G., 1995. Biochemical effects of stress. In: Hochashka, P.W., Momm-sen, T.P. (Eds.), Biochemistry and Molecular Biology of Fishes, vol. 5. Elsevier, Amsterdam, pp. 349 – 379.
Pickering, A.D., Pottinger, T.G., Sumpter, J.P., 1986. Independence of the pituitary-interrenal axis and melanotroph activity in the brown trout, Salmo truttaL., under conditions of environmental stress. Gen. Comp. Endocr. 64, 206 – 211.
Powell, K.A., Baker, B.I., 1988. Structural studies of nerve terminal containing melanin-concentrating hormone in the eel,Anguilla anguilla. Cell Tissue Res. 251, 433 – 439.
Sokal, R.R., Rohlf, F.J., 1995. Biometry. W.H. Freedman and Company, New York, p. 887. Sugimoto, M., 1993. Morphological color changes in the medaka, Oryzias latipes, after prolonged
background adaptation. I. Changes in the population and morphology of melanophores. Comp. Biochem. Physiol. 104A, 513 – 518.
Van Eys, G.J.J.M., Peters, P.T.W., 1981. Evidence for a direct role of a-MSH in morphological background adaptation of the skin inSarotherodon mossambicus. Cell Tissue Res. 217, 361 – 372. Vijayan, M.M., Mommsen, T.P, Gle´met, H.C., Moon, T.W., 1996. Metabolic effects of cortisol
treatment in a marine teleost, the sea raven. J. Exp. Biol. 199, 1509 – 1514.